Epidemiological Mapping of Canine Angiostrongylosis in Portugal: Findings from a Nationwide Prevalence Survey
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Eco-Climatic Characteristics
2.2. Sample Collection and Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Canine angiostrongylosis |
| L1 | First-stage larvae |
| L3 | Third-stage larvae |
| BAL | Bronchoalveolar lavage |
| ELISA | Enzyme-linked immunosorbent assay |
| PCR | Polymerase chain reaction |
| NUTS | Nomenclature of Units for Territorial Statistics |
| Csa | Hot-summer Mediterranean climate |
| Csb | Warm-summer Mediterranean climate |
| Cfb | Temperate oceanic climate |
| HWD | Heartworm disease |
References
- Helm, J.R.; Morgan, E.R.; Jackson, M.W.; Wotton, P.; Bell, R. Canine angiostrongylosis: An emerging disease in Europe. J. Vet. Emerg. Crit. Care 2010, 20, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Traversa, D.; Grillotti, E.; Pezzuto, C.; De Tommaso, C.; Pampurini, F.; Schaper, R.; Drake, J.; Crisi, P.E.; Russi, I.; et al. Highly Variable Clinical Pictures in Dogs Naturally Infected with Angiostrongylus vasorum. Pathogens 2021, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, Y.; McGarry, J.W.; Denk, D.; Dukes-McEwan, J.; Macdonald, N.; Mas, A.; McConnell, F.; Tatton, B.; Valentine, E.G.; Wayne, J.; et al. Emerging canine angiostrongylosis in northern England: Five fatal cases. Vet. Rec. 2009, 164, 149–152. [Google Scholar] [CrossRef]
- Borgeat, K.; Sudunagunta, S.; Kaye, B.; Stern, J.; Luis Fuentes, V.; Connolly, D.J. Retrospective evaluation of moderate-to-severe pulmonary hypertension in dogs naturally infected with Angiostrongylus vasorum. J. Small Anim. Pract. 2015, 56, 196–202. [Google Scholar] [CrossRef]
- Chapman, P.S.; Boag, A.K.; Guitian, J.; Boswood, A. Angiostrongylus vasorum infection in 23 dogs (1999–2002). J. Small Anim. Pract 2004, 45, 435–440. [Google Scholar] [CrossRef]
- Schnyder, M.; Stebler, K.; Naucke, T.J.; Lorentz, S.; Deplazes, P. Evaluation of a rapid device for serological in-clinic diagnosis of canine angiostrongylosis. Parasit. Vectors 2014, 7, 72. [Google Scholar] [CrossRef]
- Liu, J.; Schnyder, M.; Willesen, J.L.; Potter, A.; Chandrashekar, R. Performance of the Angio DetectTM in-clinic test kit for detection of Angiostrongylus vasorum infection in dog samples from Europe. Vet. Parasitol. Reg. Stud. Rep. 2017, 7, 45–47. [Google Scholar] [CrossRef]
- Morgan, E.; Shaw, S. Angiostrongylus vasorum infection in dogs: Continuing spread and developments in diagnosis and treatment. J. Small. Anim. Pract. 2010, 51, 616–621. [Google Scholar] [CrossRef]
- Koch, J.; Willesen, J.L. Canine pulmonary angiostrongylosis: An update. Vet. J. 2009, 179, 348–359. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Holmes, S.A.; Wright, I.; Morgan, E.R.; Lacher, D.W. Recent advances in the epidemiology, clinical and diagnostic features, and control of canine cardio-pulmonary angiostrongylosis. Vet. Res. 2014, 45, 92. [Google Scholar] [CrossRef]
- Morgan, E.R.; Modry, D.; Paredes-Esquivel, C.; Foronda, P.; Traversa, D. Angiostrongylosis in Animals and Humans in Europe. Pathogens 2021, 10, 1236. [Google Scholar] [CrossRef]
- Schnyder, M.; Schaper, R.; Bilbrough, G.; Morgan, E.R.; Deplazes, P. Seroepidemiological survey for canine angiostrongylosis in dogs from Germany and the UK using combined detection of Angiostrongylus vasorum antigen and specific antibodies. Parasitology 2013, 140, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Martins, Â.; Brancal, H.; Vilhena, H.; Silva, P.; Pimenta, P.; Diz-Lopes, D.; Neves, N.; Coimbra, M.; Alves, A.C.; et al. Parasitic zoonoses associated with dogs and cats: A survey of Portuguese pet owners’ awareness and deworming practices. Parasit. Vectors 2016, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Esteves-Guimarães, J.; Matos, J.I.; Leal-Sousa, B.; Oliveira, P.; Lobo, L.; Silvestre-Ferreira, A.C.; Soares, C.S.; Rodríguez-Escolar, I.; Carretón, E.; Morchón, R.; et al. Current State of Canine Heartworm in Portugal. Animals 2024, 14, 1300. [Google Scholar] [CrossRef]
- Alho, A.M.; Schnyder, M.; Schaper, R.; Meireles, J.; Belo, S.; Deplazes, P.; de Carvalho, L.M. Seroprevalence of circulating Angiostrongylus vasorum antigen and parasite-specific antibodies in dogs from Portugal. Parasitol. Res 2016, 115, 2567–2572. [Google Scholar] [CrossRef]
- Carretón, E.; Morchón, R.; Falcón-Cordón, Y.; Matos, J.; Costa-Rodríguez, N.; Montoya-Alonso, J.A. First epidemiological survey of Angiostrongylus vasorum in domestic dogs from Spain. Parasit. Vectors 2020, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Carretón, E.; Morchón, R.; García-Rodríguez, S.N.; Rodríguez-Escolar, I.; Matos, J.I.; Costa-Rodríguez, N.; Montoya-Alonso, J.A. Comprehensive Map of Canine Angiostrongylosis in Dogs in Spain. Animals 2022, 12, 2217. [Google Scholar] [CrossRef]
- Tachmazidou, A.; Papaioannou, N.; Diakou, A.; Savvas, I.; Patsikas, M.; Stylianaki, I.; Morelli, S.; Di Cesare, A.; Mylonakis, M.E. First report of fatal autochthonous angiostrongylosis in a dog in Greece. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100519. [Google Scholar] [CrossRef]
- Agencia Estatal de Meteorología; Ministerio de Agricultura; Alimentación y Medio Ambiente. Iberian Climate Atlas. Air Temperature and Precipitation (1971–2000). Available online: https://www.aemet.es/documentos/es/conocermas/publicaciones/Atlas-climatologico/Atlas.pdf (accessed on 19 April 2024).
- Alho, A.M.; Meireles, J.; Schnyder, M.; Cardoso, L.; Belo, S.; Deplazes, P.; de Carvalho, L.M. Dirofilaria immitis and Angiostrongylus vasorum: The current situation of two major canine heartworms in Portugal. Vet. Parasitol. 2018, 252, 120–126. [Google Scholar] [CrossRef]
- Alho, A.M.; Pita, J.; Amaro, A.; Amaro, F.; Schnyder, M.; Grimm, F.; Custódio, A.C.; Cardoso, L.; Deplazes, P.; de Carvalho, L.M. Seroprevalence of vector-borne pathogens and molecular detection of Borrelia afzelii in military dogs from Portugal. Parasit. Vectors 2016, 9, 225. [Google Scholar] [CrossRef]
- Madeira de Carvalho, L.; Alho, A.M.; Matos, M.; Sousa, S.; Miranda, L.M.; Anastácio, S.; Otero, D.; Gomes, L.; Nunes, T.; Otranto, D.; et al. Some emerging canine vector borne diseases and antiparasitic control measures in companion animals in Portugal—Recent updates. In Proceedings of the XVIII Congreso de la Sociedad Española de Parasitologia (SOCEPA), Las Palmas de Gran Canaria, Spain, 17–20 September 2013; p. 100. [Google Scholar]
- Oliveira, H.D.; Lobo, L.L.; Simões, P.B.; Sargo, T.; Coutinho, T.; Machado, J.P.; Cardoso, L.; Fontes Sousa, A.P. Angiostrongylus vasorum infection in dogs from the North of Portugal. In Proceedings of the 2nd Bayer Angiostrongylosis Forum, Parma, Italy, 22 June 2012; p. 19. [Google Scholar]
- Serrão, I.; Braz, B.S.; Figueiredo, M.D.; Coimbra, M.; Brancal, H.; Fernandes, M.C.; Lopes, A.P.; Pimenta, P.; Martins, Â.; Pereira, A.; et al. Preliminary report on the prevalence of Angiostrongylus vasorum infection in dogs from Portugal adopting a commercially available test kit for serological analysis. Vet. Parasitol. Reg. Stud. Rep. 2016, 3–4, 57–59. [Google Scholar] [CrossRef]
- Nabais, J.; Alho, A.M.; Gomes, L.; da Silva, J.F.; Nunes, T.; Vicente, G.; de Carvalho, L.M. Aelurostongylus abstrusus in cats and Angiostrongylus vasorum in dogs from Lisbon, Portugal. Acta Parasitol. Port. 2014, 20, 35–40. [Google Scholar]
- Alho, A.M.; Schnyder, M.; Meireles, J.; Belo, S.; Deplazes, P.; Madeira de Carvalho, L. Preliminary results on the seroprevalence of Angiostrongylus vasorum and co-infection with Dirofilaria immitis in shelter dogs from Portugal. Parasit. Vectors 2014, 7, O26. [Google Scholar] [CrossRef]
- Alho, A.M.P.V.A. Dirofilaria Immitis and Angiostrongylus Vasorum: Epidemiology and Impact of Major Heartworms in Carnivores in Portugal. Ph.D. Thesis, Faculdade de Medicina Veterinária, Lisbon University, Lisbon, Portugal, 2017. [Google Scholar]
- Segovia Arias, J.; Torres, J.; Miquel, J. Helminth parasites of the red fox (Vulpes vulpes L., 1758) in the Iberian Peninsula: An ecological study. Acta Parasitol. 2004, 49, 67–79. [Google Scholar]
- Eira, C.; Vingada, J.; Torres, J.; Miquel, J. The Helminth Community of the Red Fox, Vulpes Vulpes, In Dunas de Mira (Portugal) and its effect on host condition. Wildl. Biol. Pract. 2006, 2, 26–36. [Google Scholar] [CrossRef]
- Segovia, J.M.; Torres, J.; Miquel, J.; Llaneza, L.; Feliu, C. Helminths in the wolf, Canis lupus, from north-western Spain. J. Helminthol. 2001, 75, 183–192. [Google Scholar] [CrossRef]
- Figueiredo, A.; Oliveira, L.; Madeira de Carvalho, L.; Fonseca, C.; Torres, R.T. Parasite species of the endangered Iberian wolf (Canis lupus signatus) and a sympatric widespread carnivore. Int. J. Parasitol. Parasites. Wildl. 2016, 5, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Morchón, R.; García-Rodríguez, S.N.; Falcón-Cordón, Y.; Costa-Rodríguez, N.; Matos, J.I.; Rodríguez Escolar, I.; Carretón, E. Expansion of Canine Heartworm in Spain. Animals 2022, 12, 1268. [Google Scholar] [CrossRef]
- Maia, C.; Coimbra, M.; Ramos, C.; Cristóvão, J.M.; Cardoso, L.; Campino, L. Serological investigation of Leishmania infantum, Dirofilaria immitis and Angiostrongylus vasorum in dogs from southern Portugal. Parasit. Vectors 2015, 8, 152. [Google Scholar] [CrossRef]
- Teixeira, R.P.C. Rastreio de parasitas gastrointestinais e pulmonares em canídeos e felídeos da Região Autónoma dos Açores, ilhas de São Miguel e Terceira. Master’s Thesis, Faculdade de Medicina Veterinária, Lisbon University, Lisbon, Portugal, 2020. [Google Scholar]
- Gomes, B.C.G. Contribuição para o estudo dos parasitas gastrointestinais, pulmonares e hemáticos em cães na cidade do Funchal, ilha da Madeira. Master’s Thesis, Faculdade de Medicina Veterinária, Lisbon University, Lisbon, Portugal, 2019. [Google Scholar]
- Morgan, E.R.; Jefferies, R.; Krajewski, M.; Ward, P.; Shaw, S.E. Canine pulmonary angiostrongylosis: The influence of climate on parasite distribution. Parasitol. Int. 2009, 58, 406–410. [Google Scholar] [CrossRef]
- Rosen, L.; Ash, L.R.; Wallace, G.D. Life history of the canine lungworm Angiostrongylus vasorum (Baillet). Am. J. Vet. Res. 1970, 31, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Guilhon, J.; Afghahi, A. [Larval development of Angiostrongylus vasorum (Baillet, 1866) in the body of various species of terrestrial mollusks]. C. R. Acad. Hebd. Seances. Acad. Sci. D. 1969, 268, 434–436. [Google Scholar] [PubMed]
- Holyoak, D.; Holyoak, G.; Mendes, R. A revised check-list of the land and freshwater Mollusca (Gastropoda and Bivalvia) of mainland Portugal. Iberus 2019, 37, 113–168. [Google Scholar]
- Quinteiro, J.; Rodríguez-Castro, J.; Castillejo, J.; Iglesias, J.; Rey-Méndez, M. Phylogeny of slug species of the genus Arion: Evidence of monophyly of Iberian endemics and of the existence of relict species in Pyrenean refuges. J. Zool. Syst. Evol. Res. 2005, 43, 139–148. [Google Scholar] [CrossRef]
- GBIF. The Global Biodiversity Information Facility Portugal—Portal de Dados de Biodiversidade de Portugal. Available online: https://registos.gbif.pt/occurrences/search?q=arion+ater#tab_mapView; https://registos.gbif.pt/occurrences/search?q=arion+rufus#tab_mapView (accessed on 2 May 2024).
- De Oliveira, Á. Fauna Malacológica da cidade de Coimbra (Beira Litoral). Moluscos “urbanos” de Portugal. 1. Iberus 2010, 28, 39–50. [Google Scholar]
- Morchón, R.; Montoya-Alonso, J.A.; Sánchez-Agudo, J.; de Vicente-Bengochea, J.; Murcia-Martínez, X.; Carretón, E. Angiostrongylus vasorum in Domestic Dogs in Castilla y León, Iberian Peninsula, Spain. Animals 2021, 11, 1513. [Google Scholar] [CrossRef]
- Traversa, D.; Morelli, S.; Cesare, A.; Astuti, C.; Barlaam, A.; Colombo, M.; Veronesi, F.; Paoletti, B.; Iorio, R.; Maggi, R.; et al. Current Enzooticity of Dirofilaria immitis and Angiostrongylus vasorum in Central and Southern Italy. Animals 2025, 15, 172. [Google Scholar] [CrossRef]
- Mozzer, L.R.; Lima, W.S. Gallus gallus domesticus: Paratenic host of Angiostrongylus vasorum. Vet. Parasitol. 2015, 207, 81–84. [Google Scholar] [CrossRef]
- Morgan, E.R.; Jefferies, R.; van Otterdijk, L.; McEniry, R.B.; Allen, F.; Bakewell, M.; Shaw, S.E. Angiostrongylus vasorum infection in dogs: Presentation and risk factors. Vet. Parasitol. 2010, 173, 255–261. [Google Scholar] [CrossRef]
- Di Cesare, A.; Traversa, D. Canine angiostrongylosis: Recent advances in diagnosis, prevention, and treatment. Vet. Med. 2014, 5, 181–192. [Google Scholar] [CrossRef]
- Di Cesare, A.; Traversa, D.; Manzocchi, S.; Meloni, S.; Grillotti, E.; Auriemma, E.; Pampurini, F.; Garofani, C.; Ibba, F.; Venco, L. Elusive Angiostrongylus vasorum infections. Parasite. Vectors 2015, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Becskei, C.; Willesen, J.L.; Schnyder, M.; Wozniakiewicz, M.; Miroshnikova, N.; Mahabir, S.P. Field safety and efficacy of an orally administered combination of sarolaner, moxidectin and pyrantel (Simparica Trio(®)) for the prevention of angiostrongylosis in dogs presented as veterinary patients. Parasit. Vectors 2020, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- ESCCAP. Worm Control in Dogs and Cats. ESCCAP Guideline 01 Sixth Edition—May 2021. Available online: https://www.esccap.org/uploads/docs/oc1bt50t_0778_ESCCAP_GL1_v15_1p.pdf (accessed on 1 May 2024).
- Colella, V.; Lia, R.P.; Premont, J.; Gilmore, P.; Cervone, M.; Latrofa, M.S.; D’Anna, N.; Williams, D.; Otranto, D. Angiostrongylus vasorum in the eye: New case reports and a review of the literature. Parasit Vectors 2016, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Bank, R.A. Fauna Europaea Project: Checklist of the Land and Freshwater Gastropoda of the Iberian Peninsula. Available online: https://mvhn.wordpress.com/wp-content/uploads/2012/11/fauna_europaea_-_gastropoda_of_iberian_peninsula.pdf (accessed on 1 May 2024).
- Schnyder, M.; Tanner, I.; Webster, P.; Barutzki, D.; Deplazes, P. An ELISA for sensitive and specific detection of circulating antigen of Angiostrongylus vasorum in serum samples of naturally and experimentally infected dogs. Vet. Parasitol. 2011, 179, 152–158. [Google Scholar] [CrossRef]
- Al-Sabi, M.N.S.; Deplazes, P.; Webster, P.; Willesen, J.L.; Davidson, R.K.; Kapel, C.M. PCR detection of Angiostrongylus vasorum in faecal samples of dogs and foxes. Parasitol. Res. 2010, 107, 135–140. [Google Scholar] [CrossRef]

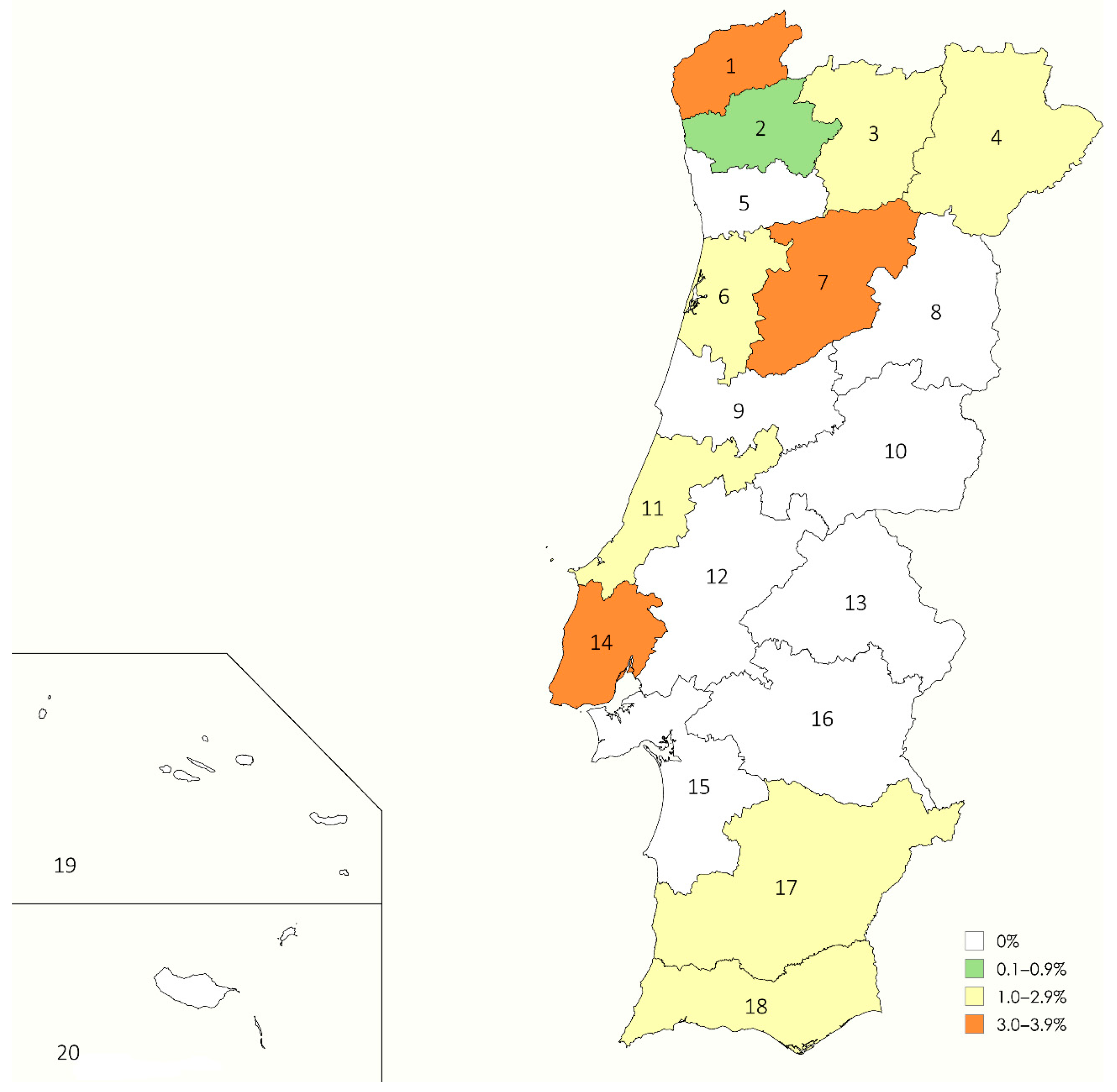
| Region Number | District | Climate | Geographical Area | n | + | % |
|---|---|---|---|---|---|---|
| Overall | 1059 | 12 | 1.13 | |||
| 1 | Viana do Castelo | Csb | Coastal | 51 | 2 | 3.9 |
| 2 | Braga | Csb | Coastal | 59 | 0 | 0.0 |
| 3 | Vila Real | Csb | Inland | 34 | 1 | 2.9 |
| 4 | Bragança | Csb | Inland | 51 | 1 | 2.0 |
| 5 | Porto | Csb | Coastal | 61 | 0 | 0.0 |
| 6 | Aveiro | Csb | Coastal | 65 | 1 | 1.5 |
| 7 | Viseu | Csb | Inland | 55 | 2 | 3.6 |
| 8 | Guarda | Csb | Inland | 79 | 0 | 0.0 |
| 9 | Coimbra | Csb | Coastal | 47 | 0 | 0.0 |
| 10 | Castelo Branco | Csa | Inland | 56 | 0 | 0.0 |
| 11 | Leiria | Csb | Coastal | 50 | 1 | 2.0 |
| 12 | Santarém | Csa | Inland | 50 | 0 | 0.0 |
| 13 | Portalegre | Csa | Inland | 69 | 0 | 0.0 |
| 14 | Lisbon | Csa | Coastal | 53 | 2 | 3.8 |
| 15 | Setúbal | Csa | Coastal | 39 | 0 | 0.0 |
| 16 | Évora | Csa | Inland | 44 | 0 | 0.0 |
| 17 | Beja | Csa | Inland | 69 | 1 | 1.4 |
| 18 | Faro | Csa | Coastal | 72 | 1 | 1.4 |
| 19 | Azores | Cfb | Insular | 25 | 0 | 0.0 |
| 20 | Madeira | Csb | Insular | 30 | 0 | 0.0 |
| Test Result | |||||
|---|---|---|---|---|---|
| Total | Positive | p-Value Chi2 | |||
| n | n | % | |||
| Geographical Area | Total | 1004 | 2 | 1.2 | 0.538 |
| Coastal | 497 | 7 | 1.4 | ||
| Inland | 507 | 5 | 1.0 | ||
| Climate | Total | 1059 | 12 | 1.1 | 0.658 |
| Csa | 452 | 4 | 0.9 | ||
| Csb | 582 | 8 | 1.4 | ||
| Cfb | 25 | 0 | 0.0 | ||
| NUTS-II | Total | 1059 | 12 | 1.1 | 0.587 |
| North | 276 | 4 | 1.4 | ||
| Centre | 356 | 4 | 1.1 | ||
| Lisbon | 92 | 2 | 2.2 | ||
| Alentejo | 208 | 1 | 0.5 | ||
| Algarve | 72 | 1 | 1.4 | ||
| Madeira | 30 | 0 | 0.0 | ||
| Azores | 25 | 0 | 0.0 | ||
| Test Result | ||||||
|---|---|---|---|---|---|---|
| Total | Positive | p-Value Chi2 | ||||
| n | % | n | % | |||
| Sex | Total | 1059 | 100.0 | 12 | 100.0 | 0.034 * |
| Female | 499 | 47.1 | 2 | 16.7 | ||
| Male | 560 | 52.9 | 10 | 83.3 | ||
| Age | Total | 1047 | 100.0 | 12 | 100.0 | 0.309 |
| <5 years | 411 | 39.3 | 3 | 25.0 | ||
| ≥5 years | 636 | 60.7 | 9 | 75.0 | ||
| Lifestyle | Total | 1054 | 100.0 | 12 | 100.0 | 0.010 * |
| Indoor | 107 | 10.2 | 4 | 33.3 | ||
| Mixed | 624 | 59.2 | 3 | 25.0 | ||
| Outdoor | 323 | 30.6 | 5 | 41.7 | ||
| Climate | Total | 1059 | 100.0 | 12 | 100.0 | 0.658 |
| Csa | 452 | 42.7 | 4 | 33.3 | ||
| Csb | 582 | 54.9 | 8 | 66.7 | ||
| Cfb | 25 | 2.4 | 0 | 0.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal-Sousa, B.; Esteves-Guimarães, J.; Matos, J.I.; Oliveira, P.; Lobo, L.; Silvestre-Ferreira, A.C.; Soares, C.S.; Carretón, E.; Morchón, R.; Fontes-Sousa, A.P.; et al. Epidemiological Mapping of Canine Angiostrongylosis in Portugal: Findings from a Nationwide Prevalence Survey. Vet. Sci. 2025, 12, 647. https://doi.org/10.3390/vetsci12070647
Leal-Sousa B, Esteves-Guimarães J, Matos JI, Oliveira P, Lobo L, Silvestre-Ferreira AC, Soares CS, Carretón E, Morchón R, Fontes-Sousa AP, et al. Epidemiological Mapping of Canine Angiostrongylosis in Portugal: Findings from a Nationwide Prevalence Survey. Veterinary Sciences. 2025; 12(7):647. https://doi.org/10.3390/vetsci12070647
Chicago/Turabian StyleLeal-Sousa, Beatriz, Joana Esteves-Guimarães, Jorge Isidoro Matos, Pedro Oliveira, Luís Lobo, Ana Cristina Silvestre-Ferreira, Carla S. Soares, Elena Carretón, Rodrigo Morchón, Ana Patrícia Fontes-Sousa, and et al. 2025. "Epidemiological Mapping of Canine Angiostrongylosis in Portugal: Findings from a Nationwide Prevalence Survey" Veterinary Sciences 12, no. 7: 647. https://doi.org/10.3390/vetsci12070647
APA StyleLeal-Sousa, B., Esteves-Guimarães, J., Matos, J. I., Oliveira, P., Lobo, L., Silvestre-Ferreira, A. C., Soares, C. S., Carretón, E., Morchón, R., Fontes-Sousa, A. P., & Montoya-Alonso, J. A. (2025). Epidemiological Mapping of Canine Angiostrongylosis in Portugal: Findings from a Nationwide Prevalence Survey. Veterinary Sciences, 12(7), 647. https://doi.org/10.3390/vetsci12070647












