Identification of the Pathogen Dorcadia ioffi Smit and Evaluation of the Effect of Different Drugs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Main Chemicals
2.3. Morphological Identification
2.4. Molecular Biological Identification
2.5. Pharmacodynamics Experiment
2.6. Data Processing and Statistical Analysis
3. Results
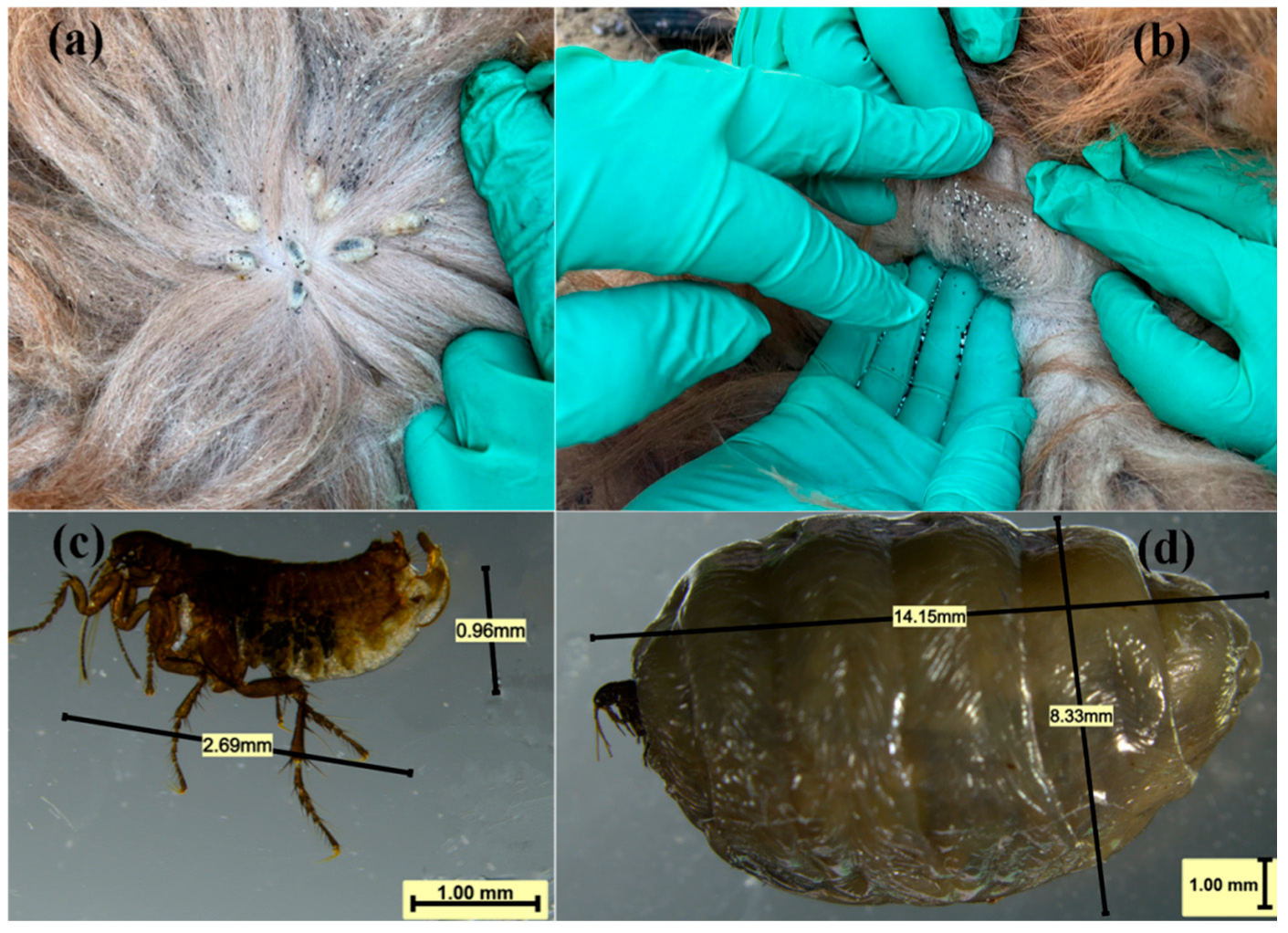
3.1. Parasitic Characteristics and Infection Phenotypes
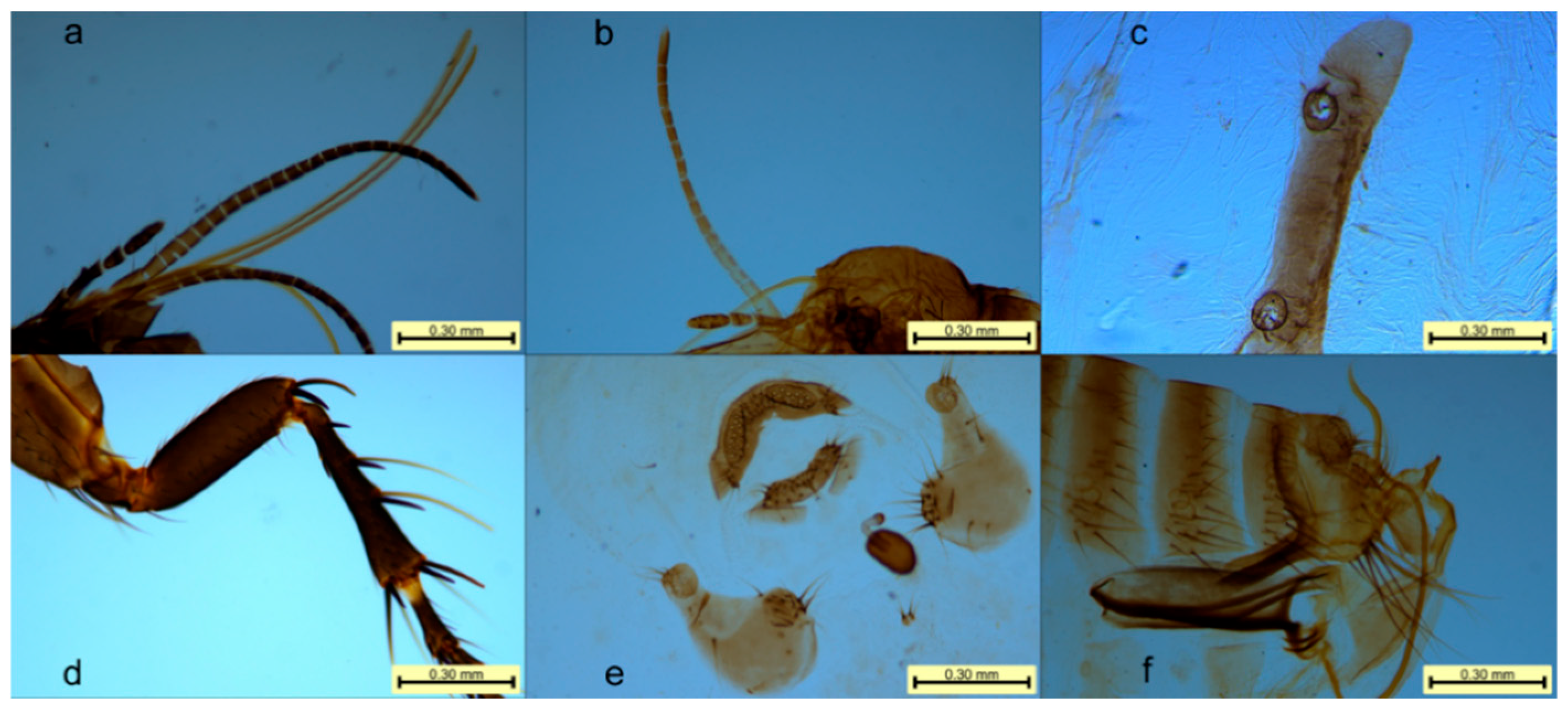
3.2. Morphological Result
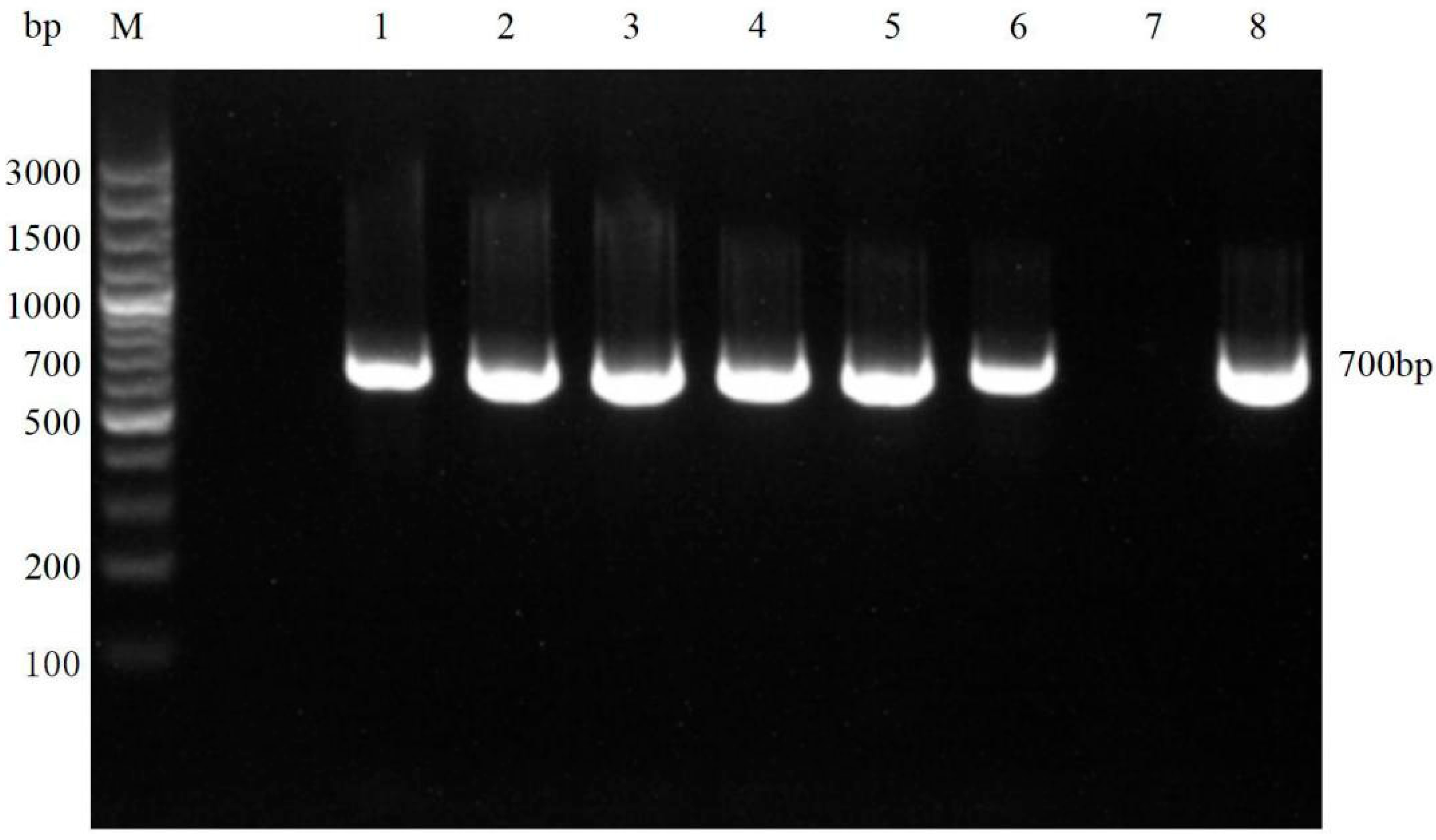
3.3. Molecular Biological Result
3.4. Statistical Analysis of Efficacy of Different Drugs
3.4.1. D. ioffi Infestation Counts
3.4.2. Statistical Analysis of D. ioffi Population Reduction Rate
3.4.3. Therapeutic Cure Rate for D. ioffi Infection in Sheep
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bossard, R.L.; Lareschi, M.; Urdapilleta, M.; Cutillas, C.; Zurita, A. Flea (Insecta: Siphonaptera) Family Diversity. Diversity 2023, 15, 1096. [Google Scholar] [CrossRef]
- Bernard, E.C.; Whittington, A.E. Papers and new species of minor insect orders published in Zootaxa, 2001–2020. Zootaxa 2021, 4979, 232–235. [Google Scholar] [CrossRef]
- Tarekegn, Z.S.; Leta, S.; Nigatu, S.D.; Mekonnen, S.A.; Molla, W. Modeling the spatial distribution and environmental factors of dominant ixodid tick species parasitizing cattle in northwest Ethiopia. Vet. Parasitol. 2025, 335, 110436. [Google Scholar] [CrossRef]
- Vinokurov, Y.I.; Krasnoyarova, B.A. Greater Altai: Features of Development and International Cooperation. Reg. Res. Russ. 2023, 13, 758–768. [Google Scholar] [CrossRef]
- Jordan, G.; Goenster, S.; Munkhnasan, T.; Shabier, A.; Buerkert, A.; Schlecht, E. Spatio-temporal patterns of herbage availability and livestock movements: A cross-border analysis in the Chinese-Mongolian Altay. Pastoralism 2016, 6, 12. [Google Scholar] [CrossRef]
- Xingxing, L.; Shi, G.; Li, L.; Zhang, R.; Qiao, J. Detection and molecular typing of epidemic Brucella strains among camels, sheep, and cattle in Xinjiang, China. PLoS ONE 2024, 19, e0311933. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.A.; Yang, M.; Pi, M.; Zhang, Y.; Su, Z. Prevalence and Associated Risk Factors of Helminth Infections in the Digestive Tract of Camels in Xinjiang, China. J. Vet. Sci. 2024, 11, 503. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, Z.; Guo, Y.; Guo, G.; Wang, H.Y.; Ma, J.J.; Chen, R.G.; Zheng, X.T.; Bao, J.L.; Li, H.; et al. High endemicity of alveolar echinococcosis in Yili Prefecture, Xinjiang Autonomous Region, the People’s Republic of China: Infection status in different ethnic communities and in small mammals. PLoS Negl. Trop. Dis. 2021, 15, e0008891. [Google Scholar] [CrossRef]
- Li, F.; Zhao, S.; Li, E.; Xie, S.; Wang, N.; Tan, W.; Wang, Y. Detection of Rickettsia raoultii in Vermipsylla alakurt-Like Fleas of Sheep in Northwestern China. Acta Parasitol. 2024, 69, 776–784. [Google Scholar] [CrossRef]
- Zhao, S.S.; Li, H.Y.; Yin, X.P.; Liu, Z.Q.; Chen, C.F.; Wang, Y.Z. First detection of Candidatus Rickettsia barbariae in the flea Vermipsylla alakurt from north-western China. Parasites Vectors 2016, 9, 325. [Google Scholar] [CrossRef]
- Mauchline, T.H.; Knox, R.; Mohan, S.; Powers, S.J.; Kerry, B.R.; Davies, K.G.; Hirsch, P.R. Identification of new single nucleotide polymorphism-based markers for inter-and intraspecies discrimination of obligate bacterial parasites (Pasteuria spp.) of invertebrates. Appl. Environ. Microbiol. 2011, 77, 6388–6394. [Google Scholar] [CrossRef]
- Yang, X.; Fan, Y.Y.; Yang, D.J.; Huang, S.; Wang, J.W.; Chen, X.; Zhang, M.; Liu, Y.W.; Li, Q.; Song, J.K.; et al. High genotype diversity and zoonotic potential of Enterocytozoon bieneusi in yaks (Bos grunniens) from Ganzi Tibetan Autonomous Prefecture, Sichuan Province. Parasite 2023, 30, 39. [Google Scholar] [CrossRef]
- Solomon, L.; Haile, G.; Ahmed, N.A.; Abdeta, D.; Galalcha, W.; Hailu, Y. Epidemiology and field efficacy of anthelmintic drugs associated with gastrointestinal nematodes of sheep in Nejo district, Oromia, Ethiopia. Sci. Rep. 2024, 14, 6841. [Google Scholar] [CrossRef]
- Bassetto, C.C.; Albuquerque, A.C.A.; Lins, J.G.G.; Marinho-Silva, N.M.; Chocobar, M.L.; Bello, H.J.; Mena, M.O.; Niciura, S.M.; Amarante, A.T.; Chagas, A.S. Revisiting anthelmintic resistance in sheep flocks from São Paulo State, Brazil. Int. J. Parasitol. Drugs Drug Resist. 2024, 24, 100527. [Google Scholar] [CrossRef]
- Antunes, M.I.; Lima, M.S.; Stilwell, G.; Romeiras, M.I.; Fragoso, L.; Madeira de Carvalho, L.M. Anthelmintic efficacy in sheep and goats under different management and deworming systems in the region of Lisbon and Tagus Valley, Portugal. Pathogens 2022, 11, 1457. [Google Scholar] [CrossRef]
- Wu, H. Insecta: Siphonaptera. In Fauna Sinica, 2nd ed.; Science Press: Beijing, China, 2007; pp. 57–63. [Google Scholar]
- Yu, X.; Ye, R.; Xie, X. Siphonaptera of Xinjiang. In Xinjiang People’s Publishing House; Xinjiang Science Press Publishing: Xinjiang, China, 1990; pp. 115–138. [Google Scholar]
- Zhao, C.G.; Zhang, J.S.; Meng, Q.L.; Qiao, J.; Shi, G.J.; Cai, X.P.; Chen, C.F. Morphological identification of Vermipsyllidae parasiting in sheep collected from Tacheng region in xinjiang. Chi. J. Prev. Med. 2012, 34, 539–542. [Google Scholar]
- Liu, Q.; Wu, H.Y.; Wu, F.L. Description of a new species of Dorcadia ioffi Smit, 1946 in China and a revision of the generic characters (Siphonaptera: Vermipsyllidae). Acta Parasitol. Et. Medica Entomol. Sin. 2003, 10, 52–56. [Google Scholar]
- Benedek, A.M.; Boeraș, I.; Lazăr, A.; Sandu, A.; Cocîrlea, M.D.; Stănciugelu, M.; Cic, N.V.; Postolache, C. Effects of season, habitat, and host characteristics on ectoparasites of wild rodents in a mosaic rural landscape. Animals 2024, 14, 304. [Google Scholar] [CrossRef]
- Ji, M.; Qiao, Z. Investigation on the Sheep Erythrocytic Fleas in Xueshan Township. Chin. J. Anim. Husb. Vet. Med. 2008, 1, 79–80. [Google Scholar]
- Li, D.; Gong, Z.; Gao, Z.; Ju, J.; Lian, H.; Duan, X.D.; Zhang, L.Y. Relationship between species, family or genus in number of fleas (Siphonaptera) and spatial distribution patterns in Hengduan Mountains of Yunnan Province, China. Acta Parasitol. Et. Medica Entomol. Sin. 2014, 24, 23–31. [Google Scholar]
- Zhang, L.; Wang, Z.; Chang, N.; Shang, M.; Wei, X.H.; Li, K.; Li, J.Y.; Lun, X.C.; Ji, H.Q.; Liu, Q.Y. Relationship between climatic factors and the flea index of two plague hosts in bilingol League, Inner Mongolia Autonomous Region. Biosaf. Health 2024, 6, 244–250. [Google Scholar] [CrossRef]
- El-Saber, B.G.; Alqahtani, A.; Ilesanmi, O.B.; Saati, A.A.; El-Mleeh, A.; Hetta, H.F.; Magdy, B.A. Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects. Pharmaceuticals 2020, 13, 196. [Google Scholar] [CrossRef]
- Campbell, W. History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr. Pharm. Biotechnol. 2012, 13, 853–865. [Google Scholar] [CrossRef]
- Marchiondo, A.A.; Holdsworth, P.A.; Green, P.; Blagburn, B.L.; Jacobs, D.E. World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of parasiticides for the treatment, prevention and control of flea and tick infestation on dogs and cats. Vet. Parasitol. 2007, 145, 332–344. [Google Scholar] [CrossRef]
- Lifschitz, A.; Nava, S.; Miró, V.; Canton, C.; Alvarez, L.; Lanusse, C. Macrocyclic lactones and ectoparasites control in livestock: Efficacy, drug resistance and therapeutic challenges. Int. J. Parasitol. Drugs Drug Resist. 2024, 26, 100559. [Google Scholar] [CrossRef]



| Group | Treatment | Dose |
|---|---|---|
| Ctr (untreated control group) | Physiological saline | 0.2 mL/(kg·BW) |
| I (avermectin injection group) | Subcutaneous injection | 0.2 mg/(kg·BW) |
| II (ivermectin injection group) | Subcutaneous injection | 0.2 mg/(kg·BW) |
| III (moxidectin pour-on group) | Pour-on along the dorsal midline | 0.05 mL/(kg·BW) |
| IV (deltamethrin solution pour-on group) | Pour-on along the neck, abdomen, and chest | 0.1 mL/(kg·BW) |
| V (trichlorfon pour-on group) | Pour-on along the neck, abdomen, and chest | 0.2 mL/(kg·BW) |
| Group | Time (d) | ||
|---|---|---|---|
| 3 | 7 | 14 | |
| Ctr | −9.3 ± 7.86 cdF | −22.88 ± 9.21 BCDEF | −27.12 ± 10.08 BCDEF |
| I | 1.71 ± 5.13 F | 18.86 ± 4.60 AEF | 10.86 ± 4.81 AEF |
| II | 3.99 ± 5.70 F | 15.94 ± 7.43 AEF | 15.76 ± 6.36 AEF |
| III | 14.24 ± 4.79 aF | 26.50 ± 4.34 AEF | 30.13 ± 3.68 AEF |
| IV | 13.59 ± 5.87 aF | 100.00 ± 0 ABCD | 100.00 ± 0 ABCD |
| V | 95.59 ± 1.18 ABCDE | 100.00 ± 0 ABCD | 100.00 ± 0 ABCD |
| p | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Qin, Z.; Wang, H.; Xia, J.; Zhao, Y.; Ma, X.; Li, N.; Yao, G. Identification of the Pathogen Dorcadia ioffi Smit and Evaluation of the Effect of Different Drugs. Vet. Sci. 2025, 12, 641. https://doi.org/10.3390/vetsci12070641
Li X, Qin Z, Wang H, Xia J, Zhao Y, Ma X, Li N, Yao G. Identification of the Pathogen Dorcadia ioffi Smit and Evaluation of the Effect of Different Drugs. Veterinary Sciences. 2025; 12(7):641. https://doi.org/10.3390/vetsci12070641
Chicago/Turabian StyleLi, Xin, Zihang Qin, Haiyan Wang, Jiangtao Xia, Yukang Zhao, Xuelian Ma, Na Li, and Gang Yao. 2025. "Identification of the Pathogen Dorcadia ioffi Smit and Evaluation of the Effect of Different Drugs" Veterinary Sciences 12, no. 7: 641. https://doi.org/10.3390/vetsci12070641
APA StyleLi, X., Qin, Z., Wang, H., Xia, J., Zhao, Y., Ma, X., Li, N., & Yao, G. (2025). Identification of the Pathogen Dorcadia ioffi Smit and Evaluation of the Effect of Different Drugs. Veterinary Sciences, 12(7), 641. https://doi.org/10.3390/vetsci12070641






