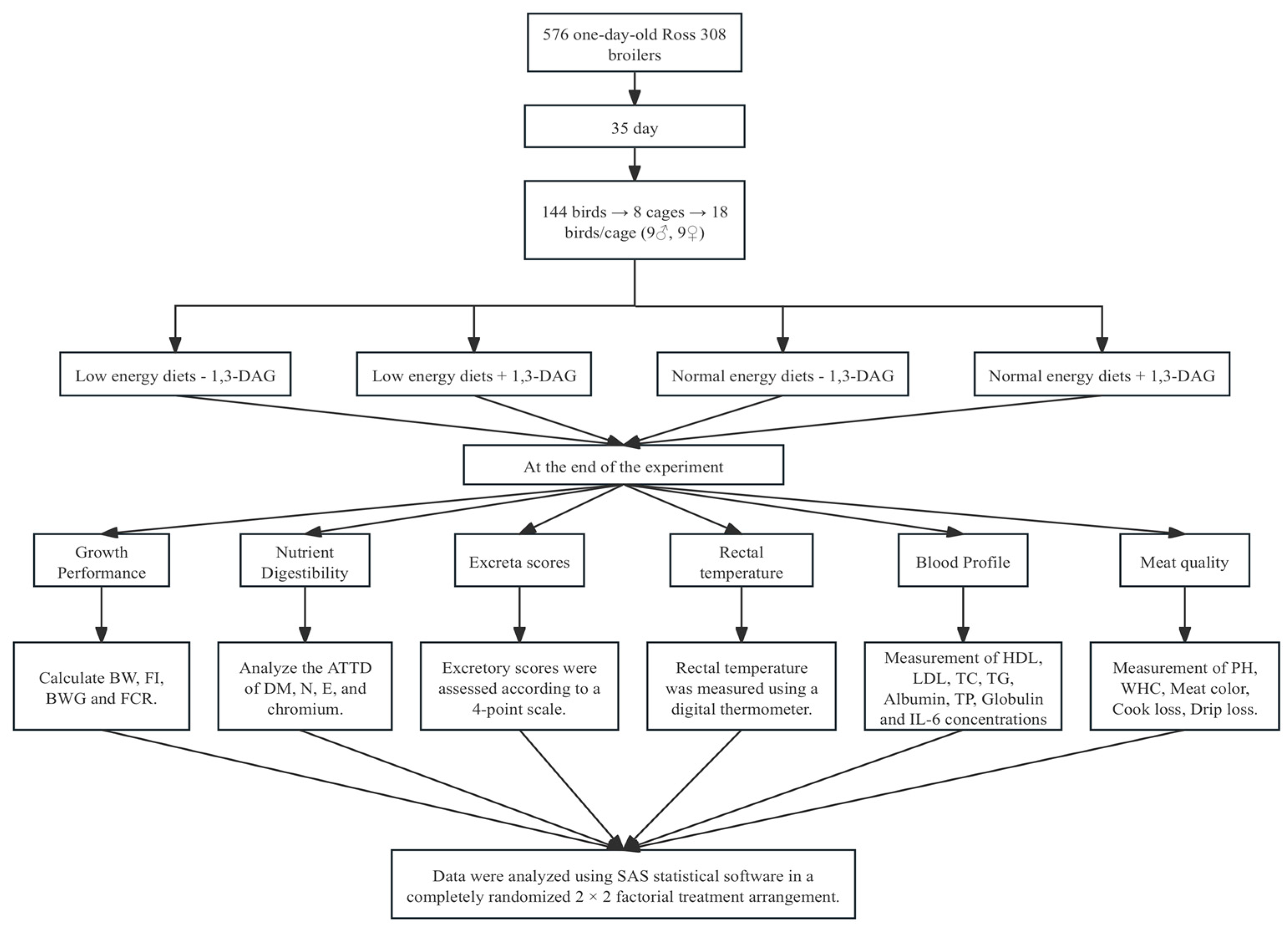
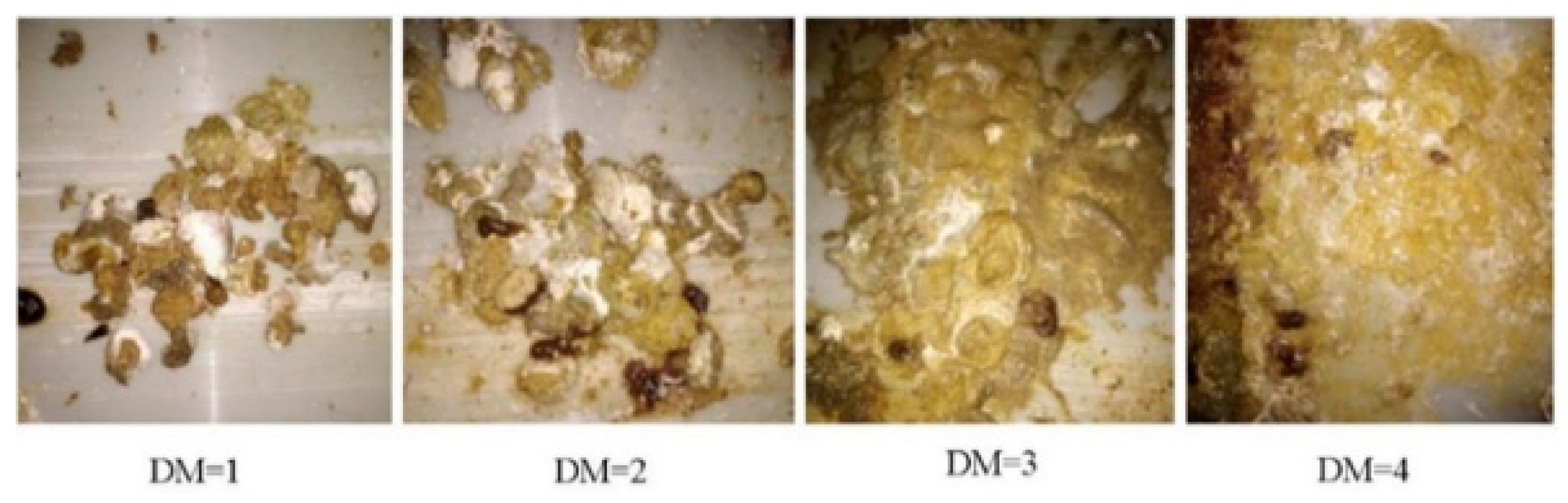
The development of modern broiler genetic lines has enhanced their growth potential and increased their demand for high energy intake. To meet this requirement, high-energy feed is essential, with fats and oils commonly incorporated into broiler diets to enhance energy density. This is because fats provide at least twice the energy of carbohydrates and proteins [
12]. Previous studies have demonstrated that the apparent ME and total fat metabolizable in broilers are influenced by age, particularly during the first week after hatching, but they typically improve after two weeks [
13]. Consequently, the inclusion of exogenous emulsifiers in diets has emerged as a potential supplement to enhance fat utilization and maintain the high productivity of broilers [
14]. Emulsifiers are especially important in commercial broiler production under energy-limited dietary conditions [
15]. Previous studies have shown that adding emulsifiers can partially compensate for the negative effects of low-energy diets on growth performance, bringing broiler growth close to that of the normal-energy diet group and also reducing feeding costs [
16]. Studies have shown that incorporating 1,3-DAG emulsifiers at concentrations of 0.075%, 0.10%, and 0.15% in low-energy diets positively affects body weight gain, feed intake, and the feed conversion ratio in broilers [
7]. Our findings are consistent with these results, as the supplementation of 1,3-DAG led to a significant improvement in growth performance. However, some reports suggest that adding 0.1% DAG emulsifiers to the diet had no significant impact on the growth performance of pigs [
17]. This discrepancy may be related to differences in animal species or the specific type of DAG emulsifier used. The isomers of DAG mainly include 1,2-DAG and 1,3-DAG, and structural differences between these isomers may affect their emulsifying properties and fat metabolism efficiency. Additionally, these two isomers differ in their synthesis and metabolic pathways, as 1,2-DAG is primarily generated during the breakdown of dietary triacylglycerols and can be incorporated into cellular membranes, affecting membrane fluidity and signaling pathways. On the other hand, 1,3-DAG is often produced during the synthesis of specific lipids and may be preferentially utilized in energy production or fat storage, influencing lipid metabolism differently [
18]. Studies have also shown that supplementing low-energy diets with 1,3-DAG can significantly improve energy digestibility by reducing feed energy wastage through the rapid metabolism of medium-chain fatty acids (MCFAs) [
7]. This is consistent with our study, where supplementation with 1,3-DAG significantly increased the digestibility of energy. The improvement in energy may be attributed to the MCFA present in DAG, which are more readily metabolized for energy. However, studies have indicated that broilers fed a diet with a mixture of emulsifiers showed no differences in dry matter, nitrogen, or energy digestibility [
4]. Other products or differences in the source of 1,3-DAG occur. In poultry farming, dietary energy levels can be adjusted to regulate growth performance and feed costs [
19]. Lowering dietary energy levels has been shown to reduce growth performance in broilers, although no significant difference in FI is typically observed [
20,
21], which is consistent with our findings. In our study, although the energy difference between the normal- and low-energy diets was only 100 kcal/kg, the broilers fed the low-energy diet exhibited significantly lower BWG throughout the experimental period compared with those on the normal-energy diet. Additionally, the FCR was significantly higher during days 1–9, days 10–20, days 21–35, and the overall experimental period. The energy provided by the diet is primarily used for maintenance and production. When broilers are fed a low-energy diet, the energy is mainly allocated for maintenance, which can impair growth. This may help explain the decrease in BWG and the increase in FCR observed in this study [
22]. Dietary energy concentration also influences broiler FI. Broilers can adjust their feed intake to regulate energy consumption in response to changes in diet energy concentration [
23,
24]. Therefore, broilers fed a reduced-energy diet tend to consume more feed to compensate for the energy deficit. However, in this study, there was no significant difference in FI based on energy levels, which may be due to environmental factors, such as the temperature, humidity, and stocking density, influencing broiler feeding behavior. Under consistent rearing conditions, broilers may not adjust their feed intake in response to changes in energy levels [
25]. In this study, dietary supplementation with 1,3-DAG had no significant effect on the rectal temperatures or fecal scores in the broiler chickens. This result may be attributed to the primary role of 1,3-DAG in enhancing fat digestion, absorption, and energy utilization rather than directly influencing thermoregulation or gut health-related physiological mechanisms. Although fat metabolism can impact the body’s heat balance, the relatively low supplementation level of 1,3-DAG in this study may not have been sufficient to induce significant changes in rectal temperature [
26]. Additionally, fecal scores are mainly influenced by dietary composition, gut microbiota, and water metabolism. Since 1,3-DAG primarily functions in fat emulsification and absorption, it may not have significantly altered the physical characteristics of the excreta [
27]. Future research should explore whether different levels of 1,3-DAG supplementation under higher ambient temperatures or varying dietary formulations could have potential effects on the rectal temperature and fecal quality.
Studies have reported that compared with broilers fed a normal-energy diet, those on a low-energy diet may experience increased protein catabolism due to energy deficiency. This can lead to the degradation of muscle cell structures, reducing the water-binding capacity of muscle tissue and consequently increasing drip loss in broilers [
28]. Our findings align with this, as feeding a low-energy diet significantly increased drip loss. Additionally, although reduced-energy diets led to lower final body weights in the broilers, they did not affect carcass composition when compared with the control diet [
29,
30]. This contrasts with our study, where changes in the ratio of other nutrients (such as protein and fat) in the low-energy diet may explain the observed differences. Studies have also shown that high-energy diets typically contain more fats and oils, which enhance the intestinal absorption of dietary lipids and increase the availability of cholesterol precursors [
31]. This, in turn, promotes hepatic cholesterol synthesis, elevating the total cholesterol, HDL cholesterol, and LDL cholesterol levels in broiler chickens. Additionally, low cholesterol may impair immune response, thereby increasing the risk of disease infection in broilers [
32]. This is consistent with our findings, where a low-energy diet significantly reduced the total cholesterol levels in the broilers, likely due to alterations in lipid metabolism resulting from reduced dietary fat and energy intake. When energy intake is restricted, stored lipids may be utilized more efficiently, leading to lower circulating cholesterol levels [
33]. However, some studies have reported that the dietary energy content does not affect the HDL, LDL, total cholesterol, and triglyceride concentrations in broilers [
23,
34]. This is inconsistent with our study results, which may be attributed to the total cholesterol measurement technique, a method that is difficult to standardize and characterized by variability and high coefficients of variation, which reduces the consistency of the results. In the poultry industry, low-energy diets can help reduce production costs by modifying the energy composition and supplementing emulsifiers. In our study, supplementation with 1,3-DAG and varying dietary energy levels showed no interactive effects on the growth performance, nutrient digestibility, excreta scores, rectal temperature, meat quality, or blood lipid indicators in broiler chickens. This is consistent with previous studies that also found no interaction between emulsifiers and dietary energy levels regarding growth performance, nutrient digestibility, blood lipid indicators, and meat quality [
4]. However, some studies have reported that emulsifiers interact with dietary energy levels to influence broiler growth performance [
35]. This difference may be attributed to the chemical composition of the emulsifier and its distinct mechanisms of action in fat emulsification and absorption. Moreover, lower supplementation levels of emulsifiers may not be sufficient to significantly improve lipid digestibility, which could explain the absence of notable interaction.