Evaluation of Cytokine Profile in Canine Malignant Oral Melanoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nance, R.L.; Sajib, A.M.; Smith, B.F. Canine models of human cancer: Bridging the gap to improve precision medicine. Prog. Mol. Biol. Transl. Sci. 2022, 189, 67–99. [Google Scholar] [CrossRef]
- Khanna, C.; Lindblad-Toh, K.; Vail, D.; London, C.; Bergman, P.; Barber, L.; Breen, M.; Kitchell, B.; McNeil, E.; Modiano, J.F.; et al. The dog as a cancer model. Nat. Biotechnol. 2006, 24, 1065–1066. [Google Scholar] [CrossRef]
- Wu, K.; Rodrigues, L.; Post, G.; Harvey, G.; White, M.; Miller, A.; Lambert, L.; Lewis, B.; Lopes, C.; Zou, J. Analyses of canine cancer mutations and treatment outcomes using real-world clinico-genomics data of 2119 dogs. npj Precis. Oncol. 2023, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. Med. Rev. J. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. Med. Rev. J. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Paoloni, M.; Davis, S.; Lana, S.; Withrow, S.; Sangiorgi, L.; Picci, P.; Hewitt, S.; Triche, T.; Meltzer, P.; Khanna, C. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genom. 2009, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.C.; Sarver, A.L.; Gavin, K.J.; Thayanithy, V.; Getzy, D.M.; Newman, R.A.; Cutter, G.R.; Lindblad-Toh, K.; Kisseberth, W.C.; Hunter, L.E.; et al. Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone 2011, 49, 356–367. [Google Scholar] [CrossRef]
- Gardner, H.L.; Fenger, J.M.; London, C.A. Dogs as a Model for Cancer. Annu. Rev. Anim. Biosci. 2016, 4, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, B.; Adissu, H.A.; Wei, B.R.; Michael, H.T.; Merlino, G.; Simpson, R.M. Naturally Occurring Canine Melanoma as a Predictive Comparative Oncology Model for Human Mucosal and Other Triple Wild-Type Melanomas. Int. J. Mol. Sci. 2018, 19, 394. [Google Scholar] [CrossRef]
- Almela, R.M.; Ansón, A. A Review of Immunotherapeutic Strategies in Canine Malignant Melanoma. Vet. Sci. 2019, 6, 15. [Google Scholar] [CrossRef]
- Deguchi, T.; Maekawa, N.; Konnai, S.; Owaki, R.; Hosoya, K.; Morishita, K.; Nakamura, M.; Okagawa, T.; Takeuchi, H.; Kim, S.; et al. Enhanced Systemic Antitumour Immunity by Hypofractionated Radiotherapy and Anti-PD-L1 Therapy in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Cancers 2023, 15, 3013. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Asano, Y.; Sajiki, Y.; Deguchi, T.; Okagawa, T.; Watari, K.; Takeuchi, H.; Takagi, S.; Hosoya, K.; et al. Exploration of serum biomarkers in dogs with malignant melanoma receiving anti-PD-L1 therapy and potential of COX-2 inhibition for combination therapy. Sci. Rep. 2022, 12, 9265. [Google Scholar] [CrossRef] [PubMed]
- Iivanainen, S.; Ahvonen, J.; Knuuttila, A.; Tiainen, S.; Koivunen, J.P. Elevated CRP levels indicate poor progression-free and overall survival on cancer patients treated with PD-1 inhibitors. ESMO Open 2019, 4, e000531. [Google Scholar] [CrossRef] [PubMed]
- Laino, A.S.; Woods, D.; Vassallo, M.; Qian, X.; Tang, H.; Wind-Rotolo, M. Jeffrey Weber Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 2020, 8, e000842. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mahoney, K.M.; Giobbie-Hurder, A.; Zhao, F.; Lee, S.; Liao, X.; Rodig, S.; Li, J.; Wu, X.; Butterfield, L.H.; et al. Soluble P.D-L1 as a Biomarker in Malignant Melanoma Treated with Checkpoint Blockade. Cancer Immunol. Res. 2017, 5, 480–492. [Google Scholar] [CrossRef]
- MacEwen, E.G.; Patnaik, A.K.; Harvey, H.J.; Hayes, A.A.; Matus, R. Canine oral melanoma: Comparison of surgery versus surgery plus Corynebacterium parvum. Cancer Investig. 1986, 4, 397–402. [Google Scholar] [CrossRef]
- Button, K.S.; Ioannidis, J.P.A.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.J.; Munafò, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef]
- These, M.S.; Ronna, B.; Ott, U. P value interpretations and considerations. J. Thorac. Dis. 2016, 8, E928–E931. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Spencer, S.A.; Lydiatt, W. Mucosal melanoma: A clinically and biologically unique disease entity. J. Natl. Compr. Cancer Netw. 2012, 10, 345–356. [Google Scholar] [CrossRef]
- Gillard, M.; Cadieu, E.; De Brito, C.; Abadie, J.; Vergier, B.; Devauchelle, P.; Degorce, F.; Dréano, S.; Primot, A.; Dorso, L.; et al. Naturally occurring melanomas in dogs as models for non-UV pathways of human melanomas. Pigment. Cell Melanoma Res. 2014, 27, 90–102. [Google Scholar] [CrossRef]
- Simpson, R.M.; Bastian, B.C.; Michael, H.T.; Webster, J.D.; Prasad, M.L.; Conway, C.M.; Prieto, V.M.; Gary, J.M.; Goldschmidt, M.H.; Esplin, D.G.; et al. Sporadic naturally occurring melanoma in dogs as a preclinical model for human melanoma. Pigment. Cell Melanoma Res. 2014, 27, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef]
- Stinson, J.A.; Barbosa, M.M.P.; Sheen, A.; Momin, N.; Fink, E.; Hampel, J.; Selting, K.A.; Kamerer, R.L.; Bailey, K.L.; Wittrup, K.D.; et al. Tumor-localized interleukin-2 and interleukin-12 combine with radiation therapy to safely potentiate regression of advanced malignant melanoma in pet dogs. Clin. Cancer Res. 2024, 30, 4029–4043. [Google Scholar] [CrossRef]
- Wigginton, J.M.; Wiltrout, R.H. IL-12/IL-2 combination cytokine therapy for solid tumours: Translation from bench to bedside. Expert Opin. Biol. Ther. 2002, 2, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, L.M.; Fiszman, G.L.; Karara, A.L.; Glikin, G.C. Suicide gene and cytokines combined nonviral gene therapy for spontaneous canine melanoma. Cancer Gene Ther. 2008, 15, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Tominaga, M.; Ichikawa, M.; Yamashita, M.; Okano, K.; Jikumaru, Y.; Nariai, Y.; Nakajima, Y.; Kuwabara, M.; Yukawa, M. Relationship between regulatory and type 1 T cells in dogs with oral malignant melanoma. Microbiol. Immunol. 2010, 54, 152–159. [Google Scholar] [CrossRef]
- Günel, N.; Coşkun, U.; Sancak, B.; Günel, U.; Hasdemir, O.; Bozkurt, S. Clinical importance of serum interleukin-18 and nitric oxide activities in breast carcinoma patients. Cancer 2002, 95, 663–667. [Google Scholar] [CrossRef]
- Graves, D.T.; Barnhill, R.; Galanopoulos, T.; Antoniades, H.N. Expression of monocyte chemotactic protein-1 in human melanoma in vivo. Am. J. Pathol. 1992, 140, 9–14. [Google Scholar]
- Manome, Y.; Wen, P.Y.; Hershowitz, A.; Tanaka, T.; Rollins, B.J.; Kufe, D.W.; Fine, H.A. Monocyte chemoattractant protein-1 (MCP-1) gene transduction: An effective tumor vaccine strategy for non-intracranial tumors. Cancer Immunol. Immunother. 1995, 41, 227–235. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sozzani, S.; Vecchi, A.; Locati, M.; Sica, A. Chemokines in the recruitment and shaping of the leukocyte infiltrate of tumors. Semin. Cancer Biol. 2004, 14, 155–160. [Google Scholar] [CrossRef]
- Nesbit, M.; Schaider, H.; Miller, T.H.; Herlyn, M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J. Immunol. 2001, 166, 6483–6490. [Google Scholar] [CrossRef] [PubMed]
- Rollins, B.J.; Sunday, M.E. Suppression of tumor formation in vivo by expression of the JE gene in malignant cells. Mol. Cell. Biol. 1991, 11, 3125–3131. [Google Scholar] [CrossRef][Green Version]
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308. [Google Scholar] [CrossRef]
- Yee, C.; Thompson, J.A.; Byrd, D.; Riddell, S.R.; Roche, P.; Celis, E.; Greenberg, P.D. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. USA 2002, 99, 16168–16173. [Google Scholar] [CrossRef]
- Alpdogan, Ö.; Muriglan, S.J.; Eng, J.M.; Willis, L.M.; Greenberg, A.S.; Kappel, B.J.; Brink, M.R.v.D. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J. Clin. Investig. 2003, 112, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Sportès, C.; Ahmadzadeh, M.; Fry, T.J.; Ngo, L.T.; Schwarz, S.L.; Stetler-Stevenson, M.; Morton, K.E.; Mavroukakis, S.A.; Morre, M.; et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother. 2006, 29, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Sportès, C.; Hakim, F.T.; Memon, S.A.; Zhang, H.; Chua, K.S.; Brown, M.R.; Fleisher, T.A.; Krumlauf, M.C.; Babb, R.R.; Chow, C.K.; et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 2008, 205, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Santana, C.G.; Jiménez-Alonso, A.A.; Rodríguez-Esparragón, F.; Cazorla-Rivero, S.; Rodríguez Grau-Bassas, E. Canine Oral Melanoma: Questioning the Existing Information through a Series of Clinical Cases. Vet. Sci. 2024, 11, 226. [Google Scholar] [CrossRef]
- Li, D.-X.; Wang, X.-M.; Tang, Y.; Yang, Y.-B.; Feng, D.-C.; Li, A.; Zhang, F.-C.; Bai, Y.-J.; Han, P. Prognostic value of preoperative neutrophil-to-lymphocyte ratio in histological variants of non-muscle-invasive bladder cancer. Investig. Clin. Urol. 2021, 62, 641–649. [Google Scholar] [CrossRef]
- Mjaess, G.; Chebel, R.; Karam, A.; Moussa, I.; Pretot, D.; Tayeh, G.A.; Sarkis, J.; Semaan, A.; Peltier, A.; Aoun, F.; et al. Prognostic role of neutrophil-to-lymphocyte ratio (NLR) in urological tumors: An umbrella review of evidence from systematic reviews and meta-analyses. Acta. Oncol. 2021, 60, 704–713. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Ni, J.; Zhang, H.; Yin, L.; Zhang, Y.; Shi, H.; Zhang, T.; Zhou, N.; Mao, W.; et al. Combination of C-Reactive Protein and Neutrophil-to-Lymphocyte Ratio as a Novel Prognostic Index in Patients with Bladder Cancer After Radical Cystectomy. Front. Oncol. 2021, 11, 762470. [Google Scholar] [CrossRef] [PubMed]
- Kool, R.; Marcq, G.; Shinde-Jadhav, S.; Mansure, J.J.; Saleh, R.; Rajan, R.; Aprikian, A.; Tanguay, S.; Cury, F.L.; Brimo, F.; et al. Role of Serum Lymphocyte-derived Biomarkers in Nonmetastatic Muscle-invasive Bladder Cancer Patients Treated with Trimodal Therapy. Eur. Urol. Open Sci. 2021, 36, 26–33. [Google Scholar] [CrossRef] [PubMed]
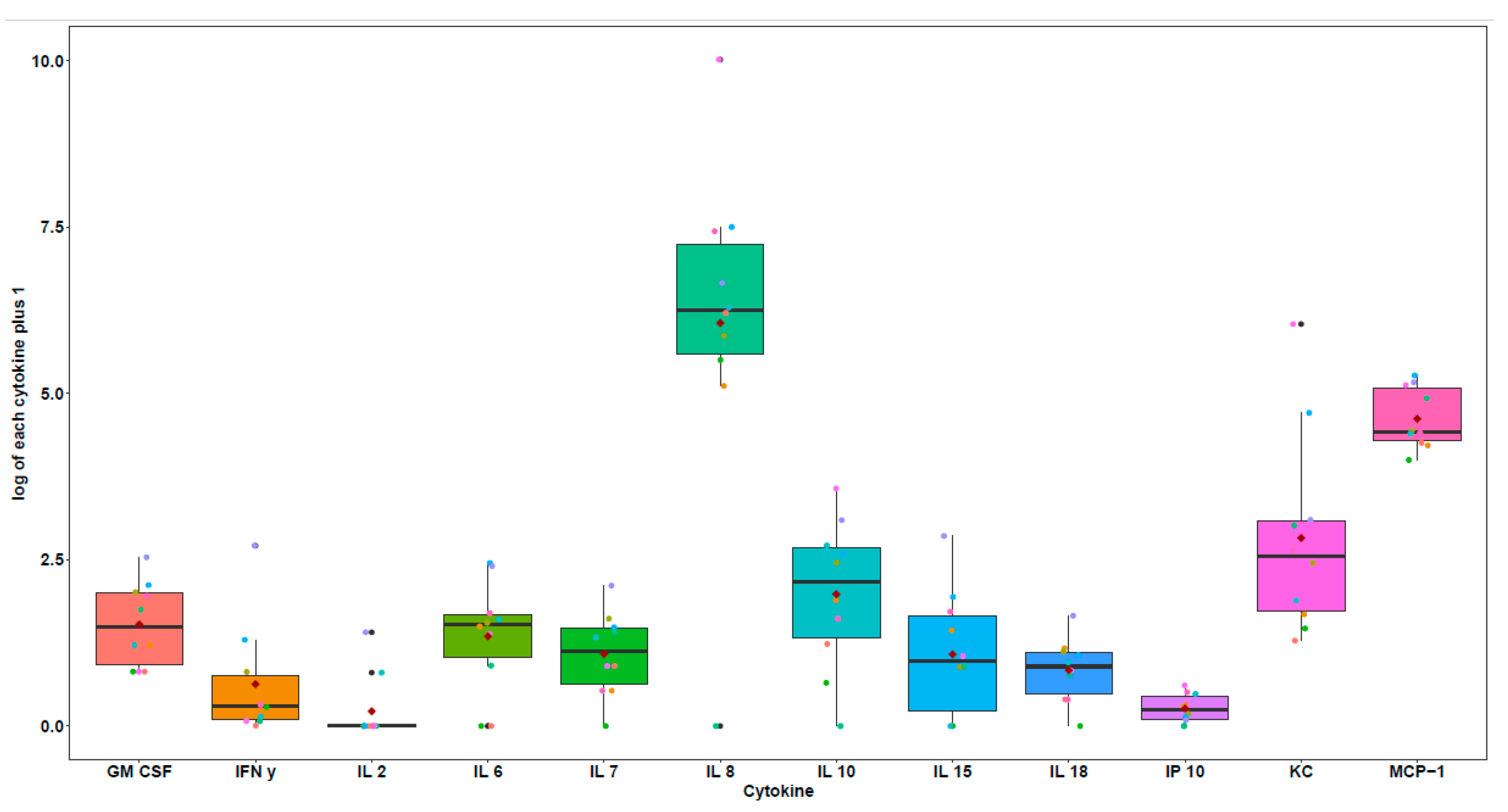
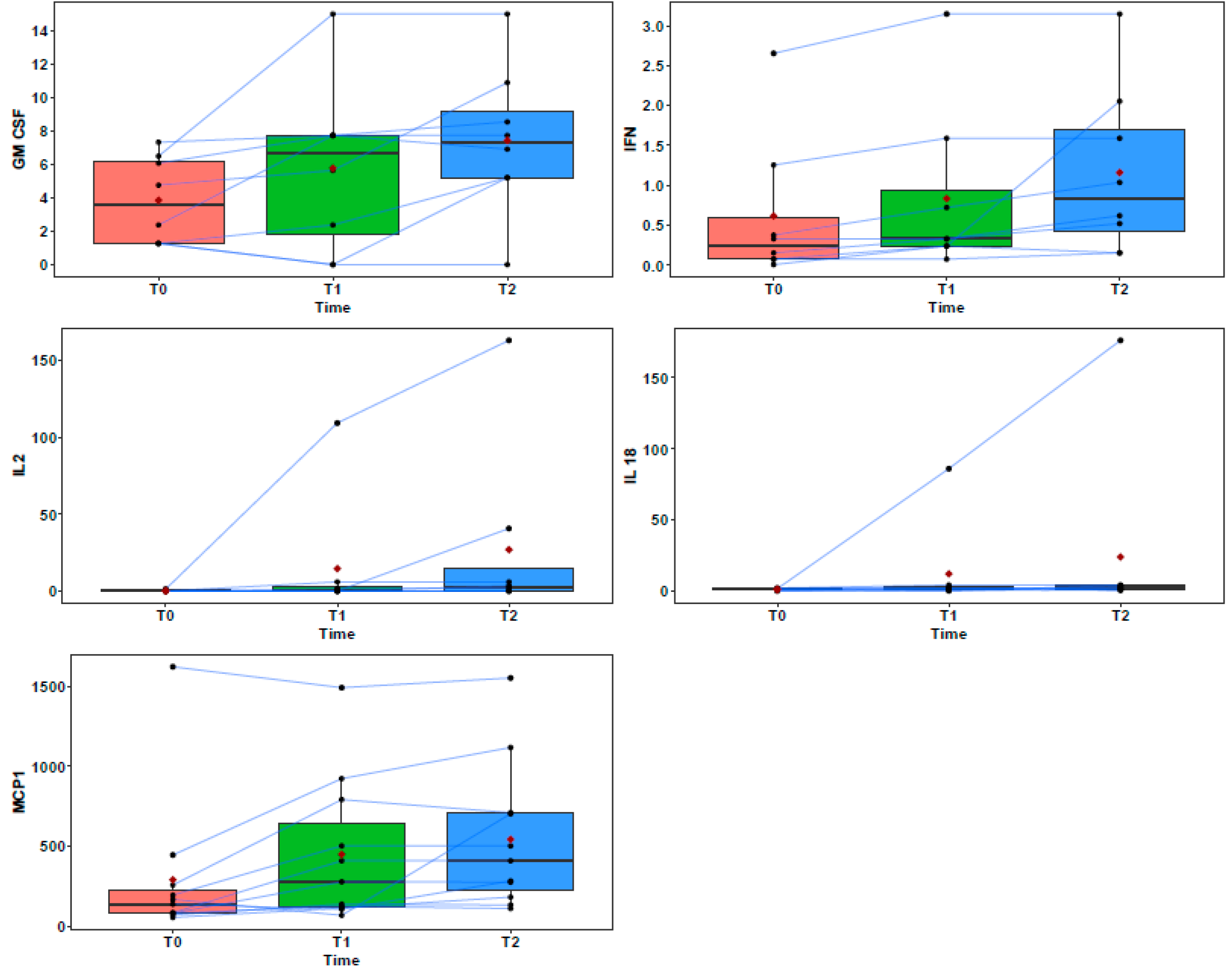
| Pt | Breed | Sex | Age (y) | Reproductive State | Diagnosis | TNM | Tumor Size (cm) |
|---|---|---|---|---|---|---|---|
| 1 | American staffordshire | F | 10 | Spayed | Oral melanoma | Stage II | 2 |
| 2 | Beagle | F | 13 | Spayed | Oral amelanotic melanoma | Stage III | 3.2 |
| 3 | Cocker spaniel | M | 13 | Spayed | Oral melanoma | Stage III | 2.5 |
| 4 | Labrador retriever | M | 14 | Spayed | Oral melanoma | Stage II | 2 |
| 5 | Mixed | F | 12 | Spayed | Oral melanoma | Stage I | 1.5 |
| 6 | Presa canario | M | 9 | Intact | Oral amelanotic melanoma | Stage III | 7.7 |
| 7 | Yorkshire terrier | M | 14 | Intact | Oral amelanotic melanoma | Stage II | 2.1 |
| 8 | Yorkshire terrier | M | 12 | Spayed | Oral amelanotic melanoma | Stage II | 2.8 |
| 9 | Yorkshire terrier | F | 14 | Spayed | Oral amelanotic melanoma | Stage III | 2.5 |
| 10 | Yorkshire terrier | M | 14 | Spayed | Oral amelanotic melanoma | Stage II | 2 |
| Pt | Diagnosis | Survival Time (days) |
|---|---|---|
| 1 | OMM | 365 |
| 2 | OAM | 43 |
| 3 | OMM | 131 |
| 4 | OMM | 310 |
| 5 | OMM | 365 |
| 6 | OAM | 365 |
| 7 | OAM | 365 |
| 8 | OAM | 155 |
| 9 | OAM | 240 |
| 10 | OAM | 365 |
| Pt | T0 | T1 | T2.1 | T2.2 |
|---|---|---|---|---|
| 1 | OMM | Remission | Remission | Remission |
| 2 | OMM | |||
| 3 | OMM | Metastasis | ||
| 4 | OMM | Remission | OMM | Metastasis |
| 5 | OMM | Remission | Remission | OMM |
| 6 | OMM | Remission | Remission | Remission |
| 7 | OMM | Remission | Remission | Remission |
| 8 | OMM | |||
| 9 | OMM | Remission | Metastasis | |
| 10 | OMM | Remission | Remission | Remission |
| Pt | C0 | T0 | C1 | T1 | C2 | T2 |
|---|---|---|---|---|---|---|
| 1 | OMM | 1.264 | Remission | 2.367 | Remission | 5.190 |
| 2 * | ||||||
| 3 | OMM | 6.477 | Metastasis | 4.969 | ||
| 4 | OMM | 1.264 | Remission | 0.000 | OMM | 0.000 |
| 5 | OMM | 4.747 | Remission | 5.625 | Remission | 10.868 |
| 6 | OMM | 2.367 | Remission | 7.717 | Remission | 8.523 |
| 7 | OMM | 7.308 | Remission | 7.717 | Remission | 7.717 |
| 8 * | ||||||
| 9 | OMM | 1.264 | Remission | 0.000 | Metastasis | 0.000 |
| 10 | OMM | 6.054 | Remission | 7.717 | Remission | 6.895 |
| Pt | C0 | T0 | C1 | T1 | C2 | T2 |
|---|---|---|---|---|---|---|
| 1 | OMM | 0.007 | Remission | 0.240 | Remission | 0.154 |
| 2 * | ||||||
| 3 | OMM | 1.255 | Metastasis | 1.591 | ||
| 4 | OMM | 0.330 | Remission | 0.330 | OMM | 0.620 |
| 5 | OMM | 0.075 | Remission | 0.240 | Remission | 2.055 |
| 6 | OMM | 0.154 | Remission | 0.330 | Remission | 0.521 |
| 7 | OMM | 2.655 | Remission | 3.149 | Remission | 3.149 |
| 8 * | ||||||
| 9 | OMM | 0.075 | Remission | 0.075 | Metastasis | 0.154 |
| 10 | OMM | 0.377 | Remission | 0.722 | Remission | 1.037 |
| Pt | C0 | T0 | C1 | T1 | C2 | T2 |
|---|---|---|---|---|---|---|
| 1 | OMM | 0.000 | Remission | 0.000 | Remission | 3.366 |
| 2 * | ||||||
| 3 | OMM | 0.00 | Metastasis | 5.930 | ||
| 4 | OMM | 0.00 | Remission | 0.000 | OMM | 0.000 |
| 5 | OMM | 0.000 | Remission | 0.000 | Remission | 40.683 |
| 6 | OMM | 1.23 | Remission | 109.302 | Remission | 163.074 |
| 7 | OMM | 0.000 | Remission | 0.000 | Remission | 0.000 |
| 8 * | ||||||
| 9 | OMM | 0.000 | Remission | 0.000 | Metastasis | 0.000 |
| 10 | OMM | 0.000 | Remission | 1.583 | Remission | 1.911 |
| Pt | C0 | T0 | C1 | T1 | C2 | T2 |
|---|---|---|---|---|---|---|
| 1 | OMM | 0.490 | Remission | 1.019 | Remission | 2.869 |
| 2 * | ||||||
| 3 | OMM | 2.061 | Metastasis | 4.244 | ||
| 4 | OMM | 0.000 | Remission | 0.256 | OMM | 1.304 |
| 5 | OMM | 1.160 | Remission | 0.055 | Remission | 3.892 |
| 6 | OMM | 1.600 | Remission | 86.041 | Remission | 176.235 |
| 7 | OMM | 1.905 | Remission | 2.061 | Remission | 2.061 |
| 8 * | ||||||
| 9 | OMM | 1.304 | Remission | 2.219 | Metastasis | 0.747 |
| 10 | OMM | 0.490 | Remission | 1.304 | Remission |
| Pt | C0 | T0 | C1 | T1 | C2 | T2 |
|---|---|---|---|---|---|---|
| 1 | OMM | 69.391 | Remission | 136.491 | Remission | 131.637 |
| 2 * | ||||||
| 3 | OMM | 83.387 | Metastasis | 408.552 | ||
| 4 | OMM | 53.473 | Remission | 112.197 | OMM | 180.804 |
| 5 | OMM | 136.491 | Remission | 104.868 | Remission | 281.735 |
| 6 | OMM | 79.710 | Remission | 276.785 | Remission | 274.679 |
| 7 | OMM | 193.172 | Remission | 502.113 | Remission | 502.113 |
| 8 * | ||||||
| 9 | OMM | 166.879 | Remission | 66.819 | Metastasis | 702.880 |
| 10 | OMM | 80.457 | Remission | 123.636 | Remission | 109.529 |
| Cytokines | OMM-P50 (pg/mL) | OMM P25-P75 (pg/mL) | DIR-P50 (pg/mL) | DIR P25-P75 (pg/mL) | p-Value |
|---|---|---|---|---|---|
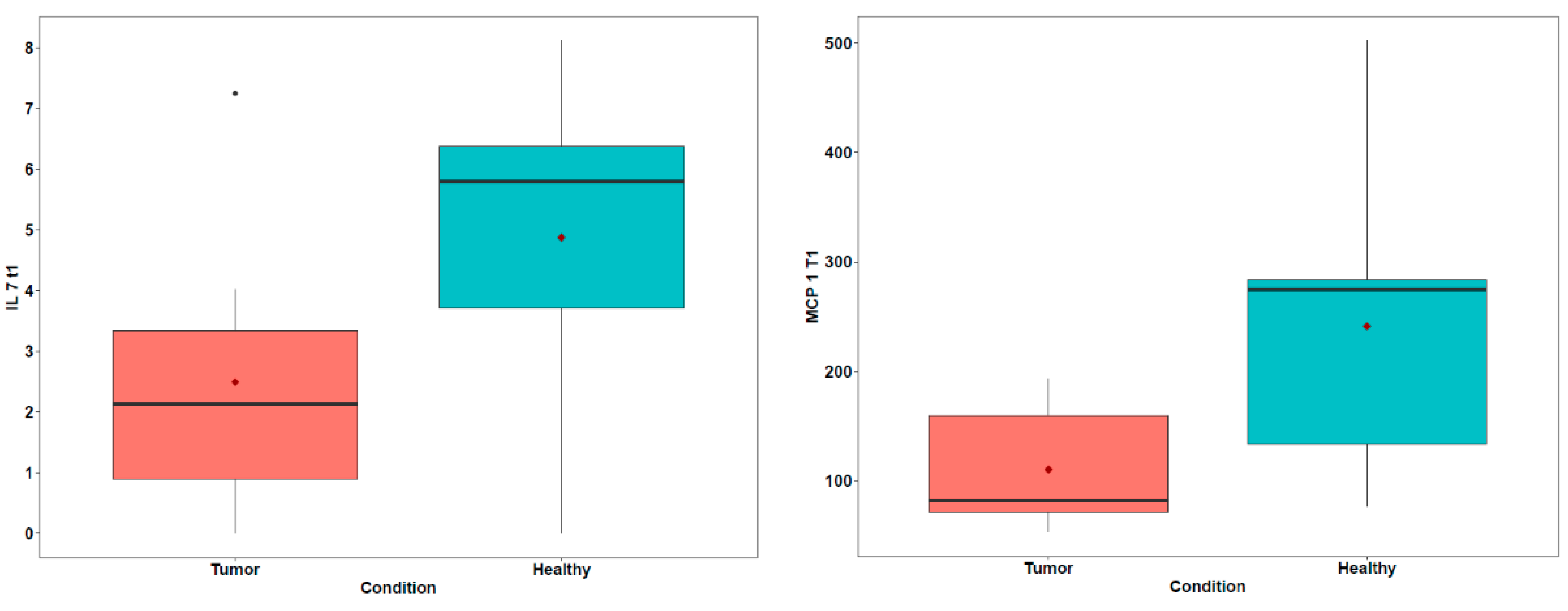
| IL-7 | 2.12 | 0.89–3.33 | 5.8 | 3.71–6.38 | 0.078 |
| MCP-1 | 81.92 | 71.97–159.28 | 274.68 | 134.08–283.85 | 0.051 |
| Cytokines | OMMr-P50 (pg/mL) | OMMr P25-P75 (pg/mL) | MET-P50 (pg/mL) | MET P25-P75 (pg/mL) | p-Value |
|---|---|---|---|---|---|
| MCP-1 | 136.49 | 79.71–193.17 | 813.18 | 629.3–972.26 | 0.015 |
| Cytokines | Tumor | P50 (pg/mL) | P25–P75 (pg/mL) | Tumor | P50 (pg/mL) | P25-P75 (pg/mL) | p-Value |
|---|---|---|---|---|---|---|---|
| IL-6 | A | 4.22 | 3.59–8.66 | M | 0.74 | 0–2.04 | 0.042 |
| IL-10 | A | 13.21 | 7.31–19.3 | M | 1.67 | 0.68–4.49 | 0.038 |
| IL-15 | A | 3.89 | 2.2–5.61 | M | 0.71 | 0–1.42 | 0.066 |
| Pt | NEU (K/µL) | LYM (K/µL) | NLR |
|---|---|---|---|
| 1 | 7.14 | 1.93 | 3.699 |
| 2 | 4.68 | 0.6 | 7.800 |
| 3 | 8.26 | 1.29 | 6.403 |
| 4 | 2.308 | 0.89 | 2.593 |
| 5 * | 1.2 | ||
| 6 | 8.73 | 3.21 | 2.719 |
| 7 | 13.53 | 1.76 | 7.687 |
| 8 | 7.07 | 1.4 | 5.050 |
| 9 | 5.8 | 1.79 | 3.240 |
| 10 | 8.27 | 2.34 | 3.534 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Santana, C.G.; Cazorla-Rivero, S.E.; Jiménez-Alonso, A.A.; Rodríguez-Esparragón, F.; González Martín, J.M.; Henríquez-Cabrera, R.; Clavo-Varas, B.; Rodríguez Grau-Bassas, E. Evaluation of Cytokine Profile in Canine Malignant Oral Melanoma. Vet. Sci. 2025, 12, 627. https://doi.org/10.3390/vetsci12070627
Pérez-Santana CG, Cazorla-Rivero SE, Jiménez-Alonso AA, Rodríguez-Esparragón F, González Martín JM, Henríquez-Cabrera R, Clavo-Varas B, Rodríguez Grau-Bassas E. Evaluation of Cytokine Profile in Canine Malignant Oral Melanoma. Veterinary Sciences. 2025; 12(7):627. https://doi.org/10.3390/vetsci12070627
Chicago/Turabian StylePérez-Santana, Carmen G., Sara E. Cazorla-Rivero, Ana A. Jiménez-Alonso, Francisco Rodríguez-Esparragón, Jesús María González Martín, Ruth Henríquez-Cabrera, Bernardino Clavo-Varas, and Enrique Rodríguez Grau-Bassas. 2025. "Evaluation of Cytokine Profile in Canine Malignant Oral Melanoma" Veterinary Sciences 12, no. 7: 627. https://doi.org/10.3390/vetsci12070627
APA StylePérez-Santana, C. G., Cazorla-Rivero, S. E., Jiménez-Alonso, A. A., Rodríguez-Esparragón, F., González Martín, J. M., Henríquez-Cabrera, R., Clavo-Varas, B., & Rodríguez Grau-Bassas, E. (2025). Evaluation of Cytokine Profile in Canine Malignant Oral Melanoma. Veterinary Sciences, 12(7), 627. https://doi.org/10.3390/vetsci12070627








