Heart Rate and Heart Rate Variability Are Affected by Age and Activity Level in Athletic Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
2.2. Experimental Protocols
2.3. Data Analysis
3. Results
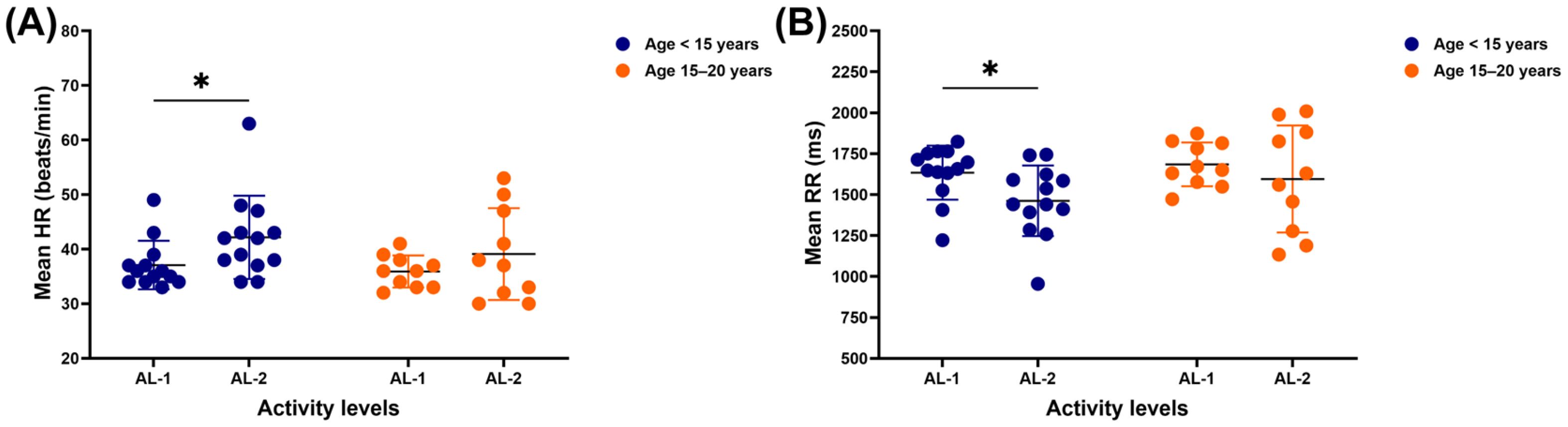
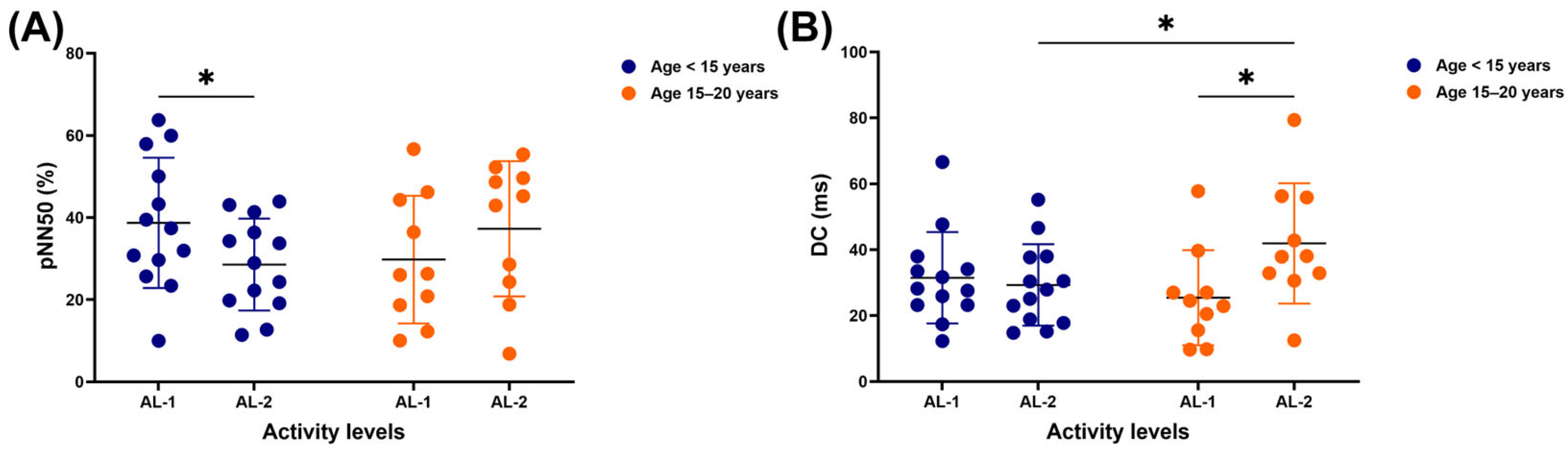
3.1. Time-Domain Results
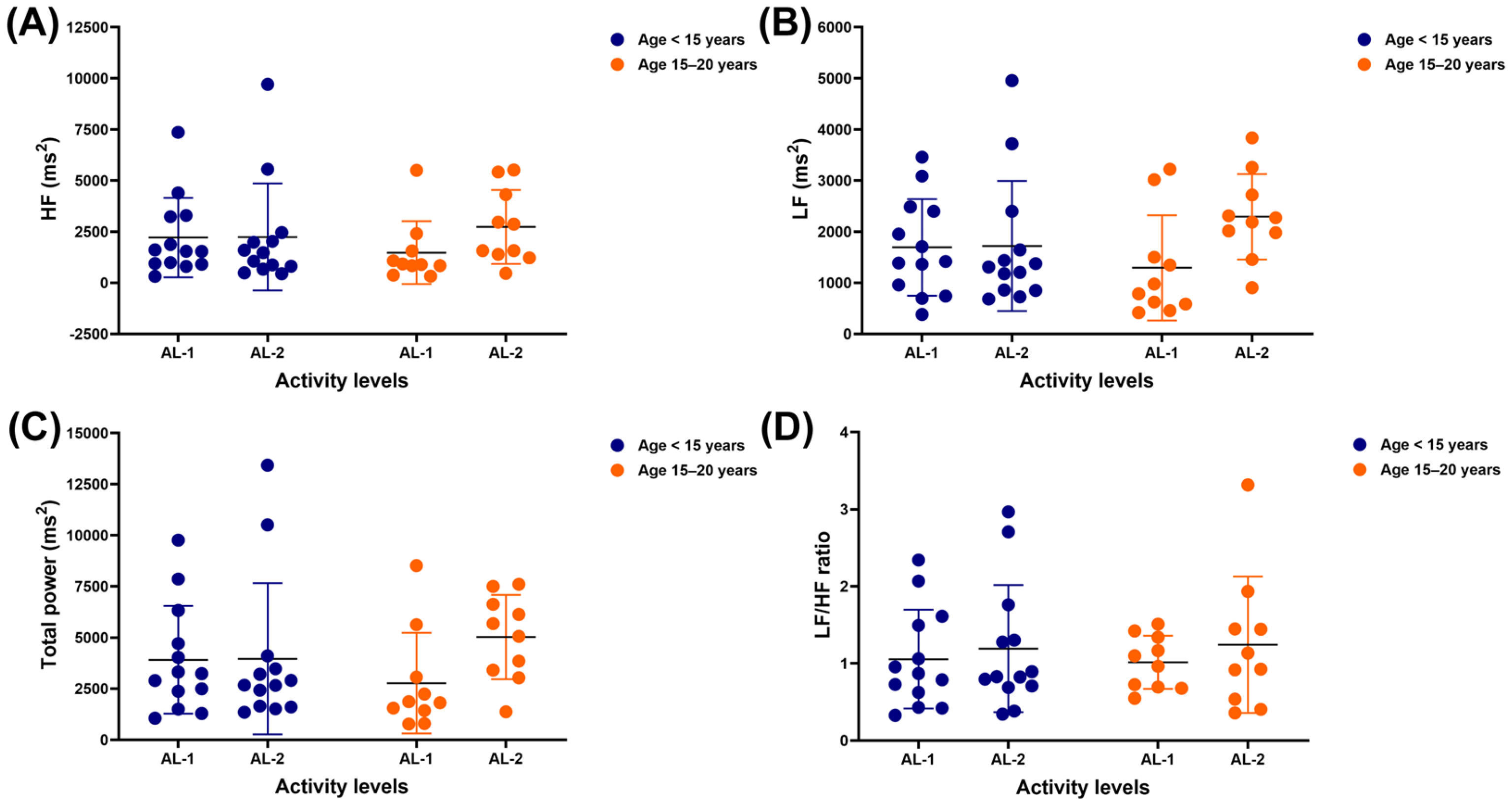
3.2. Frequency-Domain Results
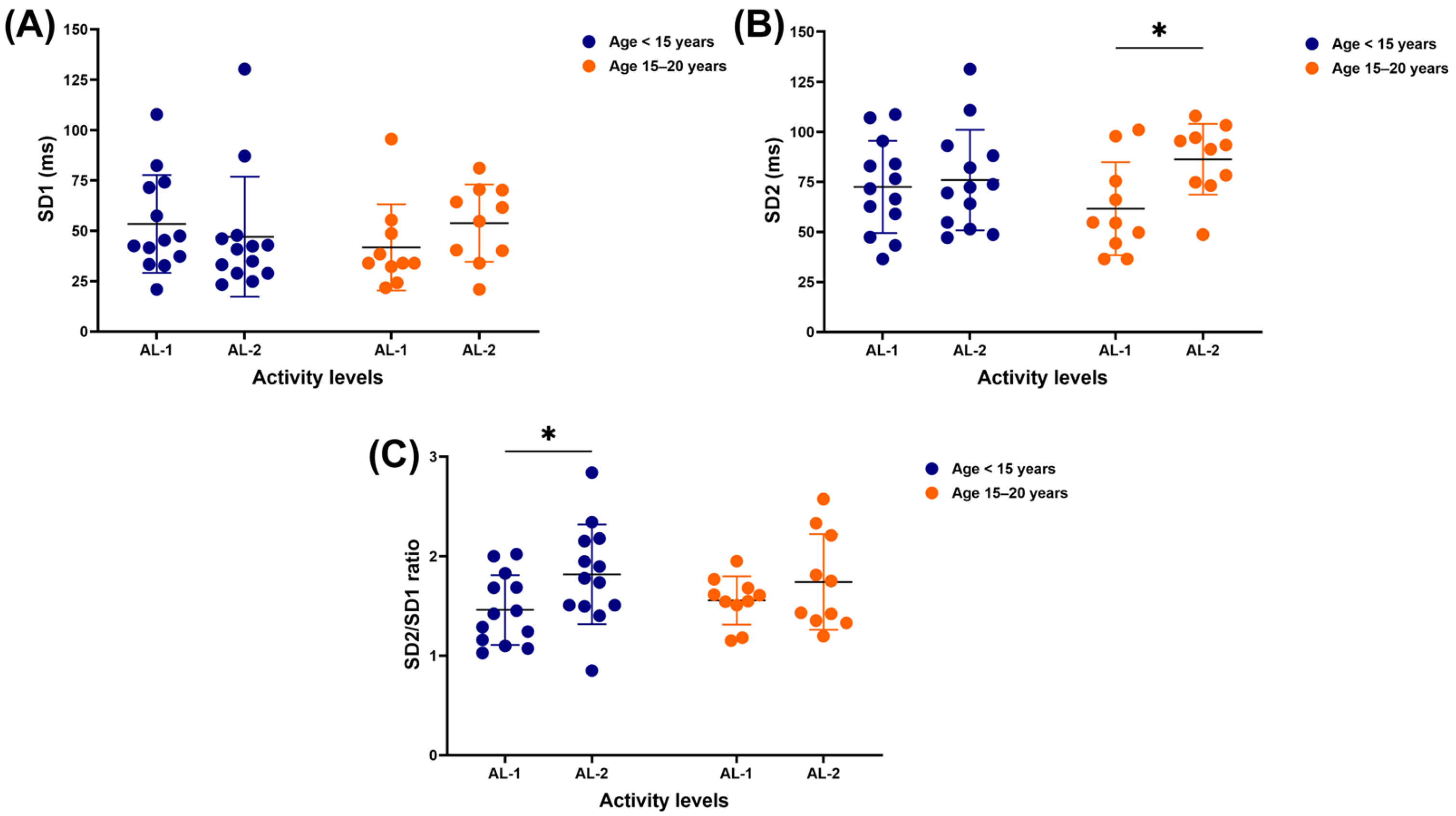
3.3. Nonlinear Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL-1 | structured exercise programme for equestrian dressage practise three to five days/week |
| AL-2 | school riding practice one or less than one day/week |
| ANOVA | analysis of variance |
| CSV | comma-separated values |
| DC | deceleration capacity of heart rate computed as a four-point difference |
| HF | high-frequency band |
| HR | heart rate |
| HRM | heart rate monitoring |
| HRV | heart rate variability |
| LF | low-frequency band |
| LSD | least significant difference |
| pNN50 | number of successive RR interval pairs that differ more than 50 ms divided by the total number of RR intervals |
| RMSSD | square root of the mean squared differences between successive RR intervals |
| RR | beat-to-beat |
| SDNN | standard deviation of normal-to-normal RR intervals |
| SDANN | standard deviation of the averages of normal-to-normal RR intervals in 5 min segments |
| SDNNI | mean of the standard deviations of normal-to-normal RR intervals in 5 min segments |
| SD1 | standard deviation in Poincaré plot perpendicular to the line of identity |
| SD2 | standard deviation in Poincaré plot along the line of identity |
References
- Fédération Equestre Internationale. The FEI Database. 2025. Available online: https://data.fei.org/default.aspx (accessed on 17 April 2025).
- McGowan, C. Welfare of Aged Horses. Animals 2011, 1, 366–376. [Google Scholar] [CrossRef] [PubMed]
- McKeever, K.H.; Eaton, T.L.; Geiser, S.; Kearns, C.F.; Lehnhard, R.A. Age related decreases in thermoregulation and cardiovascular function in horses. Equine Vet. J. 2010, 42, 220–227. [Google Scholar] [CrossRef]
- Walker, A.; Arent, S.M.; McKeever, K.H. Maximal aerobic capacity (VO2max) in horses: A retrospective study to identify the age-related decline. Comp. Exerc. Physiol. 2009, 6, 177–181. [Google Scholar] [CrossRef]
- Kim, J.-s.; Hinchcliff, K.W.; Yamaguchi, M.; Beard, L.A.; Markert, C.D.; Devor, S.T. Age-related changes in metabolic properties of equine skeletal muscle associated with muscle plasticity. Vet. J. 2005, 169, 397–403. [Google Scholar] [CrossRef]
- Fukuda, T.; Kikuchi, M.; Kurotaki, T.; Oyamada, T.; Yoshikawa, H.; Yoshikawa, T. Age-related changes in the testes of horses. Equine Vet. J. 2001, 33, 20–25. [Google Scholar] [CrossRef]
- Paradis, M.R. Demographics of health and disease in the geriatric horse. Vet. Clin. Equine Pract. 2002, 18, 391–401. [Google Scholar] [CrossRef]
- Harrington McKeever, K. Aging and how it affects the physiological response to exercise in the horse. Clin. Tech. Equine Pract. 2003, 2, 258–265. [Google Scholar] [CrossRef]
- Rietmann, T.R.; Stuart, A.E.A.; Bernasconi, P.; Stauffacher, M.; Auer, J.A.; Weishaupt, M.A. Assessment of mental stress in warmblood horses: Heart rate variability in comparison to heart rate and selected behavioural parameters. Appl. Anim. Behav. Sci. 2004, 88, 121–136. [Google Scholar] [CrossRef]
- Wonghanchao, T.; Sanigavatee, K.; Poochipakorn, C.; Huangsaksri, O.; Chanda, M. Dynamic Adaptation of Heart Rate and Autonomic Regulation During Training and Recovery Periods in Response to a 12-Week Structured Exercise Programme in Untrained Adult and Geriatric Horses. Animals 2025, 15, 1122. [Google Scholar] [CrossRef]
- Nyerges-Bohák, Z.; Nagy, K.; Rózsa, L.; Póti, P.; Kovács, L. Heart rate variability before and after 14 weeks of training in Thoroughbred horses and Standardbred trotters with different training experience. PLoS ONE 2021, 16, e0259933. [Google Scholar] [CrossRef]
- Sanigavatee, K.; Poochipakorn, C.; Huangsaksri, O.; Vichitkraivin, S.; Pakdeelikhit, S.; Chotiyothin, W.; Wongkosoljit, S.; Wonghanchao, T.; Chanda, M. A structured exercise regimen enhances autonomic function compared to unstructured physical activities in geriatric horses. Sci. Rep. 2025, 15, 2493. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- Schiweck, C.; Piette, D.; Berckmans, D.; Claes, S.; Vrieze, E. Heart rate and high frequency heart rate variability during stress as biomarker for clinical depression. A systematic review. Psychol. Med. 2019, 49, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Levy, W.C.; Cerqueira, M.D.; Harp, G.D.; Johannessen, K.-A.; Abrass, I.B.; Schwartz, R.S.; Stratton, J.R. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am. J. Cardiol. 1998, 82, 1236–1241. [Google Scholar] [CrossRef]
- Gehlen, H.; Faust, M.-D.; Grzeskowiak, R.M.; Trachsel, D.S. Association between Disease Severity, Heart Rate Variability (HRV) and Serum Cortisol Concentrations in Horses with Acute Abdominal Pain. Animals 2020, 10, 1563. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Schwarzwald, C.C. Heart rate variability analysis in horses for the diagnosis of arrhythmias. Vet. J. 2021, 268, 105590. [Google Scholar] [CrossRef]
- Stucke, D.; Große Ruse, M.; Lebelt, D. Measuring heart rate variability in horses to investigate the autonomic nervous system activity—Pros and cons of different methods. Appl. Anim. Behav. Sci. 2015, 166, 1–10. [Google Scholar] [CrossRef]
- von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed]
- McCraty, R.; Zayas, M.A. Cardiac coherence, self-regulation, autonomic stability, and psychosocial well-being. Front. Psychol. 2014, 5, 1090. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Moneghetti, K.J.; Christle, J.W.; Hadley, D.; Froelicher, V.; Plews, D. Heart Rate Variability: An Old Metric with New Meaning in the Era of Using mHealth technologies for Health and Exercise Training Guidance. Part Two: Prognosis and Training. Arrhythmia Electrophysiol. Rev. 2018, 7, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.; Rogers, C.W.; Gronqvist, G.; Gee, E.K.; Bolwell, C.F. Anxiety and pain in horses measured by heart rate variability and behavior. J. Vet. Behav. 2017, 22, 1–6. [Google Scholar] [CrossRef]
- Ohmura, H.; Jones, J.H. Changes in heart rate and heart rate variability as a function of age in Thoroughbred horses. J. Equine Sci. 2017, 28, 99–103. [Google Scholar] [CrossRef]
- Janczarek, I.; Kędzierski, W.; Wilk, I.; Wnuk–Pawlak, E.; Rakowska, A. Comparison of daily heart rate variability in old and young horses: A preliminary study. J. Vet. Behav. 2020, 38, 1–7. [Google Scholar] [CrossRef]
- Sanigavatee, K.; Poochipakorn, C.; Huangsaksri, O.; Wonghanchao, T.; Rodkruta, N.; Chanprame, S.; Wiwatwongwana, T.; Chanda, M. Comparison of daily heart rate and heart rate variability in trained and sedentary aged horses. J. Equine Vet. Sci. 2024, 137, 105094. [Google Scholar] [CrossRef]
- Frippiat, T.; van Beckhoven, C.; Moyse, E.; Art, T. Accuracy of a heart rate monitor for calculating heart rate variability parameters in exercising horses. J. Equine Vet. Sci. 2021, 104, 103716. [Google Scholar] [CrossRef]
- Kapteijn, C.M.; Frippiat, T.; van Beckhoven, C.; van Lith, H.A.; Endenburg, N.; Vermetten, E.; Rodenburg, T.B. Measuring heart rate variability using a heart rate monitor in horses (Equus caballus) during groundwork. Front. Vet. Sci. 2022, 9, 939534. [Google Scholar] [CrossRef]
- Ille, N.; Erber, R.; Aurich, C.; Aurich, J. Comparison of heart rate and heart rate variability obtained by heart rate monitors and simultaneously recorded electrocardiogram signals in nonexercising horses. J. Vet. Behav. 2014, 9, 341–346. [Google Scholar] [CrossRef]
- Mott, R.; Dowell, F.; Evans, N. Use of the Polar V800 and Actiheart 5 heart rate monitors for the assessment of heart rate variability (HRV) in horses. Appl. Anim. Behav. Sci. 2021, 241, 105401. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013; p. 567. [Google Scholar]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart Rate Variability. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Eggensperger, B.H.; Schwarzwald, C.C. Influence of 2nd-degree AV blocks, ECG recording length, and recording time on heart rate variability analyses in horses. J. Vet. Cardiol. 2017, 19, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Berntson, G.G.; Stowell, J.R. ECG artifacts and heart period variability: Don’t miss a beat! Psychophysiology 1998, 35, 127–132. [Google Scholar] [CrossRef]
- Lipponen, J.A.; and Tarvainen, M.P. A robust algorithm for heart rate variability time series artefact correction using novel beat classification. J. Med. Eng. Technol. 2019, 43, 173–181. [Google Scholar] [CrossRef]
- Gleerup, K.B.; Forkman, B.; Lindegaard, C.; Andersen, P.H. An equine pain face. Vet. Anaesth. Analg. 2015, 42, 103–114. [Google Scholar] [CrossRef]
- Dalla Costa, E.; Dai, F.; Lebelt, D.; Scholz, P.; Barbieri, S.; Canali, E.; Zanella, A.J.; Minero, M. Welfare assessment of horses: The AWIN approach. Anim. Welf. 2016, 25, 481–488. [Google Scholar] [CrossRef]
- Visser, E.K.; Ellis, A.D.; Van Reenen, C.G. The effect of two different housing conditions on the welfare of young horses stabled for the first time. Appl. Anim. Behav. Sci. 2008, 114, 521–533. [Google Scholar] [CrossRef]
- Visser, E.K.; van Reenen, C.G.; van der Werf, J.T.N.; Schilder, M.B.H.; Knaap, J.H.; Barneveld, A.; Blokhuis, H.J. Heart rate and heart rate variability during a novel object test and a handling test in young horses. Physiol. Behav. 2002, 76, 289–296. [Google Scholar] [CrossRef]
- Porges, S.W. Cardiac vagal tone: A physiological index of stress. Neurosci. Biobehav. Rev. 1995, 19, 225–233. [Google Scholar] [CrossRef]
- Mendonça, T.; Bienboire-Frosini, C.; Kowalczyk, I.; Leclercq, J.; Arroub, S.; Pageat, P. Equine Activities Influence Horses’ Responses to Different Stimuli: Could This Have an Impact on Equine Welfare? Animals 2019, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Nyerges-Bohák, Z.; Kovács, L.; Povázsai, Á.; Hamar, E.; Póti, P.; Ladányi, M. Heart rate variability in horses with and without severe equine asthma. Equine Vet. J. 2025, 57, 611–618. [Google Scholar] [CrossRef]
- Berger, M.; Pichot, V.; Solelhac, G.; Marques-Vidal, P.; Haba-Rubio, J.; Vollenweider, P.; Waeber, G.; Preisig, M.; Barthélémy, J.-C.; Roche, F.; et al. Association between nocturnal heart rate variability and incident cardiovascular disease events: The HypnoLaus population-based study. Heart Rhythm 2022, 19, 632–639. [Google Scholar] [CrossRef]
- Takase, B. Role of Heart Rate Variability in Non-Invasive Electrophysiology: Prognostic Markers of Cardiovascular Disease. J. Arrhythm. 2010, 26, 227–237. [Google Scholar] [CrossRef]
- Ireland, J.L.; Clegg, P.D.; McGowan, C.M.; Duncan, J.S.; McCall, S.; Platt, L.; Pinchbeck, G.L. Owners’ perceptions of quality of life in geriatric horses: A cross-sectional study. Anim. Welf. 2011, 20, 483–495. [Google Scholar] [CrossRef]
- Pérez Manrique, L.; Hudson, R.; Bánszegi, O.; Szenczi, P. Individual differences in behavior and heart rate variability across the preweaning period in the domestic horse in response to an ecologically relevant stressor. Physiol. Behav. 2019, 210, 112652. [Google Scholar] [CrossRef]
- Ellis, A.D.; Stephenson, M.; Preece, M.; Harris, P. A novel approach to systematically compare behavioural patterns between and within groups of horses. Appl. Anim. Behav. Sci. 2014, 161, 60–74. [Google Scholar] [CrossRef]
- Physick-Sheard, P.W.; Marlin, D.J.; Thornhill, R.; Schroter, R.C. Frequency domain analysis of heart rate variability in horses at rest and during exercise. Equine Vet. J. 2000, 32, 253–262. [Google Scholar] [CrossRef]
- Kuwahara, M.; Hiraga, A.; Kai, M.; Tsubone, H.; Sugano, S. Influence of training on autonomic nervous function in horses: Evaluation by power spectral analysis of heart rate variability. Equine Vet. J. 1999, 31, 178–180. [Google Scholar] [CrossRef]
- Ohmura, H.; Boscan, P.L.; Solano, A.M.; Stanley, S.D.; Jones, J.H. Changes in heart rate, heart rate variability, and atrioventricular block during withholding of food in Thoroughbreds. J. Am. Vet. Med. Assoc. 2012, 73, 508–514. [Google Scholar] [CrossRef]

| Age Ranges | Age Groups (Years) (Mean ± SD) | Activity Levels | |
|---|---|---|---|
| <15 years | 10.8 ± 2.9 (N = 13) | AL-1 | - |
| 10.4 ± 2.8 (N = 13) | - | AL-2 | |
| 15–20 years | 17.5 ± 2.3 (N = 10) | AL-1 | - |
| 18.2 ± 1.9 (N = 10) | - | AL-2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wonghanchao, T.; Sanigavatee, K.; Petchdee, S.; Chettaratanont, K.; Thongyen, T.; Wanichayanon, B.; Poochipakorn, C.; Chanda, M. Heart Rate and Heart Rate Variability Are Affected by Age and Activity Level in Athletic Horses. Vet. Sci. 2025, 12, 624. https://doi.org/10.3390/vetsci12070624
Wonghanchao T, Sanigavatee K, Petchdee S, Chettaratanont K, Thongyen T, Wanichayanon B, Poochipakorn C, Chanda M. Heart Rate and Heart Rate Variability Are Affected by Age and Activity Level in Athletic Horses. Veterinary Sciences. 2025; 12(7):624. https://doi.org/10.3390/vetsci12070624
Chicago/Turabian StyleWonghanchao, Thita, Kanokpan Sanigavatee, Soontaree Petchdee, Kulpreeya Chettaratanont, Thitakorn Thongyen, Boonbaramee Wanichayanon, Chanoknun Poochipakorn, and Metha Chanda. 2025. "Heart Rate and Heart Rate Variability Are Affected by Age and Activity Level in Athletic Horses" Veterinary Sciences 12, no. 7: 624. https://doi.org/10.3390/vetsci12070624
APA StyleWonghanchao, T., Sanigavatee, K., Petchdee, S., Chettaratanont, K., Thongyen, T., Wanichayanon, B., Poochipakorn, C., & Chanda, M. (2025). Heart Rate and Heart Rate Variability Are Affected by Age and Activity Level in Athletic Horses. Veterinary Sciences, 12(7), 624. https://doi.org/10.3390/vetsci12070624








