Assessment of Pulmonary Vein Diameters in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Echocardiography
2.3. Acquisition and Reproducibility of PV Diameters
2.4. Thoracic Radiography
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Variability of PV Diameter Measurements
3.3. Pulmonary Vein Diameters and BW
3.4. Measurements of PV2 Diameter According to the ACVIM Stage and Correlations with Echocardiographic Parameters
3.5. Radiographic Score and PV2 Diameter
3.6. Diameter of PV2 as a Diagnostic Marker for Differentiating Stages B1, B2, and C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Peak velocity of late diastolic transmitral flow |
| ACVIM | American College of Veterinary Internal Medicine |
| AUC | Area under the ROC curve |
| BW | Body weight |
| CHF | Congestive heart failure |
| CI | Confidence interval |
| CKCSs | Cavalier King Charles Spaniels |
| CV | Coefficient of variation |
| E | Peak velocity of early diastolic transmitral flow |
| HF | Heart failure |
| ICC | Intraclass correlation coefficient |
| LA | Left atrium |
| LA:Ao | Ratio of the left atrial dimension to the aortic annulus dimension |
| LVIDDN | Left ventricular internal diameter in diastole normalized for body weight |
| LV | Left ventricle |
| MMVD | Myxomatous mitral valve disease |
| PISA | Proximal isovelocity surface area |
| PG | Pressure gradient |
| PH | Pulmonary hypertension |
| PPVs | Positive predictive values |
| PV | Pulmonary vein |
| PVs | Pulmonary veins |
| PV/PA | Pulmonary vein diameter-to-pulmonary artery diameter ratio |
| RF | Regurgitant fraction |
| ROC curve | Receiver operating characteristic curve |
| TR | Tricuspid regurgitation |
| VHS | Vertebral heart score (VHS) |
| VLAS | Vertebral left atrial size (VLAS) |
References
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Markby, G.; Summers, K.M.; MacRae, V.E.; Del-Pozo, J.; Corcoran, B.M. Myxomatous degeneration of the canine mitral valve: From gross changes to molecular events. J. Comp. Pathol. 2017, 156, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Menciotti, G.; Borgarelli, M.; Aherne, M.; Wesselowski, S.; Häggström, J.; Ljungvall, I.; Lahmers, S.M.; Abbott, J.A. Mitral valve morphology assessed by three-dimensional transthoracic echocardiography in healthy dogs and dogs with myxomatous mitral valve disease. J. Vet. Cardiol. 2017, 19, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Boswood, A.; Haggstrom, J.; Gordon, S.G.; Wess, G.; Stepien, R.L.; Oyama, M.A.; Keene, B.W.; Bonagura, J.; MacDonald, K.A.; Patteson, M.; et al. Effect of Pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: The EPIC Study—A randomized clinical trial. J. Vet. Intern. Med. 2016, 30, 1765–1779. [Google Scholar] [CrossRef]
- Hansson, K.; Häggström, J.; Kvart, C.; Lord, P. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in Cavalier King Charles Spaniels with and without left atrial enlargement. Vet. Radiol. Ultrasound 2002, 43, 568–575. [Google Scholar] [CrossRef]
- Cornell, C.C.; Kittleson, M.D.; Della Torre, P.; Häggström, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 2004, 18, 311–321. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. Clinical practice. Acute pulmonary edema. N. Engl. J. Med. 2005, 353, 2788–2796. [Google Scholar] [CrossRef]
- Zanza, C.; Saglietti, F.; Tesauro, M.; Longhitano, Y.; Savioli, G.; Balzanelli, M.G.; Romenskaya, T.; Cofone, L.; Pindinello, I.; Racca, G.; et al. Cardiogenic pulmonary edema in emergency medicine. Adv. Respir. Med. 2023, 91, 445–463. [Google Scholar] [CrossRef]
- Kirillova, V.V.; Smorgon, A.V.; Garganeeva, A.A.; Batalov, R.E.; Meshchaninov, V.N.; Sokolova, L.A.; Blagodareva, M.S.; Khlynin, M.S.; Popov, S.V. Ultrasound diagnosis of congestion in the pulmonary and systemic circulations in patients with atrial fibrillation and chronic heart failure. Russ. Open Med. J. 2021, 10, e0415. [Google Scholar] [CrossRef]
- Kirillova, V.V. Using pulmonary vein diameters for prescribing diuretic therapy and estimating its effectiveness in heart failure with preserved ejection fraction outpatients. Future Cardiol. 2022, 18, 719–729. [Google Scholar] [CrossRef]
- Birettoni, F.; Caivano, D.; Patata, V.; Moïse, N.S.; Guglielmini, C.; Rishniw, M.; Porciello, F. Canine pulmonary vein-to-pulmonary artery ratio: Echocardiographic technique and reference intervals. J. Vet. Cardiol. 2016, 18, 326–335. [Google Scholar] [CrossRef]
- Patata, V.; Caivano, D.; Porciello, F.; Rishniw, M.; Domenech, O.; Marchesotti, F.; Giorgi, M.E.; Guglielmini, C.; Poser, H.; Spina, F.; et al. Pulmonary vein to pulmonary artery ratio in healthy and cardiomyopathic cats. J. Vet. Cardiol. 2020, 27, 23–33. [Google Scholar] [CrossRef]
- Caivano, D.; Corda, A.; Rishniw, M.; Giorgi, M.E.; Pinna Parpaglia, M.L.; Conti, M.B.; Porciello, F.; Birettoni, F. Transthoracic M-mode echocardiographic assessment of pulmonary vein-to-pulmonary artery ratio in healthy horses. PLoS ONE 2019, 14, e0221154. [Google Scholar] [CrossRef]
- Merveille, A.C.; Bolen, G.; Krafft, E.; Roels, E.; Gomart, S.; Etienne, A.L.; Clercx, C.; McEntee, K. Pulmonary vein-to-pulmonary artery ratio is an echocardiographic index of congestive heart failure in dogs with degenerative mitral valve disease. J. Vet. Intern. Med. 2015, 29, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Roels, E.; Merveille, A.C.; Moyse, E.; Gomart, S.; Clercx, C.; Mc Entee, K. Diagnostic value of the pulmonary vein-to-right pulmonary artery ratio in dogs with pulmonary hypertension of precapillary origin. J. Vet. Cardiol. 2019, 24, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Roels, E.; Fastrès, A.; Merveille, A.C.; Bolen, G.; Teske, E.; Clercx, C.; McEntee, K. The prevalence of pulmonary hypertension assessed using the pulmonary vein-to-right pulmonary artery ratio and its association with survival in West Highland White Terriers with canine idiopathic pulmonary fibrosis. BMC Vet. Res. 2021, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.I.; Caro-Vadillo, A.; Falcón-Cordón, Y.; García-Rodríguez, S.N.; Costa-Rodríguez, N.; Carretón, E.; Montoya-Alonso, J.A. Echocardiographic assessment of the pulmonary vein to pulmonary artery ratio in canine heartworm disease. Animals 2023, 13, 703. [Google Scholar] [CrossRef]
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef]
- Brewer, F.C.; Moïse, N.S.; Kornreich, B.G.; Bezuidenhout, A.J. Use of computed tomography and silicon endocasts to identify pulmonary veins with echocardiography. J. Vet. Cardiol. 2012, 14, 293–300. [Google Scholar] [CrossRef]
- Holt, D.E.; Cole, S.G.; Anderson, R.B.; Miscelis, R.R.; Bridges, C.R. The canine right caudal and accessory lobe pulmonary veins: Revised anatomical description, clinical relevance, and embryological implications. Anat. Histol. Embryol. 2005, 34, 273–275. [Google Scholar] [CrossRef]
- Borgarelli, M.; Häggström, J. Canine degenerative myxomatous mitral valve disease: Natural history, clinical presentation and therapy. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 651–663. [Google Scholar] [CrossRef]
- Prieto Ramos, J.; Corda, A.; Swift, S.; Saderi, L.; De La Fuente Oliver, G.; Corcoran, B.; Summers, K.M.; French, A.T. Clinical and echocardiographic findings in an aged population of Cavalier King Charles Spaniels. Animals 2021, 11, 949. [Google Scholar] [CrossRef]
- Chetboul, V.; Tidholm, A.; Nicolle, A.; Sampedrano, C.C.; Gouni, V.; Pouchelon, J.L.; Lefebvre, H.P.; Concordet, D. Effects of animal position and number of repeated measurements on selected two-dimensional and M-mode echocardiographic variables in healthy dogs. J. Am. Vet. Med. Assoc. 2005, 227, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Schober, K.E.; Hart, T.M.; Stern, J.A.; Li, X.; Samii, V.F.; Zekas, L.J.; Scansen, B.A.; Bonagura, J.D. Detection of congestive heart failure in dogs by Doppler echocardiography. J. Vet. Intern. Med. 2010, 24, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.D.; Brown, W.A. Regurgitant fraction measured by using the proximal isovelocity surface area method in dogs with chronic myxomatous mitral valve disease. J. Vet. Intern. Med. 2003, 17, 84–88. [Google Scholar] [CrossRef]
- Johnson, L.; Boon, J.; Orton, E.C. Clinical characteristics of 53 dogs with Doppler-derived evidence of pulmonary hypertension: 1992-1996. J. Vet. Intern. Med. 1999, 13, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.H.; Hagio, M.; Okano, S. Effects of respiratory cycle on pulmonary venous flow and cardiac cycle on pulmonary venous diameter of dogs: A transesophageal echocardiography study. J. Vet. Med. Sci. 1999, 61, 155–158. [Google Scholar] [CrossRef]
- Bagardi, M.; Locatelli, C.; Manfredi, M.; Bassi, J.; Spediacci, C.; Ghilardi, S.; Zani, D.D.; Brambilla, P.G. Breed-specific vertebral heart score, vertebral left atrial size, and radiographic left atrial dimension in Cavalier King Charles Spaniels: Reference interval study. Vet. Radiol. Ultrasound 2022, 63, 156–163. [Google Scholar] [CrossRef]
- Oui, H.; Oh, J.; Keh, S.; Lee, G.; Jeon, S.; Kim, H.; Yoon, J.; Choi, J. Measurements of the pulmonary vasculature on thoracic radiographs in healthy dogs compared to dogs with mitral regurgitation. Vet. Radiol. Ultrasound 2014, 56, 251–256. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.; Seo, M.W.; Park, C. Pulmonary-vein-to-pulmonary-artery ratio can be utilized to evaluate myxomatous mitral valve disease progression in dogs. Am. J. Vet. Res. 2024, 85, 1–8. [Google Scholar] [CrossRef] [PubMed]

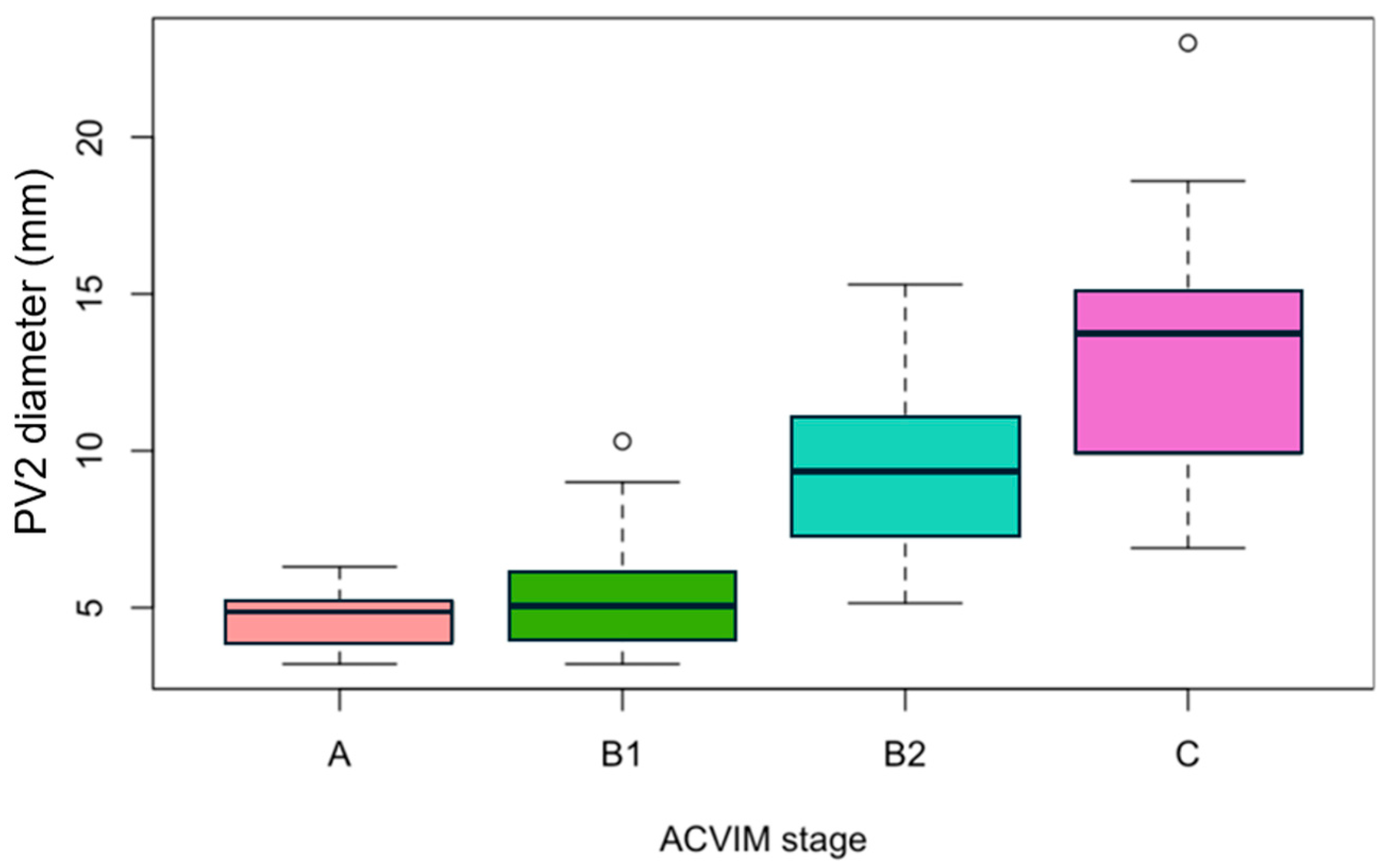
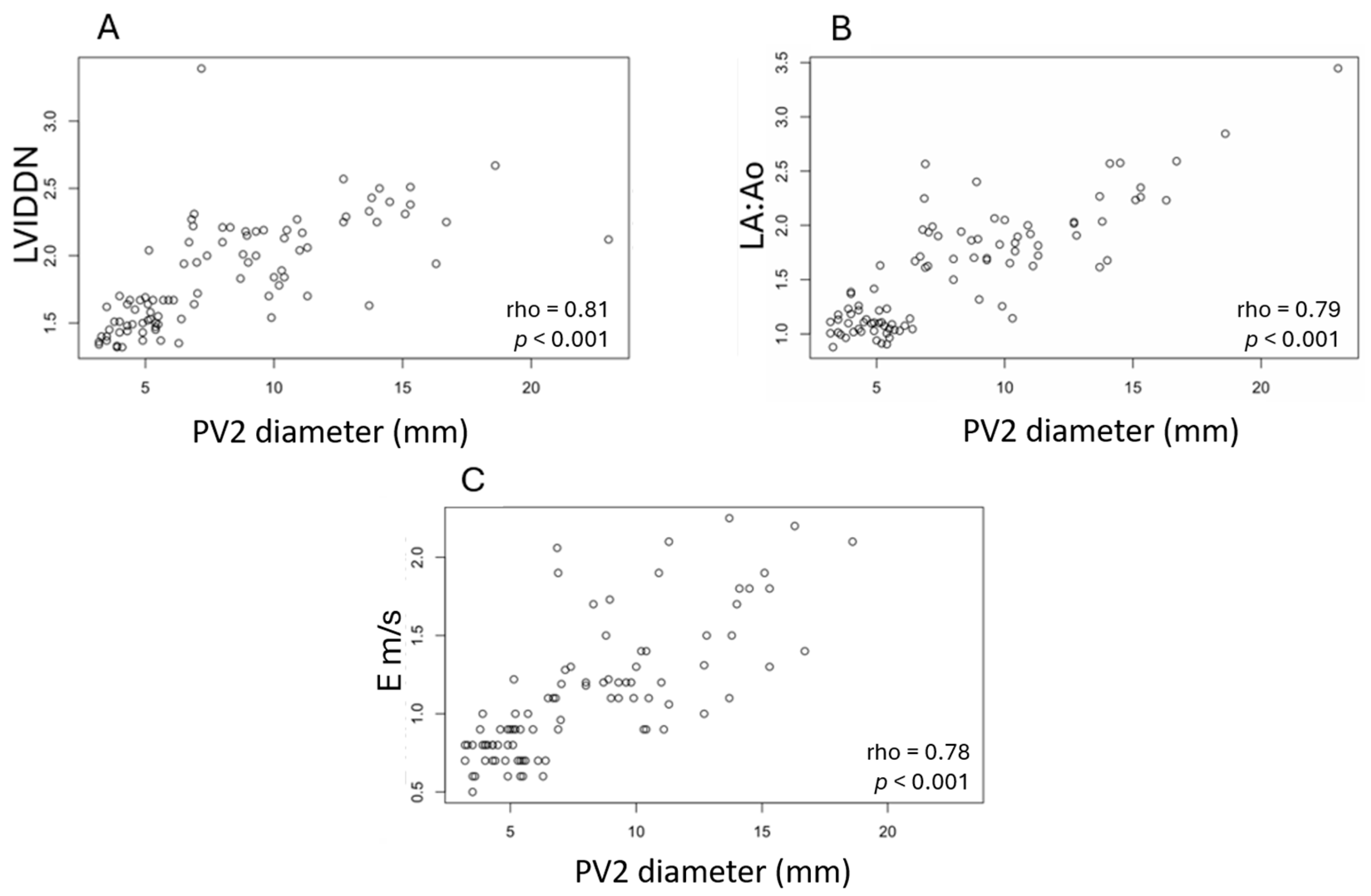

| A (n = 28) | B1 (n = 21) | B2 (n = 29) | C (n = 22) | |
|---|---|---|---|---|
| Age (years, IQR) | 2.47 (1.80–3.14) a | 6.41 (4.44–8.12) b | 8.84 (8.04–10.23) c | 9.65 (8.98–10.41) c |
| Weight (kg, IQR) | 7.60 (6.45–9.43) a | 9.00 (8.40–10.60) b | 9.80 (8.30–10.50) b | 8.70 (7.28–9.93) ab |
| Males (ratio, %) | 10/28 (35.7%) | 10/21 (47.6%) | 17/29 (58.6%) | 10/22 (45.5%) |
| Cough (ratio, %) | - | 1/21 (4.8%) | 10/29 (34.5%) | 8/22 (36.4%) |
| Exercise intolerance (ratio, %) | - | - | 9/29 (31.0%) | 5/22 (22.7%) |
| Tachypnea/Dyspnea (ratio, %) | - | - | 3/29 (10.3%) | 12/22 (54.5%) |
| Syncope (ratio, %) | - | - | 2/29 (6.9%) | 3/22 (13.6%) |
| Ascites (ratio, %) | - | - | - | 2/22 (9.1%) |
| HR (beats/min, IQR) | 128 (114–141) a | 130 (115–140) a | 140 (133–150) ab | 150 (141–155) b |
| Murmur grade 0/6 to 6/6 (IQR) | - | 3 (2–4) | 4 (4–5) | 5 (4–5) |
| Drugs | B1 (n = 21) | B2 (n = 29) | C (n = 22) |
|---|---|---|---|
| Pimobendan | 4 (19.0%) | 20 (69.0%) | 20 (90.9%) |
| Benazepril | 2 (9.5%) | 15 (51.7%) | 11 (50.0%) |
| Spironolactone | 1 (4.8%) | 11 (37.9%) | 13 (59.1%) |
| Furosemide | - | 2 (6.9%) | 15 (68.2%) |
| Torasemide | - | 2 (6.9%) | 9 (40.9%) |
| Sildenafil | - | 1 (3.4%) | 3 (13.6%) |
| Digoxin | - | - | 1 (4.5%) |
| ICC | 95% CI | p | |
|---|---|---|---|
| Interobserver PV1 diameter | 0.988 | 0.982–0.993 | <0.001 |
| Interobserver PV2 diameter | 0.997 | 0.995–0.998 | <0.001 |
| Interobserver PV3 diameter | 0.994 | 0.990–0.996 | <0.001 |
| Echocardiographic Variables | A | B1 | B2 | C | p |
|---|---|---|---|---|---|
| LVIDDN (cm/kg) | 1.51 (1.42–1.61) a * 28/28 dogs | 1.53 (1.47–1.67) a * 21/21 dogs | 2.13 (2.00–2.22) b * 29/29 dogs | 2.26 (1.98–2.38) b * 22/22 dogs | <0.001 |
| LA:Ao | 1.07 (1.00–1.13) a * 28/28 dog | 1.18 (1.08–1.32) b * 21/21 dogs | 1.81 (1.68–1.99) c * 29/29 dogs | 2.14 (1.92–2.51) d * 22/22 dogs | <0.001 |
| TR PG (mmHg) | 16.0 (11.5–18.0) a * 19/28 dogs | 26.5 (21.3–35.8) b * 18/21 dogs | 50.0 (31.0–57.5) c * 27/29 dogs | 63.0 (40.0–75.0) c * 21/22 dogs | <0.001 |
| RF (%) | / | 26.5 (13.0–36.5) a * 14/21 dogs | 58.0 (51.0–63.0) b * 19/29 dogs | 76.5 (65.5–79.8) c * 18/22 dogs | <0.001 |
| E (m/s) | 0.8 (0.7–0.9) a * 28/28 dogs | 0.8 (0.7–0.9) a * 21/21 dogs | 1.2 (1.1–1.4) b * 29/29 dogs | 1.7 (1.2–1.9) c * 21/22 dogs | <0.001 |
| E:A | 1.29 (1.16–1.43) a * 28/28 dogs | 1.17 (1.17–1.17) a * 21/21 dogs | 1.33 (1.17–1.57) a * 28/29 dogs | 2.00 (1.63–2.43) b * 21/22 dogs | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferri, C.; Besso, J.; Gaillot, H.; Ruel, Y.; Agoulon, A.; Bourguignon, C.; Mey, C.; Gouni, V. Assessment of Pulmonary Vein Diameters in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease. Vet. Sci. 2025, 12, 615. https://doi.org/10.3390/vetsci12070615
Ferri C, Besso J, Gaillot H, Ruel Y, Agoulon A, Bourguignon C, Mey C, Gouni V. Assessment of Pulmonary Vein Diameters in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease. Veterinary Sciences. 2025; 12(7):615. https://doi.org/10.3390/vetsci12070615
Chicago/Turabian StyleFerri, Carlotta, Juliette Besso, Hugues Gaillot, Yannick Ruel, Albert Agoulon, Christophe Bourguignon, Clémence Mey, and Vassiliki Gouni. 2025. "Assessment of Pulmonary Vein Diameters in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease" Veterinary Sciences 12, no. 7: 615. https://doi.org/10.3390/vetsci12070615
APA StyleFerri, C., Besso, J., Gaillot, H., Ruel, Y., Agoulon, A., Bourguignon, C., Mey, C., & Gouni, V. (2025). Assessment of Pulmonary Vein Diameters in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease. Veterinary Sciences, 12(7), 615. https://doi.org/10.3390/vetsci12070615






