Involvement of Peripheral Serotonin in Blood Cells in Healthy Cyclical Mares of Different Ages
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Samples
2.3. Analytical Procedures
2.4. Statistical Analysis
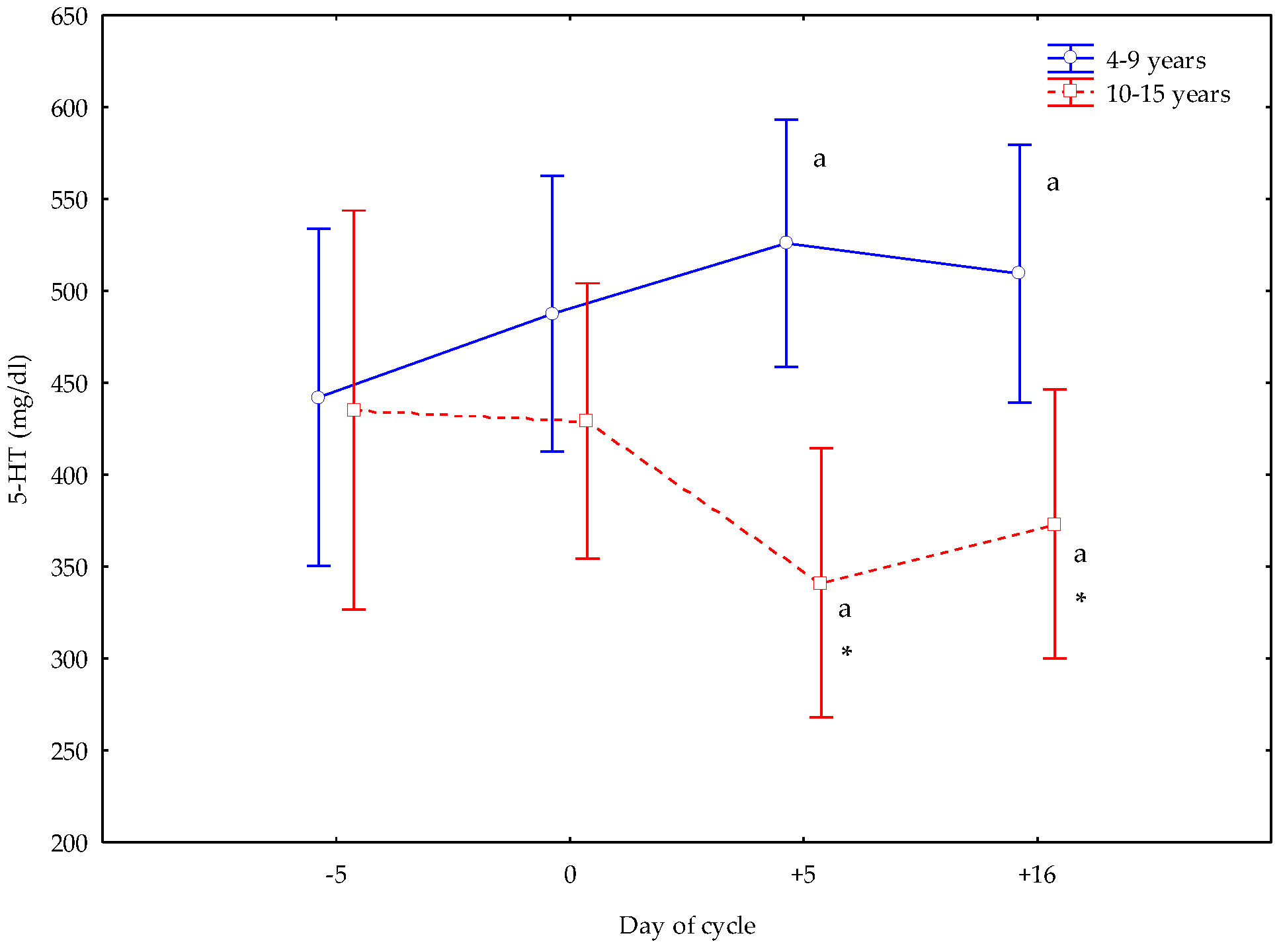
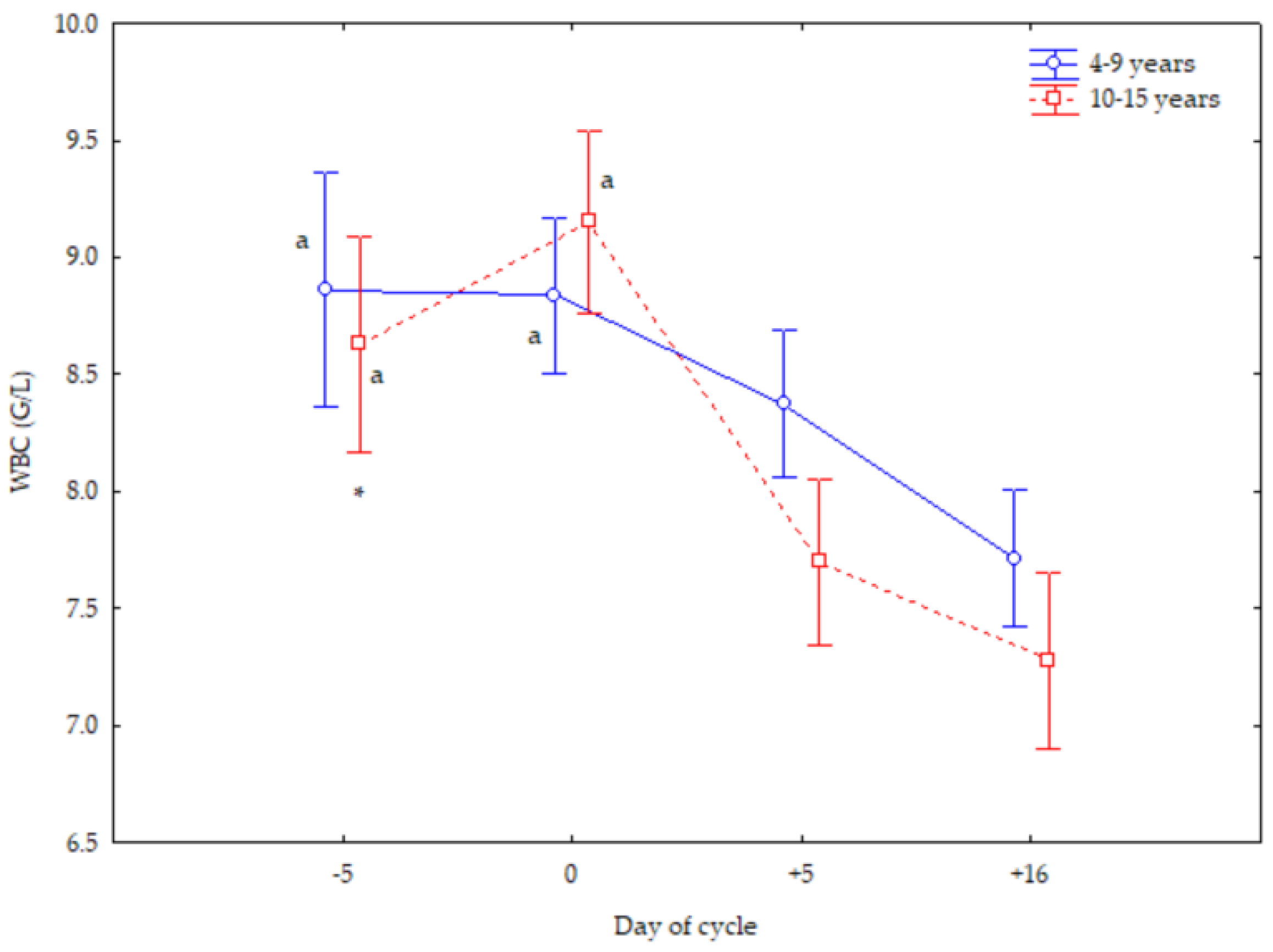
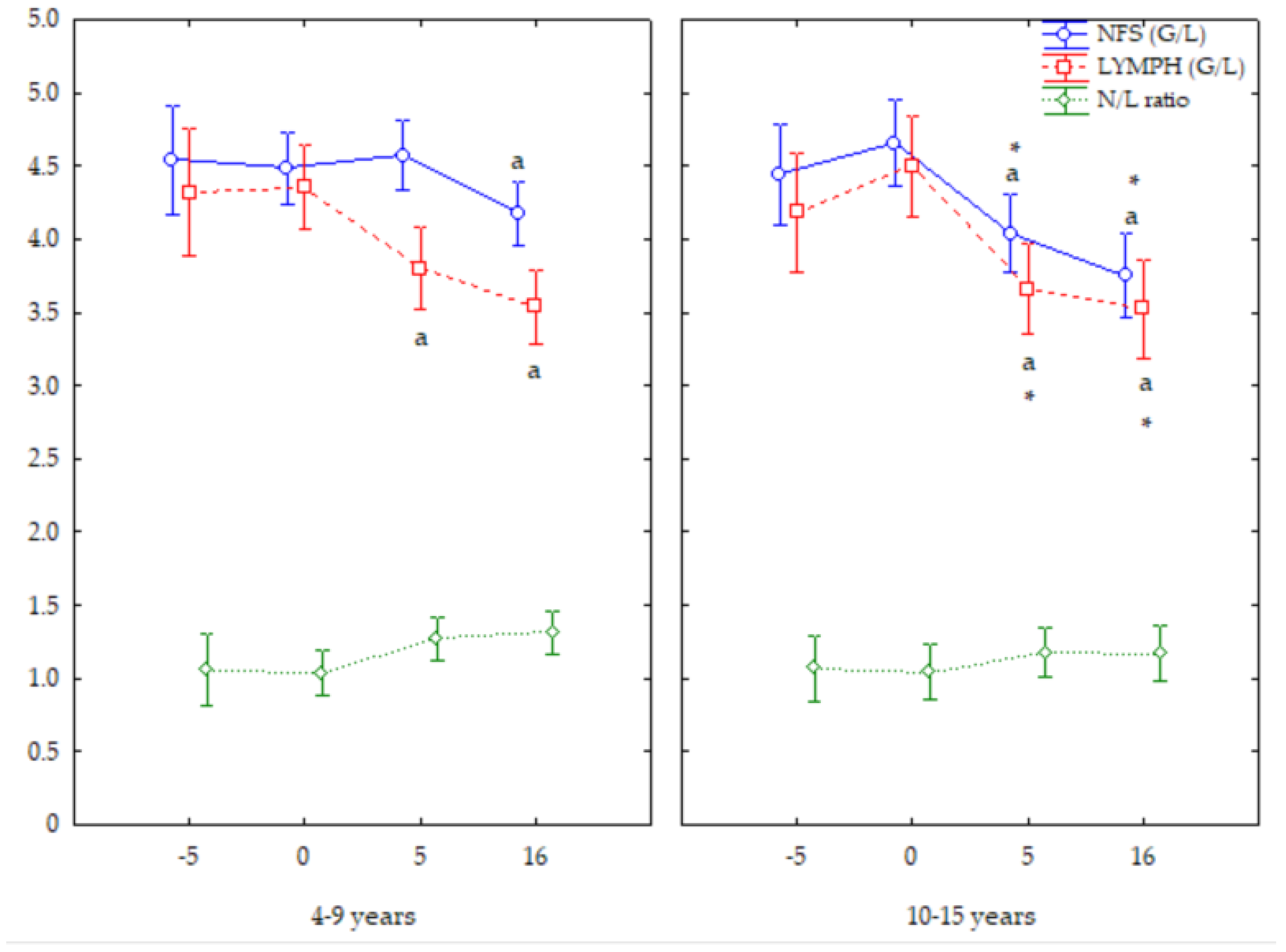
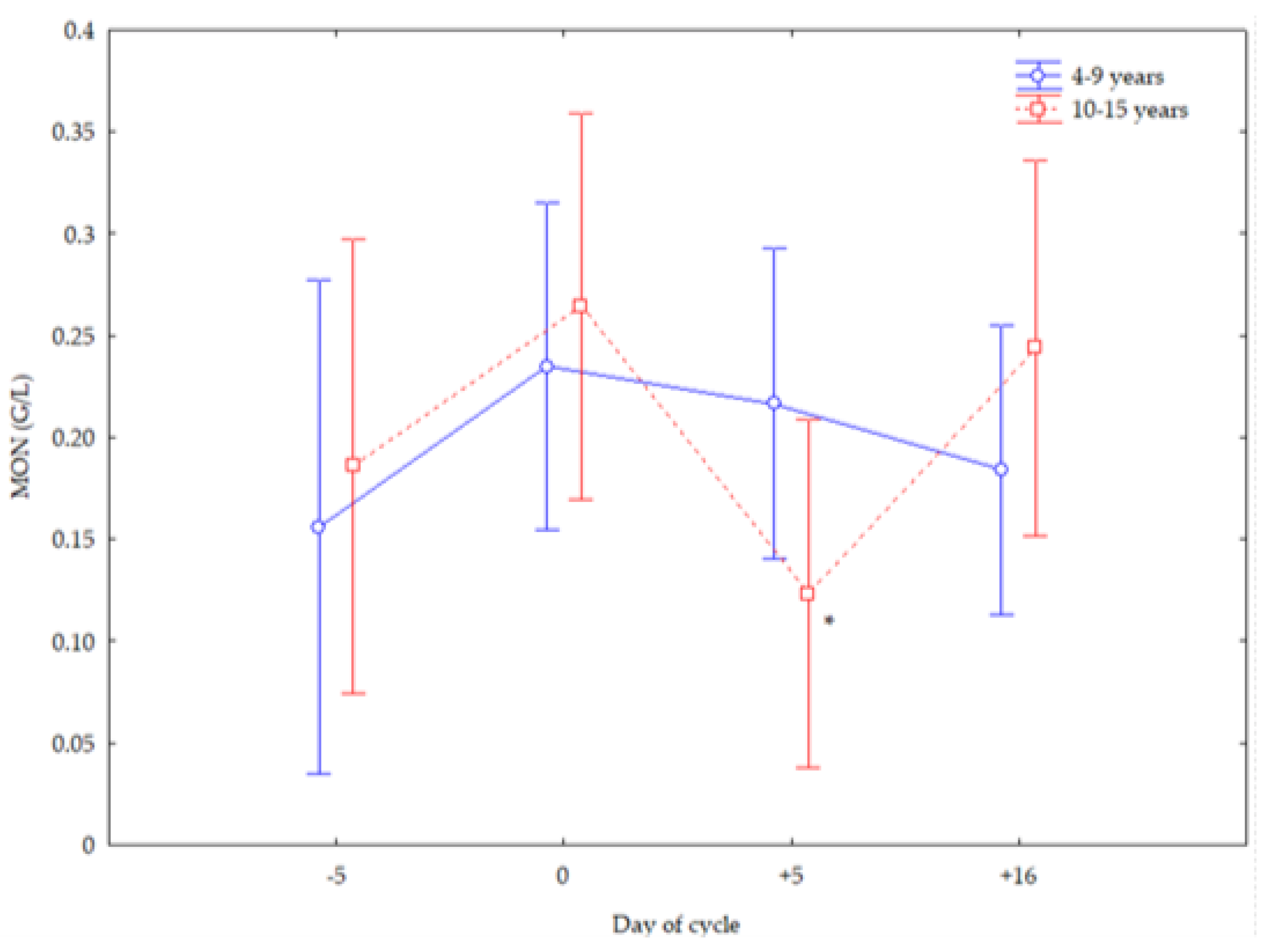
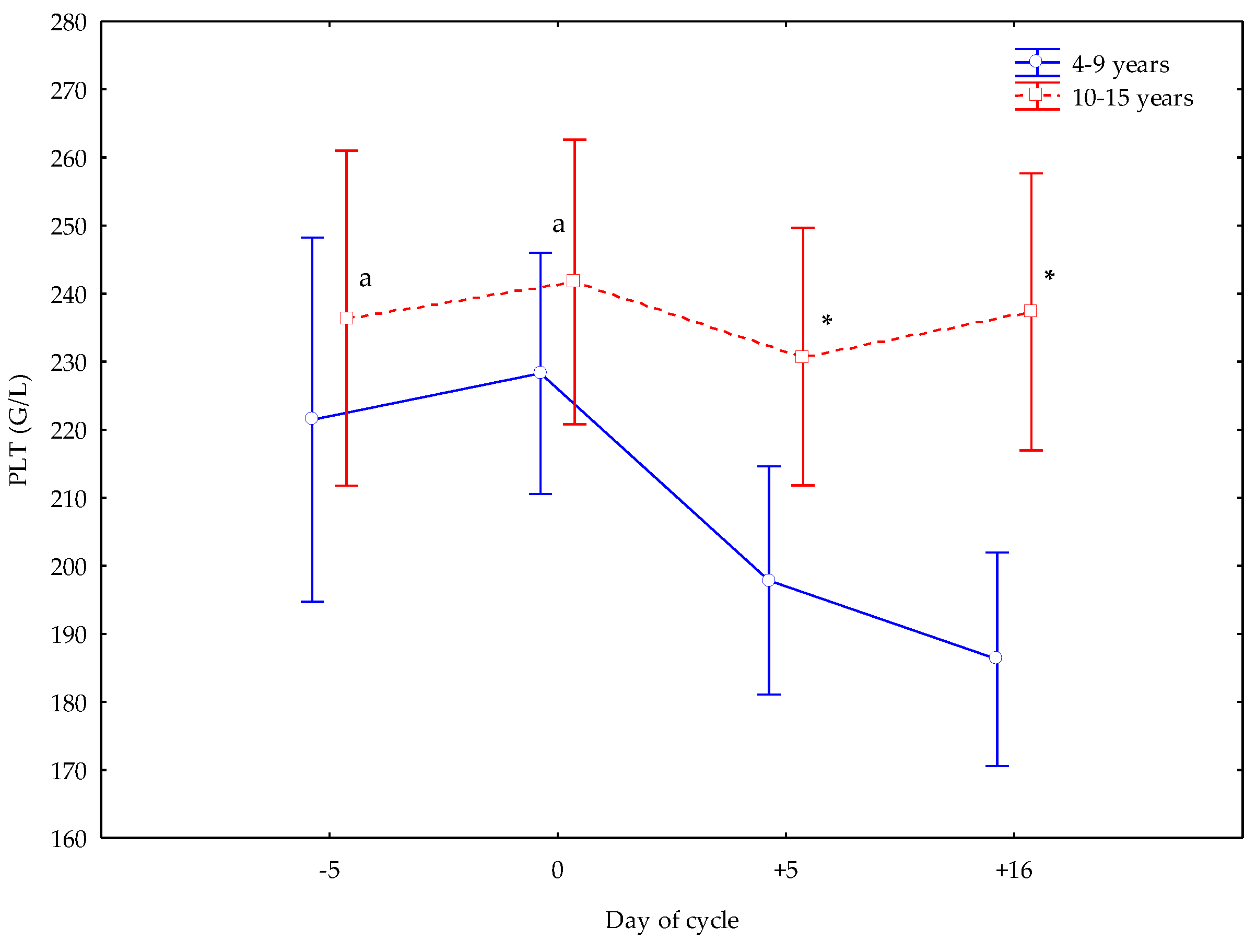
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, J.; Salamonsen, L.A. Inflammation, Leukocytes and Menstruation. Rev. Endocr. Metab. Disord. 2012, 13, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Oertelt-Prigione, S. Immunology and the Menstrual Cycle. Autoimmun. Rev. 2012, 11, A486–A492. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.M.A.; Landgren, B.-M.; Fjällström, P.; Shamekh, M.M.; Gustafsson, J.-Å.; Johansson, A.F.; Nalvarte, I. Expression of Sex Hormone Receptor and Immune Response Genes in Peripheral Blood Mononuclear Cells During the Menstrual Cycle. Front. Endocrinol. 2021, 12, 721813. [Google Scholar] [CrossRef] [PubMed]
- Kugaya, A.; Epperson, C.N.; Zoghbi, S.; Van Dyck, C.H.; Hou, Y.; Fujita, M.; Staley, J.K.; Garg, P.K.; Seibyl, J.P.; Innis, R.B. Increase in Prefrontal Cortex Serotonin2A Receptors Following Estrogen Treatment in Postmenopausal Women. Am. J. Psychiatry 2003, 160, 1522–1524. [Google Scholar] [CrossRef] [PubMed]
- Rybaczyk, L.A.; Bashaw, M.J.; Pathak, D.R.; Moody, S.M.; Gilders, R.M.; Holzschu, D.L. An Overlooked Connection: Serotonergic Mediation of Estrogen-Related Physiology and Pathology. BMC Womens Health 2005, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Kundakovic, M.; Rocks, D. Sex Hormone Fluctuation and Increased Female Risk for Depression and Anxiety Disorders: From Clinical Evidence to Molecular Mechanisms. Front. Neuroendocrinol. 2022, 66, 101010. [Google Scholar] [CrossRef]
- Arreola, R.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Velasco-Velázquez, M.A.; Garcés-Alvarez, M.E.; Hurtado-Alvarado, G.; Quintero-Fabian, S.; Pavón, L. Immunomodulatory Effects Mediated by Serotonin. J. Immunol. Res. 2015, 2015, 354957. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Bouman, A.; Moes, H.; Heineman, M.J.; De Leij, L.F.M.H.; Faas, M.M. The Immune Response during the Luteal Phase of the Ovarian Cycle: Increasing Sensitivity of Human Monocytes to Endotoxin. Fertil. Steril. 2001, 76, 555–559. [Google Scholar] [CrossRef]
- Engler-Chiurazzi, E.B.; Chastain, W.H.; Citron, K.K.; Lambert, L.E.; Kikkeri, D.N.; Shrestha, S.S. Estrogen, the Peripheral Immune System and Major Depression—A Reproductive Lifespan Perspective. Front. Behav. Neurosci. 2022, 16, 850623. [Google Scholar] [CrossRef]
- Ferlazzo, A.M.; Bruschetta, G.; Di Pietro, P.; Medica, P. The Influence of Age on Plasma Serotonin Levels in Thoroughbred Horses. Livest. Sci. 2012, 147, 203–207. [Google Scholar] [CrossRef]
- Satué, K.; Hernández, Á.; Lorente, C.; Fazio, E.; Medica, P. Age- and Sex-Related Modifications of Hematology in Spanish Purebred Horse. J. Equine Vet. Sci. 2020, 93, 103219. [Google Scholar] [CrossRef] [PubMed]
- Dapper, D.V.B.; Didia, B.C. Haemorheological Changes during the Menstrual Cycle. East Afr. Med. J. 2002, 79, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Ashwini, S. Study of Immune Profile during Different Phases of Menstrual Cycle. Int. J. Biol. Med. Res. 2012, 3, 1407–1409. [Google Scholar]
- Nandi, S.; Rani, R. A Study of Total Leukocyte Count in Different Phases of Menstrual Cycle. Natl. J. Physiol. Pharm. Pharmacol. 2015, 5, 108. [Google Scholar] [CrossRef]
- Deepti, M.R.; Valivety, U. A Study of Variation of Total Leucocyte Count and Platelet Count with Different Phases of Mestrual Cycle. J. Evid. Based Med. Healthc. 2019, 6, 389–392. [Google Scholar] [CrossRef]
- Gunasekar, M.; Joseph, C.; Sridevi, P.; Thulasiraman, S.; Jayanthi, N. Evaluation of Hematology and Serum Biochemistry in Different Stages of Estrus Cycle and Pathological Conditions in Bitches. Multilogic Sci. 2018, 7, 93–96. [Google Scholar]
- Shah, C.; Bhatt, L.; Ravichandra, B.V.; Kothule, V.; Kadam, S.; Nataraju, G.J.; Patel, J.; Ranvir, R.; Bhatnagar, U.; Sundar, S.R.; et al. Influence of Estrous Stages on Electrocardiography, Clinical Pathology and Ovarian Weight of Experimental Beagle Dogs: A Retrospective Analysis. Interdiscip. Toxicol. 2019, 12, 149–156. [Google Scholar] [CrossRef]
- Satué, K.; Fazio, E.; Ferlazzo, A.; Medica, P. Intrafollicular and Systemic Serotonin, Oestradiol and Progesterone Concentrations in Cycling Mares. Reprod. Domest. Anim. Zuchthyg. 2019, 54, 1411–1418. [Google Scholar] [CrossRef]
- Duffy, D.M.; Ko, C.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels with Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef]
- Dai, M.; Xu, Y.; Gong, G.; Zhang, Y. Roles of Immune Microenvironment in the Female Reproductive Maintenance and Regulation: Novel Insights into the Crosstalk of Immune Cells. Front. Immunol. 2023, 14, 1109122. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Riber, C.; Trigo, P.; Castejón, F. Age- and Gender-Related Variations in Hematology, Clinical Biochemistry, and Hormones in Spanish Fillies and Colts. Res. Vet. Sci. 2012, 93, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Satué, K.; Fazio, E.; Velasco-Martínez, M.G.; Fauci, D.L.; Cravana, C.; Medica, P. Effect of Age on Amplitude of Circulating Catecholamine’s Change of Healthy Cyclic Mares. Vet. Res. Commun. 2024, 48, 2863–2868. [Google Scholar] [CrossRef]
- Bruschetta, G.; D’Ascola, A.; Medica, P.; Ferlazzo, A.M. Physical Exercise Affects Serotoninergic System in Horse Leukocytes. J. Equine Vet. Sci. 2020, 88, 102969. [Google Scholar] [CrossRef] [PubMed]
- Ayala, I.; Martos, N.F.; Silvan, G.; Gutierrez-Panizo, C.; Clavel, J.G.; Illera, J.C. Cortisol, Adrenocorticotropic Hormone, Serotonin, Adrenaline and Noradrenaline Serum Concentrations in Relation to Disease and Stress in the Horse. Res. Vet. Sci. 2012, 93, 103–107. [Google Scholar] [CrossRef]
- Torfs, S.C.; Maes, A.A.; Delesalle, C.J.; Deprez, P.; Croubels, S.M. Comparative Analysis of Serotonin in Equine Plasma with Liquid Chromatography–Tandem Mass Spectrometry and Enzyme-Linked Immunosorbent Assay. J. Vet. Diagn. Investig. 2012, 24, 1035–1042. [Google Scholar] [CrossRef]
- Roberto Da Costa, R.; Carvalho, H.; Agrícola, R.; Alpoim-Moreira, J.; Martins, C.; Ferreira-Dias, G. Peripheral Blood Neutrophil Function and Lymphocyte Subpopulations in Cycling Mares. Reprod. Domest. Anim. 2003, 38, 464–469. [Google Scholar] [CrossRef]
- Subandrio, A.L.; Sheldon, I.M.; Noakes, D.E. Peripheral and Intrauterine Neutrophil Function in the Cow: The Influence of Endogenous and Exogenous Sex Steroid Hormones. Theriogenology 2000, 53, 1591–1608. [Google Scholar] [CrossRef]
- Kenney, R.M. Cyclic and Pathologic Changes of the Mare Endometrium as Detected by Biopsy, with a Note on Early Embryonic Death. J. Am. Vet. Med. Assoc. 1978, 172, 241–262. [Google Scholar] [CrossRef]
- Kumar, R.S.; Goyal, N. Estrogens as Regulator of Hematopoietic Stem Cell, Immune Cells and Bone Biology. Life Sci. 2021, 269, 119091. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Penhale, W.J. The Influence of Testosterone on the Development of Autoimmune Thyroiditis in Thymectomized and Irradiated Rats. Clin. Exp. Immunol. 1982, 48, 367–374. [Google Scholar] [PubMed]
- Sugiura, K.; Nishikawa, M.; Ishiguro, K.; Tajima, T.; Inaba, M.; Torii, R.; Hatoya, S.; Wijewardana, V.; Kumagai, D.; Tamada, H.; et al. Effect of Ovarian Hormones on Periodical Changes in Immune Resistance Associated with Estrous Cycle in the Beagle Bitch. Immunobiology 2004, 209, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, M.; Aoyama, H.; Morishita, M.; Iwamoto, Y. Effects of Sex Hormones on Chemotaxis of Human Peripheral Polymorphonuclear Leukocytes and Monocytes. J. Periodontol. 1992, 63, 28–32. [Google Scholar] [CrossRef] [PubMed]
- DeNotta, S.; McFarlane, D. Immunosenescence and Inflammaging in the Aged Horse. Immun. Ageing A 2023, 20, 2. [Google Scholar] [CrossRef]
- Satué, K.; Hernández, A.; Lorente, C.; O’Connor, J.E. Immunophenotypical Characterization in Andalusian Horse: Variations with Age and Gender. Vet. Immunol. Immunopathol. 2010, 133, 219–227. [Google Scholar] [CrossRef]
- Butcher, S.; Chahel, H.; Lord, J.M. Review Article: Ageing and the Neutrophil: No Appetite for Killing? Immunology 2000, 100, 411–416. [Google Scholar] [CrossRef]
- Satue, K.; Blanco, O.; Munoz, A. AGE-Related Differences in the Hematological Profile of Andalusian Broodmares of Carthusian Strain. Veterinární Medicína 2009, 54, 175–182. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Jang, B.; Hur, S.; Jung, U.; Kil, K.; Na, B.; Lee, M.; Choi, Y.; Fukui, A.; et al. Fluctuation of Peripheral Blood T, B, and NK Cells during a Menstrual Cycle of Normal Healthy Women. J. Immunol. 2010, 185, 756–762. [Google Scholar] [CrossRef]
- Dosiou, C.; Hamilton, A.E.; Pang, Y.; Overgaard, M.T.; Tulac, S.; Dong, J.; Thomas, P.; Giudice, L.C. Expression of Membrane Progesterone Receptors on Human T Lymphocytes and Jurkat Cells and Activation of G-Proteins by Progesterone. J. Endocrinol. 2008, 196, 67–77. [Google Scholar] [CrossRef]
- Miyaura, H.; Iwata, M. Direct and Indirect Inhibition of Th1 Development by Progesterone and Glucocorticoids. J. Immunol. 2002, 168, 1087–1094. [Google Scholar] [CrossRef]
- Ehring, G.R.; Kerschbaum, H.H.; Eder, C.; Neben, A.L.; Fanger, C.M.; Khoury, R.M.; Negulescu, P.A.; Cahalan, M.D. A Nongenomic Mechanism for Progesterone-Mediated Immunosuppression: Inhibition of K+ Channels, Ca2+ Signaling, and Gene Expression in T Lymphocytes. J. Exp. Med. 1998, 188, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Borkowska, B.; Pawlowski, B. Leukocyte Changes across Menstruation, Ovulation, and Mid-Luteal Phase and Association with Sex Hormone Variation. Am. J. Hum. Biol. Off. J. Hum. Biol. Counc. 2016, 28, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Borisenkov, M.F.; Mongalev, N.P. Comparative analysis of functions of reproductive organs in cow and female reindeer. Blood cells content in vessels of reproductive organs. Zh. Evol. Biokhimol. Fiziol. 2006, 42, 253–256. [Google Scholar]
- Hellberg, P.; Thomsen, P.; Janson, P.O.; Brännström, M. Leukocyte Supplementation Increases the Luteinizing Hormone-Induced Ovulation Rate in the in Vitro-Perfused Rat Ovary. Biol. Reprod. 1991, 44, 791–797. [Google Scholar] [CrossRef]
- Mongalev, N.P.; Borisenkov, M.F. Functional Significance of Leukocytosis in the Estrous Cycle of Cows. Aktual. Vopr. Vet. Biol. Probl. Vet. Biol. 2016, 4, 3–8. [Google Scholar]
- Mongalev, N.P.; Rubtsova, L.Y. The Role of the Leukocyte Pool in the Formation of Estral Cycles in Cows; Medicine-Health: Kishinev, Moldova, 2008; p. 283. [Google Scholar]
- Shirasuna, K.; Nitta, A.; Sineenard, J.; Shimizu, T.; Bollwein, H.; Miyamoto, A. Vascular and Immune Regulation of Corpus Luteum Development, Maintenance, and Regression in the Cow. Domest. Anim. Endocrinol. 2012, 43, 198–211. [Google Scholar] [CrossRef]
- Walusimbi, S.S.; Pate, J.L. Physiology and Endocrinology Symposium: Role of Immune Cells in the Corpus Luteum. J. Anim. Sci. 2013, 91, 1650–1659. [Google Scholar] [CrossRef]
- Fajardo, O.; Galeno, J.; Urbina, M.; Carreira, I.; Lima, L. Serotonin, Serotonin 5-HT(1A) Receptors and Dopamine in Blood Peripheral Lymphocytes of Major Depression Patients. Int. Immunopharmacol. 2003, 3, 1345–1352. [Google Scholar] [CrossRef]
- Sempere, T.; Urbina, M.; Lima, L. 5-HT1A and Beta-Adrenergic Receptors Regulate Proliferation of Rat Blood Lymphocytes. Neuroimmunomodulation 2004, 11, 307–315. [Google Scholar] [CrossRef]
- Schoenichen, C.; Bode, C.; Duerschmied, D. Role of Platelet Serotonin in Innate Immune Cell Recruitment. Front. Biosci. Landmark Ed. 2019, 24, 514–526. [Google Scholar] [CrossRef]
- Duerschmied, D.; Suidan, G.L.; Demers, M.; Herr, N.; Carbo, C.; Brill, A.; Cifuni, S.M.; Mauler, M.; Cicko, S.; Bader, M.; et al. Platelet Serotonin Promotes the Recruitment of Neutrophils to Sites of Acute Inflammation in Mice. Blood 2013, 121, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.G.; Jiang, X.; Stephensen, C.B. Monocyte Subsets Display Age-Dependent Alterations at Fasting and Undergo Non-Age-Dependent Changes Following Consumption of a Meal. Immun. Ageing 2022, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Northern, A.L.; Rutter, S.M.; Peterson, C.M. Cyclic Changes in the Concentrations of Peripheral Blood Immune Cells during the Normal Menstrual Cycle. Proc. Soc. Exp. Biol. Med. 1994, 207, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Soga, F.; Katoh, N.; Inoue, T.; Kishimoto, S. Serotonin Activates Human Monocytes and Prevents Apoptosis. J. Investig. Dermatol. 2007, 127, 1947–1955. [Google Scholar] [CrossRef]
- Willson, C.J.; Chandra, S.A.; Kimbrough, C.L.; Jordan, H.L. Effect of Estrous Cycle Phase on Clinical Pathology Values in Beagle Dogs. Vet. Clin. Pathol. 2012, 41, 71–76. [Google Scholar] [CrossRef]
- Maclean, J.A.; Schoenwaelder, S.M. Serotonin in Platelets. In Serotonin; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–119. ISBN 978-0-12-800050-2. [Google Scholar]
- Paz, L.B.; Beck Júnior, A.A.; Engelmann, A.M.; Mucha, J.V.G.; Frank, M.I.; Pereira, R.C.F.; Krause, A.; Côrte, F.D.D.L. Effects of Breed, Age and Gender on Equine Platelet Rich Plasma and Correlation of Platelet Count with Its Physical Aspect. Arq. Bras. Med. Veterinária Zootec. 2022, 74, 759–766. [Google Scholar] [CrossRef]
- Rossi, D.J.; Bryder, D.; Seita, J.; Nussenzweig, A.; Hoeijmakers, J.; Weissman, I.L. Deficiencies in DNA Damage Repair Limit the Function of Haematopoietic Stem Cells with Age. Nature 2007, 447, 725–729. [Google Scholar] [CrossRef]
- Rundberg Nilsson, A.; Soneji, S.; Adolfsson, S.; Bryder, D.; Pronk, C.J. Human and Murine Hematopoietic Stem Cell Aging Is Associated with Functional Impairments and Intrinsic Megakaryocytic/Erythroid Bias. PLoS ONE 2016, 11, e0158369. [Google Scholar] [CrossRef]
- Chmurska-Gąsowska, M.; Bojarski, B.; Sowińska, N.; Strus, M. Changes in Leukogram and Erythrogram Results in Bitches with Vaginitis. Animal 2021, 11, 1403. [Google Scholar] [CrossRef]
- Pai, V.P.; Hernandez, L.L.; Stull, M.A.; Horseman, N.D. The Type 7 Serotonin Receptor, 5-HT7, Is Essential in the Mammary Gland for Regulation of Mammary Epithelial Structure and Function. BioMed Res. Int. 2015, 2015, 364746. [Google Scholar] [CrossRef]
- Carnevale, E.M. The Mare Model for Follicular Maturation and Reproductive Aging in the Woman. Theriogenology 2008, 69, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J.; Gastal, E.L.; Gastal, M.O.; Bergfelt, D.R.; Baerwald, A.R.; Pierson, R.A. Comparative Study of the Dynamics of Follicular Waves in Mares and Women1. Biol. Reprod. 2004, 71, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
| WBCs (G/L) | LYMPHs (G/L) | NFSs (G/L) | N/L Ratio | EOSs (G/L) | MONs (G/L) | PLTs (G/L) | |
|---|---|---|---|---|---|---|---|
| 5-HT (ng/mL) | 0.20 | −0.13 | 0.47 | 0.34 | −0.06 | 0.15 | 0.15 |
| WBCs (G/L) | 0.75 | 0.61 | −0.24 | 0.33 | 0.30 | 0.26 | |
| LYMPHs (G/L) | −0.05 | −0.77 | 0.41 | 0.24 | 0.28 | ||
| NFSs (G/L) | 0.56 | 0.01 | 0.16 | 0.07 | |||
| N/L ratio | −0.17 | −0.10 | −0.23 | ||||
| EOSs (G/L) | −0.01 | −0.12 | |||||
| MONs (G/L) | −0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satué, K.; La Fauci, D.; Medica, P.; Velasco-Martínez, M.G.; Barbiera, G.; Fazio, E. Involvement of Peripheral Serotonin in Blood Cells in Healthy Cyclical Mares of Different Ages. Vet. Sci. 2025, 12, 548. https://doi.org/10.3390/vetsci12060548
Satué K, La Fauci D, Medica P, Velasco-Martínez MG, Barbiera G, Fazio E. Involvement of Peripheral Serotonin in Blood Cells in Healthy Cyclical Mares of Different Ages. Veterinary Sciences. 2025; 12(6):548. https://doi.org/10.3390/vetsci12060548
Chicago/Turabian StyleSatué, Katiuska, Deborah La Fauci, Pietro Medica, María Gemma Velasco-Martínez, Giuliana Barbiera, and Esterina Fazio. 2025. "Involvement of Peripheral Serotonin in Blood Cells in Healthy Cyclical Mares of Different Ages" Veterinary Sciences 12, no. 6: 548. https://doi.org/10.3390/vetsci12060548
APA StyleSatué, K., La Fauci, D., Medica, P., Velasco-Martínez, M. G., Barbiera, G., & Fazio, E. (2025). Involvement of Peripheral Serotonin in Blood Cells in Healthy Cyclical Mares of Different Ages. Veterinary Sciences, 12(6), 548. https://doi.org/10.3390/vetsci12060548





