Fecal Microbiota Changes in Angus Beef Cows Persistently Infected by Bovine Viral Diarrhea Virus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Epidemiological Survey and Diagnosis of BVDV-Persistent Infection
2.1.1. Epidemiological Survey
2.1.2. PI Cow Screening
2.2. Experimental Groups and Sampling
2.2.1. Grouping
2.2.2. Sampling
2.3. Gut Microbiota Analysis
2.3.1. DNA Extraction
2.3.2. 16S rRNA Gene Amplicon Sequencing
2.3.3. Bioinformatics
2.4. Statistical Analysis
3. Results
3.1. Epidemiological Survey and PI Cow Identification
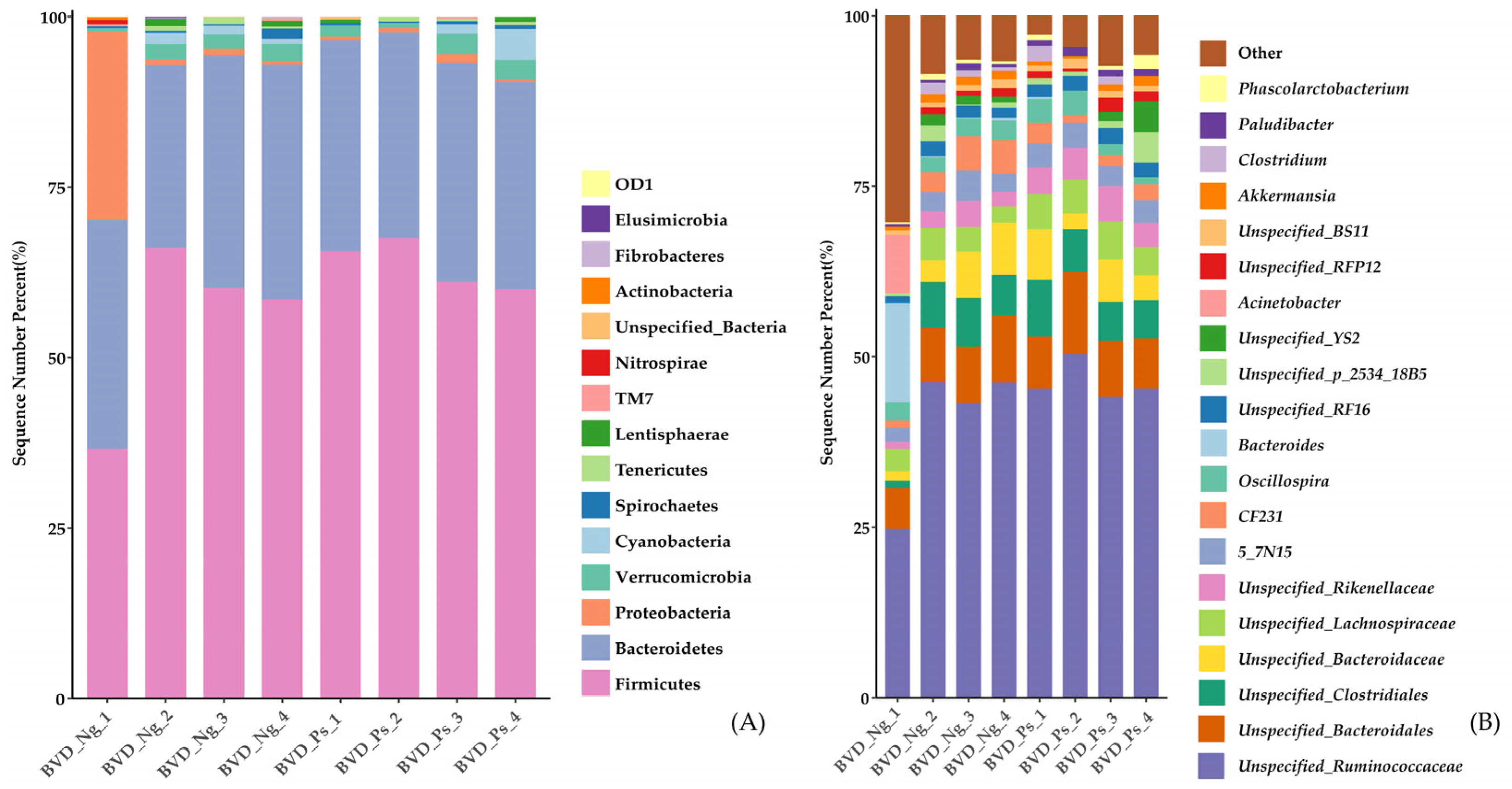
3.2. Gut Microbiota Composition
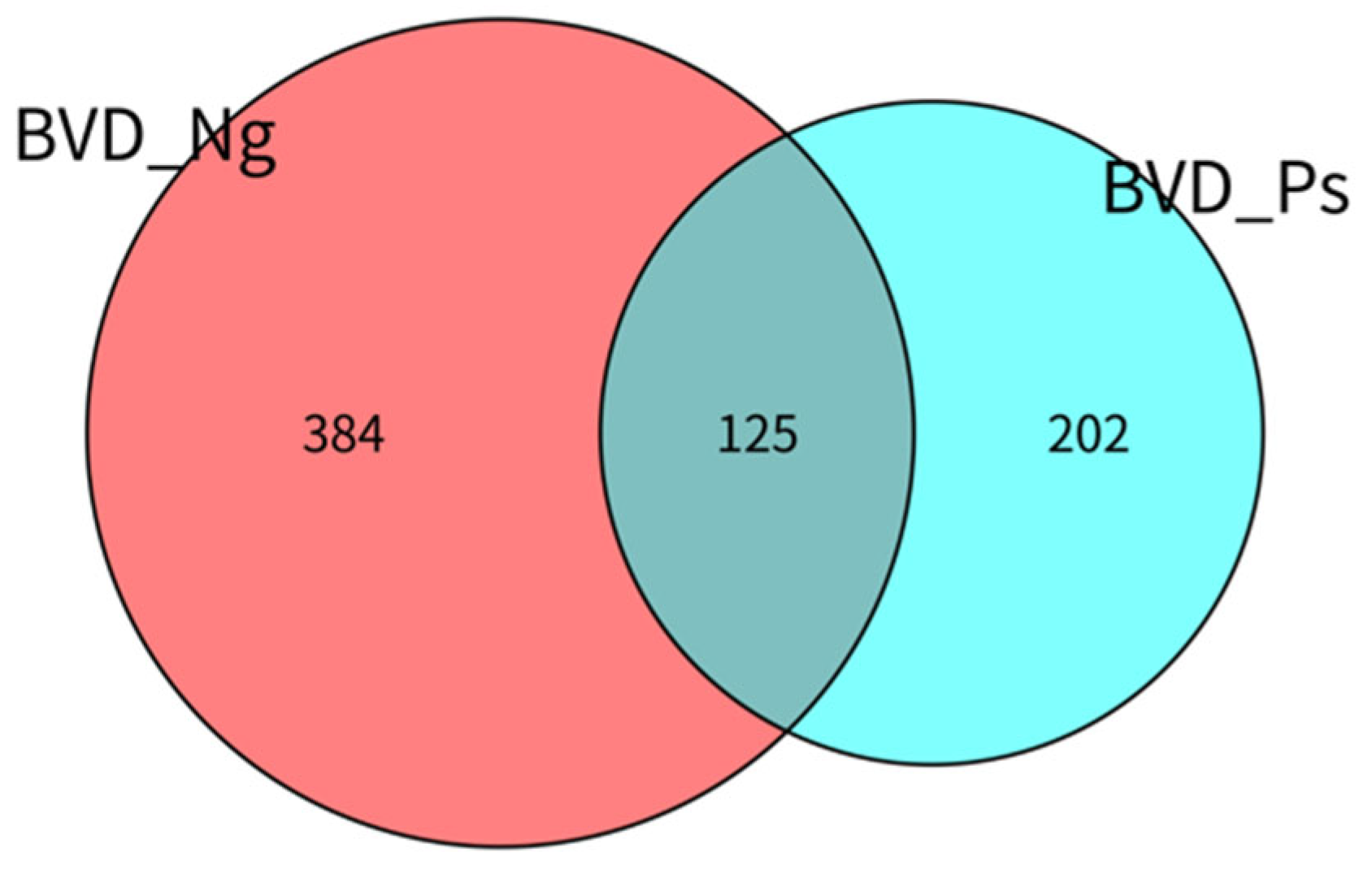
3.2.1. OTU Distribution
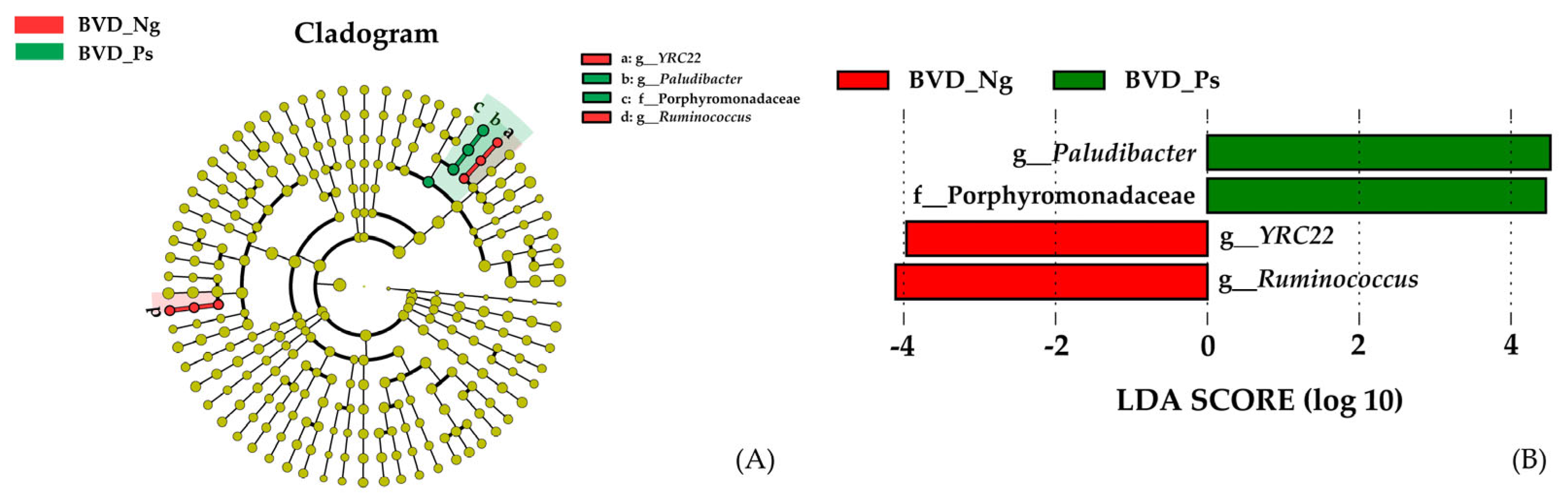
3.2.2. Taxonomic Differences
3.3. Gut Microbiota Structural Differences
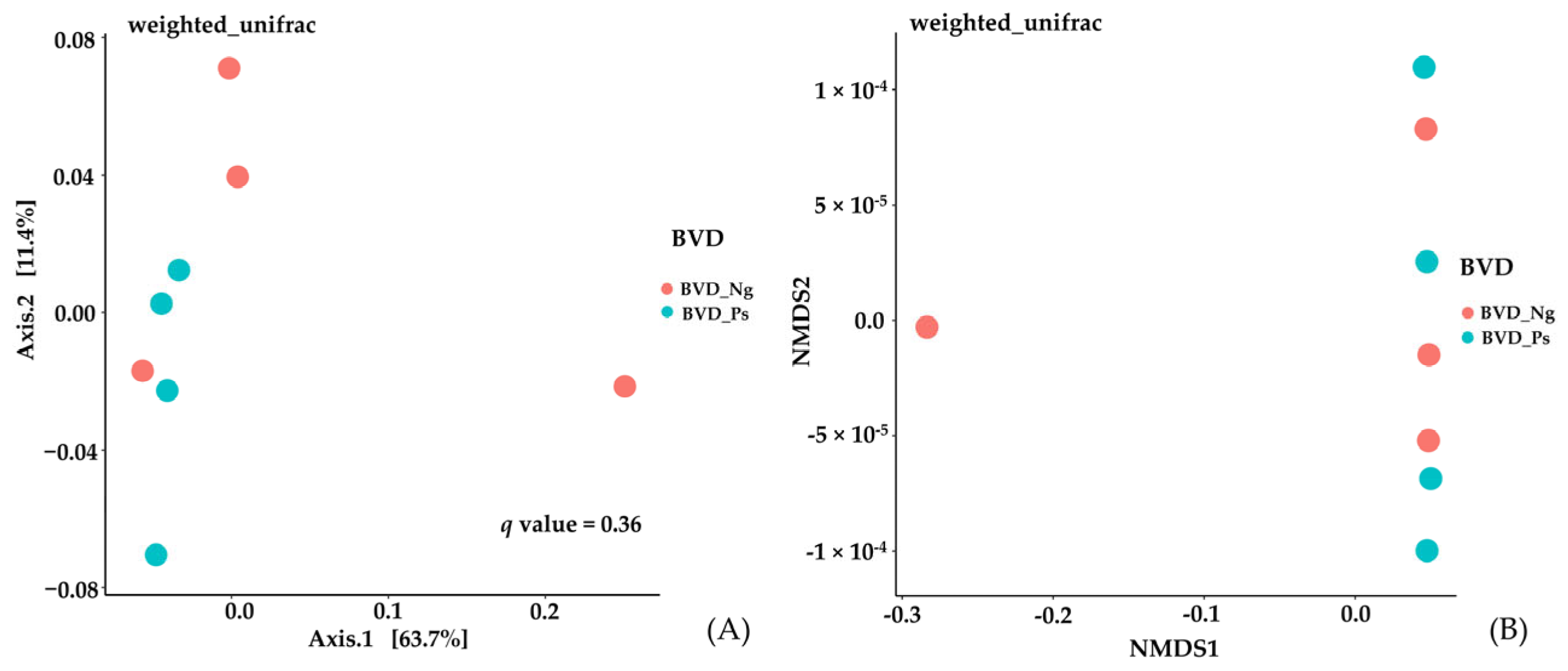
3.3.1. Alpha Diversity
3.3.2. Beta Diversity
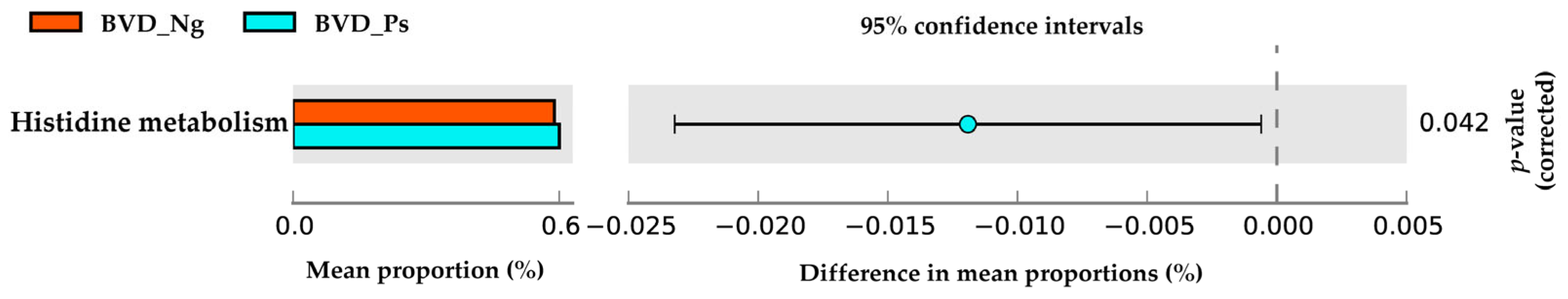
3.4. Metabolic Pathway Differences
4. Discussion
4.1. Epidemiological Survey and PI Cow Screening
4.2. Gut Microbiota Composition in PI Cows
4.3. Microbial Diversity Alterations
4.4. Metabolic Pathway Implications
4.5. Therapeutic Potential of Microbiota Modulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carman, S.; van Dreumel, T.; Ridpath, J.; Hazlett, M.; Alves, D.; Dubovi, E.; Tremblay, R.; Bolin, S.; Godkin, A.; Anderson, N. Severe acute bovine viral diarrhea in Ontario, 1993–1995. J. Vet. Diagn. Invest. 1998, 10, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liu, W.; Feng, H.; Huang, J.; Wang, Q.; Zhang, Q.; He, J.; Wang, R. Bifidobacterium animalis subsp. lactis A6 attenuates hippocampal damage and memory impairments in an ADHD rat model. Food Funct. 2024, 15, 2668–2678. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, B.; Zhang, Y.; Fan, W.; Xue, Q.; Chen, X.; Wang, J.; Qi, X. Mitochondria-mediated ferroptosis contributes to the inflammatory responses of bovine viral diarrhea virus (BVDV) in vitro. J. Virol. 2024, 98, e0188023. [Google Scholar] [CrossRef]
- McClurkin, A.W.; Littledike, E.T.; Cutlip, R.C.; Frank, G.H.; Coria, M.F.; Bolin, S.R. Production of cattle immunotolerant to bovine viral diarrhea virus. Can. J. Comp. Med. 1984, 48, 156–161. [Google Scholar]
- Newcomer, B.W.; Chamorro, M.F.; Walz, P.H. Vaccination of cattle against bovine viral diarrhea virus. Vet. Microbiol. 2017, 206, 78–83. [Google Scholar] [CrossRef]
- Lin, F.Y.; Tzeng, H.Y.; Tseng, C.Y.; Tsai, R.S.; Oba, M.; Mizutani, T.; Yamada, Y.; Chiou, H.Y.; Chuang, S.T.; Hsu, W.L. Surveillance and genetic diversity of bovine viral diarrhea virus in dairy herds across Taiwan. Vet. J. 2025, 310, 106305. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C. Employing pigs to decipher the host genetic effect on gut microbiome: Advantages, challenges, and perspectives. Gut Microbes 2023, 15, 2205410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, B.; Lu, S.; Wang, S.; Ren, X.; Liu, R.; Dong, H.; Li, K.; Fouad, D.; Ataya, F.S.; et al. Metagenomic analysis for exploring the potential of Lactobacillus yoelii FYL1 to mitigate bacterial diarrhea and changes in the gut microbiota of juvenile yaks. Microb. Pathog. 2024, 186, 106496. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, M.; Huang, Y.; Feng, F.; Luo, X. Novel Lauric Acid-Butyric Structural Lipid Inhibits Inflammation: Small Intestinal Microbes May Be Important Mediators. Mol. Nutr. Food Res. 2024, 68, e2300535. [Google Scholar] [CrossRef]
- Uchiyama, J.; Murakami, H.; Sato, R.; Mizukami, K.; Suzuki, T.; Shima, A.; Ishihara, G.; Sogawa, K.; Sakaguchi, M. Examination of the fecal microbiota in dairy cows infected with bovine leukemia virus. Vet. Microbiol. 2020, 240, 108547. [Google Scholar] [CrossRef]
- Correa, R.; de Oliveira Santos, I.; Braz-de-Melo, H.A.; de Sant’Ana, L.P.; das Neves Almeida, R.; Pasquarelli-do-Nascimento, G.; Prado, P.S.; Kobinger, G.P.; Maurice, C.F.; Magalhaes, K.G. Gut microbiota modulation induced by Zika virus infection in immunocompetent mice. Sci. Rep. 2021, 11, 1421. [Google Scholar] [CrossRef] [PubMed]
- Whon, T.W.; Kim, H.S.; Shin, N.R.; Sung, H.; Kim, M.S.; Kim, J.Y.; Kang, W.; Kim, P.S.; Hyun, D.W.; Seong, H.J.; et al. Calf Diarrhea Caused by Prolonged Expansion of Autochthonous Gut Enterobacteriaceae and Their Lytic Bacteriophages. Msystems 2021, 6, e00816-20. [Google Scholar] [CrossRef]
- Fan, P.; Kim, M.; Liu, G.; Zhai, Y.; Liu, T.; Driver, J.D.; Jeong, K.C. The Gut Microbiota of Newborn Calves and Influence of Potential Probiotics on Reducing Diarrheic Disease by Inhibition of Pathogen Colonization. Front. Microbiol. 2021, 12, 772863. [Google Scholar] [CrossRef]
- Yuan, N.; Song, Q.; Jin, Y.; Zhang, Z.; Wu, Z.; Sheng, X.; Qi, X.; Xing, K.; Xiao, L.; Wang, X. Replication of standard bovine viral diarrhea strain OregonC24Va induces endoplasmic reticulum stress-mediated apoptosis of bovine trophoblast cells. Cell Stress. Chaperones 2023, 28, 49–60. [Google Scholar] [CrossRef]
- Zhou, B.; Yuan, Y.; Zhang, S.; Guo, C.; Li, X.; Li, G.; Xiong, W.; Zeng, Z. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front. Immunol. 2020, 11, 575. [Google Scholar] [CrossRef]
- Li, S.; Hu, X.; Tian, R.; Guo, Y.; Chen, J.; Li, Z.; Zhao, X.; Kuang, L.; Ran, D.; Zhao, H.; et al. RNA-Seq-based transcriptomic profiling of primary interstitial cells of Cajal in response to bovine viral diarrhea virus infection. Vet. Res. Commun. 2019, 43, 143–153. [Google Scholar] [CrossRef]
- Mysara, M.; Njima, M.; Leys, N.; Raes, J.; Monsieurs, P. From reads to operational taxonomic units: An ensemble processing pipeline for MiSeq amplicon sequencing data. Gigascience 2017, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Fritzen, J.T.T.; Zucoloto, N.Z.; Lorenzetti, E.; Alfieri, A.F.; Alfieri, A.A. Outbreak of persistently infected heifer calves with bovine viral diarrhea virus subgenotypes 1b and 1d in a BVDV-vaccinated open dairy herd. Acta Trop. 2024, 254, 107198. [Google Scholar] [CrossRef]
- Qi, W.; Yonggang, Q.; Junshuai, C. Molecular epidemiological investigation of bovine viral diarrhea in some areas of Xinjiang. Anim. Husb. Vet. Med. 2020, 52, 105–109. [Google Scholar]
- Yang, G.; Wang, J.; Wang, S.; Zhu, Y. Forsythiaside A Improves the Inhibitory Efficiency of Recombinant Protein Vaccines against Bovine Viral Diarrhea Virus Infection. Int. J. Mol. Sci. 2022, 23, 9390. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Li, C.; Zhao, Z.; Cui, Y.; Yuan, X.; Wang, X.; Liu, Y.; Zhou, Y.; Zhu, Z. The gut microbiota contributes to the infection of bovine viral diarrhea virus in mice. J. Virol. 2024, 98, e0203523. [Google Scholar] [CrossRef]
- Chen, S.Y.; Deng, F.; Jia, X.; Liu, H.; Zhang, G.W.; Lai, S.J. Gut microbiota profiling with differential tolerance against the reduced dietary fibre level in rabbit. Sci. Rep. 2019, 9, 288. [Google Scholar] [CrossRef]
- Fang, S.; Chen, X.; Ye, X.; Zhou, L.; Xue, S.; Gan, Q. Effects of Gut Microbiome and Short-Chain Fatty Acids (SCFAs) on Finishing Weight of Meat Rabbits. Front. Microbiol. 2020, 11, 1835. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Kommadath, A.; Fouhse, J.; Wilkinson, J.; Diether, N.; Stothard, P.; Estelle, J.; Rogel-Gaillard, C.; Plastow, G.; Willing, B.P. Characterization of whole blood transcriptome and early-life fecal microbiota in high and low responder pigs before, and after vaccination for Mycoplasma hyopneumoniae. Vaccine 2019, 37, 1743–1755. [Google Scholar] [CrossRef]
- Fouhse, J.M.; Dawson, K.; Graugnard, D.; Dyck, M.; Willing, B.P. Dietary supplementation of weaned piglets with a yeast-derived mannan-rich fraction modulates cecal microbial profiles, jejunal morphology and gene expression. Animal 2019, 13, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Si, H.; Du, H.; Guo, H.; Dai, H.; Xu, S.; Wan, J. Comparison of gut microbiota structure and Actinobacteria abundances in healthy young adults and elderly subjects: A pilot study. BMC Microbiol. 2021, 21, 13. [Google Scholar] [CrossRef]
- Andersen, D.; Roager, H.M.; Zhang, L.; Moll, J.M.; Frandsen, H.L.; Danneskiold-Samsoe, N.B.; Hansen, A.K.; Kristiansen, K.; Licht, T.R.; Brix, S. Systems-wide effects of short-term feed deprivation in obese mice. Sci. Rep. 2021, 11, 5716. [Google Scholar] [CrossRef]
- Ye, Z.; Kini, A.; Tan, Q.; Woltemate, S.; Vital, M.; Nikolovska, K.; Seidler, U. Oral tributyrin treatment affects short-chain fatty acid transport, mucosal health, and microbiome in a mouse model of inflammatory diarrhea. J. Nutr. Biochem. 2025, 138, 109847. [Google Scholar] [CrossRef]
- Yang, W.; Cui, M.; Yang, P.; Liu, C.; Han, X.; Yao, W.; Li, Z. Gut microbiota and blood biomarkers in IBD-Related arthritis: Insights from mendelian randomization. Sci. Rep. 2025, 15, 514. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, X.; Xu, D.; Zhang, D.; Zhang, Y.; Song, Q.; Li, X.; Zhao, Y.; Zhao, L.; Li, W.; et al. Relationship between rumen microbial differences and traits among Hu sheep, Tan sheep, and Dorper sheep. J. Anim. Sci. 2022, 100, skac261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Li, M.; Luo, J.; Wang, S.; Liu, S.; Wang, S.; Lyu, W.; Chen, L.; Su, W.; Ding, H.; et al. Impacts of canine distemper virus infection on the giant panda population from the perspective of gut microbiota. Sci. Rep. 2017, 7, 39954. [Google Scholar] [CrossRef]
- Davis, T.; Bialy, D.; Leng, J.; La Ragione, R.; Shelton, H.; Chrzastek, K. Alteration of the Chicken Upper Respiratory Microbiota, Following H9N2 Avian Influenza Virus Infection. Pathogens 2023, 12, 1168. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, A.; Wu, F.; Wang, X. UPLC-G2Si-HDMS Untargeted Metabolomics for Identification of Yunnan Baiyao’s Metabolic Target in Promoting Blood Circulation and Removing Blood Stasis. Molecules 2022, 27, 3208. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hong, Q.; Zhang, X.; Liu, Z. Bifidobacterium animalis BD400 protects from collagen-induced arthritis through histidine metabolism. Front. Immunol. 2025, 16, 1518181. [Google Scholar] [CrossRef]
- Taxis, T.M.; Bauermann, F.V.; Ridpath, J.F.; Casas, E. Circulating MicroRNAs in Serum from Cattle Challenged with Bovine Viral Diarrhea Virus. Front. Genet. 2017, 8, 91. [Google Scholar] [CrossRef]
- Behary, J.; Amorim, N.; Jiang, X.T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef]
- Wirusanti, N.I.; Baldridge, M.T.; Harris, V.C. Microbiota regulation of viral infections through interferon signaling. Trends Microbiol. 2022, 30, 778–792. [Google Scholar] [CrossRef]
- Smillie, C.S.; Sauk, J.; Gevers, D.; Friedman, J.; Sung, J.; Youngster, I.; Hohmann, E.L.; Staley, C.; Khoruts, A.; Sadowsky, M.J.; et al. Strain Tracking Reveals the Determinants of Bacterial Engraftment in the Human Gut Following Fecal Microbiota Transplantation. Cell Host Microbe 2018, 23, 229–240.e5. [Google Scholar] [CrossRef]
- van Lingen, E.E.; Baunwall, S.; Lieberknecht, S.S.C.; Benech, N.N.; Ianiro, G.G.; Sokol, H.H.; Gasbarrini, A.A.; Cammarota, G.G.; Eriksen, M.M.K.; van der Meulen-de Jong, A.A.E.; et al. Short- and long-term follow-up after fecal microbiota transplantation as treatment for recurrent Clostridioides difficile infection in patients with inflammatory bowel disease. Therap Adv. Gastroenterol. 2023, 16, 17562848231156285. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Cheong, H.S.; Kim, B.; Sohn, W.; Cho, Y.K.; Kwon, M.J.; Kim, J.; Song, Y.; Joo, E.J. Human gut microbiota from hepatitis B virus-infected individuals is associated with reduced triglyceride level in mice: Faecal transplantation study. Microbes Infect. 2024, 26, 105281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rodriguez, F.; Navas, M.J.; Costa-Hurtado, M.; Almagro, V.; Bosch-Camos, L.; Lopez, E.; Cuadrado, R.; Accensi, F.; Pina-Pedrero, S.; et al. Fecal microbiota transplantation from warthog to pig confirms the influence of the gut microbiota on African swine fever susceptibility. Sci. Rep. 2020, 10, 17605. [Google Scholar] [CrossRef] [PubMed]
- Yitbarek, A.; Taha-Abdelaziz, K.; Hodgins, D.C.; Read, L.; Nagy, E.; Weese, J.S.; Caswell, J.L.; Parkinson, J.; Sharif, S. Gut microbiota-mediated protection against influenza virus subtype H9N2 in chickens is associated with modulation of the innate responses. Sci. Rep. 2018, 8, 13189. [Google Scholar] [CrossRef]

| Tested Samples | Positive Samples | Positive Rate (%) | Retested Samples | Positive in Retest | Positive Rate (%) |
|---|---|---|---|---|---|
| 4310 | 19 | 0.44 | 15 | 10 | 0.23 |
| Index of α-Diversity | BVD-Ng | BVD-Ps | p Value |
|---|---|---|---|
| Chao1 | 158.750 ± 21.554 | 102.750 ± 5.072 | 0.114 |
| Shannon | 6.601 ± 0.268 | 5.945 ± 0.112 | 0.200 |
| Faith’s _pd | 13.471 ± 0.722 | 10.240 ± 0.193 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, R.; Chen, Y.; Yi, P.; Sun, Y.; Chen, L.; Ma, X.; Zhong, Q.; Li, N.; Yao, G. Fecal Microbiota Changes in Angus Beef Cows Persistently Infected by Bovine Viral Diarrhea Virus. Vet. Sci. 2025, 12, 538. https://doi.org/10.3390/vetsci12060538
Xia R, Chen Y, Yi P, Sun Y, Chen L, Ma X, Zhong Q, Li N, Yao G. Fecal Microbiota Changes in Angus Beef Cows Persistently Infected by Bovine Viral Diarrhea Virus. Veterinary Sciences. 2025; 12(6):538. https://doi.org/10.3390/vetsci12060538
Chicago/Turabian StyleXia, Ruiyang, Yalu Chen, Pengfei Yi, Yawei Sun, Lijing Chen, Xuelian Ma, Qi Zhong, Na Li, and Gang Yao. 2025. "Fecal Microbiota Changes in Angus Beef Cows Persistently Infected by Bovine Viral Diarrhea Virus" Veterinary Sciences 12, no. 6: 538. https://doi.org/10.3390/vetsci12060538
APA StyleXia, R., Chen, Y., Yi, P., Sun, Y., Chen, L., Ma, X., Zhong, Q., Li, N., & Yao, G. (2025). Fecal Microbiota Changes in Angus Beef Cows Persistently Infected by Bovine Viral Diarrhea Virus. Veterinary Sciences, 12(6), 538. https://doi.org/10.3390/vetsci12060538






