Identification of Non-Tuberculous Mycobacteria in Iberian Lynx (Lynx pardinus) and Their Impact on Its Health
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Real-Time PCR
2.2. Mycobacteria Culture
2.3. Mycobacterial Isolation and DNA Extraction
2.4. Mycobacterium spp. Identification by Convencional PCR
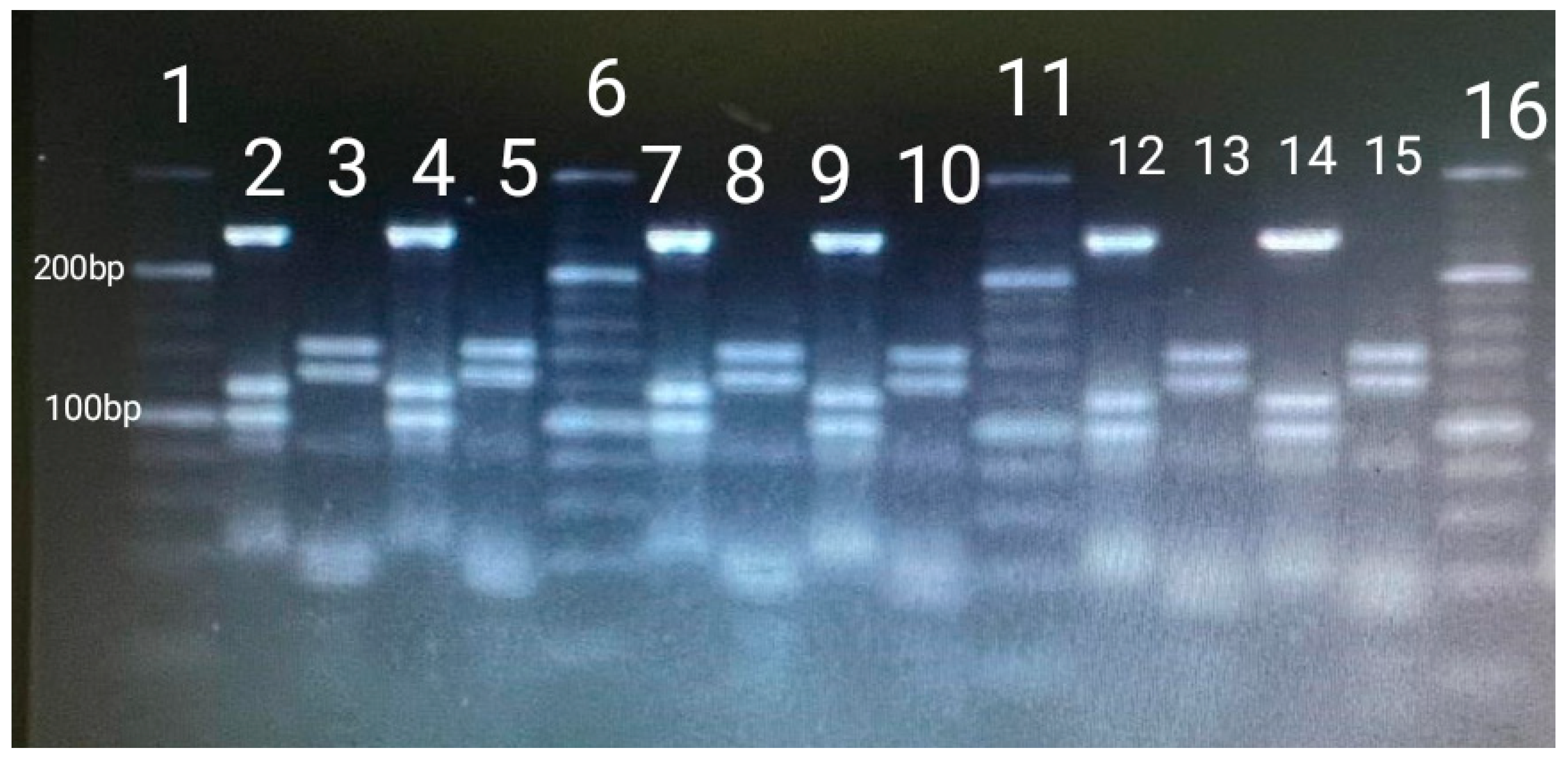
2.5. NTM Identification by PCR and Restriction Enzyme Analysis and Interpretation (PRA-hsp65)
2.6. Blood Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NTM | Non-tuberculous mycobacteria |
| TB | Tuberculosis |
| MAC | M. avium complex |
| HSP65 | 65-kDa heat shock protein gene |
| CDV | Canine distemper virus |
| SuHV-1 | Aujeszky’s disease |
| FeLV | Feline leukemia virus |
| RBC | Red blood cell |
| MCV | Mean corpuscular volume |
| MCHC | Mean corpuscular hemoglobin concentration |
| WBC | Differential leukocyte count |
| ALT/GPT | Alanine aminotransferase |
| AST/GOT | Aspartate aminotransferase |
References
- Parte, A.C.; Carbasse, J.S.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Varela-Castro, L.; Barral, M.; Arnal, M.C.; de Luco, D.F.; Gortázar, C.; Garrido, J.M.; Sevilla, I.A. Beyond tuberculosis: Diversity and implications of non-tuberculous mycobacteria at the wildlife–livestock interface. Transbound. Emerg. Dis. 2022, 69, e2978–e2993. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.W.; Collins, M.T.; Koets, A.P.; Mcguirk, S.M.; Roussel, A.J. Paratuberculosis (Johne’s Disease) in Cattle and Other Susceptible Species. J. Vet. Intern. Med. 2012, 26, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Nishiuchi, Y.; Iwamoto, T.; Maruyama, F. Infection sources of a common non-tuberculous mycobacterial pathogen, Mycobacterium avium complex. Front. Med. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed Central]
- Jagielski, T.; Minias, A.; van Ingen, J.; Rastogi, N.; Brzostek, A.; Żaczek, A.; Dziadek, J. Methodological and clinical aspects of the molecular epidemiology of Mycobacterium tuberculosis and other mycobacteria. Clin. Microbiol. Rev. 2016, 29, 239–290. [Google Scholar] [CrossRef] [PubMed Central]
- Forbes, B.A.; Hall, G.S.; Miller, M.B.; Novak, S.M.; Rowlinson, M.-C.; Salfinger, M.; Somoskövi, A.; Warshauer, D.M.; Wilson, M.L. Practice guidelines for clinical microbiology laboratories: Mycobacteria. Clin. Microbiol. Rev. 2018, 31, 10-1128. [Google Scholar] [CrossRef] [PubMed Central]
- Bo, M.; Arru, G.; Niegowska, M.; Erre, G.L.; Manchia, P.A.; Sechi, L.A. Association between Lipoprotein Levels and Humoral Reactivity to Mycobacterium avium subsp. paratuberculosis in Multiple Sclerosis, Type 1 Diabetes Mellitus and Rheumatoid Arthritis. Microorganisms 2019, 7, 423. [Google Scholar] [CrossRef] [PubMed Central]
- Biet, F.; Boschiroli, M.L. Non-tuberculous mycobacterial infections of veterinary relevance. Res. Vet. Sci. 2014, 97, S69–S77. [Google Scholar] [CrossRef]
- Tato Jiménez, Á. Infecciones Por Mycobacterium spp. en Animales Salvajes en la Provincia de Cáceres. Ph.D. Thesis, Universidad de Extremadura, Cáceres, Spain, 1999. [Google Scholar]
- Balseiro, A.; Merediz, I.; Sevilla, I.A.; García-Castro, C.; Gortázar, C.; Prieto, J.M.; Delahay, R.J. Infection of Eurasian badgers (Meles meles) with Mycobacterium avium complex (MAC) bacteria. Vet. J. 2011, 188, 231–233. [Google Scholar] [CrossRef]
- Muñoz-Mendoza, M.; Marreros, N.; Boadella, M.; Gortázar, C.; Menéndez, S.; de Juan, L.; Bezos, J.; Romero, B.; Copano, M.F.; Amado, J.; et al. Wild boar tuberculosis in Iberian Atlantic Spain: A different picture from Mediterranean habitats. BMC Vet. Res. 2013, 9, 176. [Google Scholar] [CrossRef] [PubMed Central]
- García-Jiménez, W.L.; Benítez-Medina, J.M.; Martínez, R.; Carranza, J.; Cerrato, R.; García-Sánchez, A.; Risco, D.; Moreno, J.C.; Sequeda, M.; Gómez, L.; et al. Non-tuberculous Mycobacteria in Wild Boar (Sus scrofa) from Southern Spain: Epidemiological, Clinical and Diagnostic Concerns. Transbound. Emerg. Dis. 2015, 62, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Gortazar, C.; Torres, M.J.; Acevedo, P.; Aznar, J.; Negro, J.J.; de la Fuente, J.; Vicente, J. Fine-tuning the space, time, and host distribution of mycobacteria in wildlife. BMC Microbiol. 2011, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Censo del Lince 2021. Ministerio para la Transición Ecológica y el Reto Demográfico. Available online: https://www.miteco.gob.es/content/dam/miteco/es/biodiversidad/temas/inventarios-nacionales/lince/censodelinceiberico2021_tcm30-541552.pdf (accessed on 19 May 2025).
- Censo del Lince 2022. Ministerio para la Transición Ecológica y el Reto Demográfico. Available online: https://www.miteco.gob.es/content/dam/miteco/es/biodiversidad/temas/inventarios-nacionales/lince/informecensodelinceiberico2022_tcm30-569643.pdf (accessed on 19 May 2025).
- Nájera, F.; Grande-Gómez, R.; Peña, J.; Vázquez, A.; Palacios, M.J.; Rueda, C.; Corona-Bravo, A.I.; Zorrilla, I.; Revuelta, L.; Gil-Molino, M.; et al. Disease Surveillance during the Reintroduction of the Iberian Lynx (Lynx pardinus) in Southwestern Spain. Animals 2021, 11, 547. [Google Scholar] [CrossRef] [PubMed Central]
- Luaces, I.; Doménech, A.; García-Montijano, M.; Collado, V.M.; Sónchez, C.; Tejerizo, J.G.; Galka, M.; Fernóndez, P.; Gómez-Lucía, E. Detection of Feline leukemia virus in the endangered Iberian lynx (Lynx pardinus). J. Vet. Diagn. Investig. 2008, 20, 381–385. [Google Scholar] [CrossRef]
- Gortázar, C.; Torres, M.J.; Vicente, J.; Acevedo, P.; Reglero, M.; de la Fuente, J.; Negro, J.J.; Aznar Martín, J. Bovine Tuberculosis in Doñana Biosphere Reserve: The Role of Wild Ungulates as Disease Reservoirs in the Last Iberian Lynx Strongholds. PLoS ONE 2008, 3, e2776. [Google Scholar] [CrossRef]
- Riojas, M.A.; McGough, K.J.; Rider-Riojas, C.J.; Rastogi, N.; Hazbón, M.H. Phylogenomic analysis of the species of the Mycobacterium tuberculosis complex demonstrates that Mycobacterium africanum, Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti and Mycobacterium pinnipedii are later heterotypic synonyms of Mycobacterium tuberculosis. Int. J. Syst. Evol. Microbiol. 2018, 68, 324–332. [Google Scholar] [PubMed]
- Barbier, E.; Boschiroli, M.; Gueneau, E.; Rochelet, M.; Payne, A.; de Cruz, K.; Blieux, A.; Fossot, C.; Hartmann, A. First molecular detection of Mycobacterium bovis in environmental samples from a French region with endemic bovine tuberculosis. J. Appl. Microbiol. 2016, 120, 1193–1207. [Google Scholar] [CrossRef]
- Liébana, E.; Aranaz, A.; Francis, B.; Cousins, D. Assessment of genetic markers for species differentiation within the Mycobacterium tuberculosis complex. J. Clin. Microbiol. 1996, 34, 933. [Google Scholar] [CrossRef] [PubMed Central]
- Cousins, D.V.; Wilton, S.D.; Francis, B.R. Use of DNA amplification for the rapid identification of Mycobacterium bovis. Vet. Microbiol. 1991, 27, 187–195. [Google Scholar] [CrossRef]
- Telenti, A.; Marchesi, F.; Balz, M.; Bally, F.; Bottger, E.C.; Bodmer, T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 1993, 31, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Chimara, E.; Ferrazoli, L.; Ueky, S.Y.M.; Martins, M.C.; Durham, A.M.; Arbeit, R.D.; Leão, S.C. Reliable identification of mycobacterial species by PCR-restriction enzyme analysis (PRA)-hsp65 in a reference laboratory and elaboration of a sequence-based extended algorithm of PRA-hsp65 patterns. BMC Microbiol. 2008, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, J.M.; Ballesteros-Duperón, E.; Bueno-Segura, J.F. Feeding ecology of the Iberian lynx Lynx pardinus in eastern Sierra Morena (Southern Spain). Acta Theriol. 2006, 51, 85–90. [Google Scholar] [CrossRef]
- Tortoli, E. Impact of genotypic studies on mycobacterial taxonomy: The new mycobacteria of the 1990s. Clin. Microbiol. Rev. 2003, 16, 319–354. [Google Scholar] [CrossRef]
- Marshall, H.M.; Carter, R.; Torbey, M.J.; Minion, S.; Tolson, C.; Sidjabat, H.E.; Huygens, F.; Hargreaves, M.; Thomson, R.M. Mycobacterium lentiflavum in Drinking Water Supplies, Australia. Emerg. Infect. Dis. 2011, 17, 395. [Google Scholar] [CrossRef] [PubMed Central]
- Jordão Junior, C.M.; Lopes, F.C.M.; David, S.; Farache Filho, A.; Leite, C.Q.F. Detection of nontuberculous mycobacteria from water buffalo raw milk in Brazil. Food Microbiol. 2009, 26, 658–661. [Google Scholar] [CrossRef]
- Arfaatabar, M.; Karami, P.; Khaledi, A. An update on prevalence of slow-growing mycobacteria and rapid-growing mycobacteria retrieved from hospital water sources in Iran—A systematic review. Germs 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed Central]
- Katale, B.Z.; Mbugi, E.V.; Botha, L.; Keyyu, J.D.; Kendall, S.; Dockrell, H.M.; Michel, A.L.; Kazwala, R.R.; Rweyemamu, M.M.; van Helden, P.; et al. Species diversity of non-tuberculous mycobacteria isolated from humans, livestock and wildlife in the Serengeti ecosystem, Tanzania. BMC Infect. Dis. 2014, 14, 616. [Google Scholar] [CrossRef]
- Del Olmo Izuzquiza, I.R.; Cirac, M.L.M.; Alonso, M.B.; Prades, P.B.; Laleona, C.G. Mycobacterium lentiflavum lymphadenitis: Two case reports. Arch. Argent. Pediatr. 2016, 114, e329–e332. [Google Scholar] [PubMed]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: AnOfficial ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Eur. Respir. J. 2020, 56, e1–e36. [Google Scholar] [CrossRef] [PubMed Central]
- Jarzembowski, J.A.; Young, M.B. Nontuberculous Mycobacterial Infections. Arch. Pathol. Lab. Med. 2008, 132, 1333–1341. Available online: https://meridian.allenpress.com/aplm/article/132/8/1333/460552/Nontuberculous-Mycobacterial-Infections (accessed on 14 February 2023). [CrossRef]
- Miqueleiz-Zapatero, A.; Santa Olalla-Peralta, C.; Guerrero-Torres, M.D.; Cardeñoso-Domingo, L.; Hernández-Milán, B.; Domingo-García, D. Mycobacterium lentiflavum como causa principal de linfadenitis en población pediátrica. Enferm. Infecc. Microbiol. Clin. 2018, 36, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Vise, E.; Mawlong, M.; Garg, A.; Sen, A.; Shakuntala, I.; Das, S. Recovery of Mycobacterium lentiflavum from bronchial lavage during follow-up of an extrapulmonary tuberculosis patient. Int. J. Mycobacteriol. 2017, 6, 315. Available online: https://www.ijmyco.org/article.asp?issn=2212-5531;year=2017;volume=6;issue=3;spage=315;epage=317;aulast=Vise (accessed on 16 February 2023).
- Piersimoni, C.; Goteri, G.; Nista, D.; Mariottini, A.; Mazzarelli, G.; Bornigia, S. Mycobacterium lentiflavum as an Emerging Causative Agent of Cervical Lymphadenitis. J. Clin. Microbiol. 2004, 42, 3894. [Google Scholar] [CrossRef] [PubMed Central]
- Yagi, K.; Morimoto, K.; Ishii, M.; Namkoong, H.; Okamori, S.; Asakura, T.; Suzuki, S.; Asami, T.; Uwamino, Y.; Funatsu, Y.; et al. Clinical characteristics of pulmonary Mycobacterium lentiflavum disease in adult patients. Int. J. Infect. Dis. 2018, 67, 65–69. Available online: http://www.ijidonline.com/article/S1201971217303132/fulltext (accessed on 27 October 2023). [CrossRef]
- Giménez-Anaya, A.; Herrero, J.; Rosell, C.; Couto, S.; García-Serrano, A. Food habits of wild boars (Sus scrofa) in a Mediterranean coastal wetland. Wetlands 2008, 28, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Llario, P. The sexual function of wallowing in male wild boar (Sus scrofa). J. Ethol. 2005, 23, 9–14. [Google Scholar] [CrossRef]
- Durnez, L.; Katakweba, A.; Sadiki, H.; Katholi, C.R.; Kazwala, R.R.; Machang’U, R.R.; Portaels, F.; Leirs, H. Mycobacteria in Terrestrial Small Mammals on Cattle Farms in Tanzania. Vet. Med. Int. 2011, 2011, 495074. [Google Scholar] [CrossRef] [PubMed Central]
- Varela-Castro, L.; Torrontegi, O.; Sevilla, I.A.; Barral, M. Detection of Wood Mice (Apodemus sylvaticus) Carrying Non-Tuberculous Mycobacteria Able to Infect Cattle and Interfere with the Diagnosis of Bovine Tuberculosis. Microorganisms 2020, 8, 374. [Google Scholar] [CrossRef] [PubMed Central]
| Lynx Data | Azuaga (BA) | Don Benito (BA) | Zafra (BA) | Navalmoral de la Mata/Plasencia (CA) | Logrosán (CA) | Mérida | Total |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 14 | 3 | 10 | 1 | - | 1 | 29 |
| Female | 13 | 5 | 6 | 7 | 1 | 1 | 33 |
| Age | |||||||
| Yearlings (<1) | 7 | 1 | 6 | 2 | - | - | 16 |
| Subadults (1–3) | 5 | 3 | 5 | 2 | 1 | 2 | 18 |
| Adults (>3) | 15 | 4 | 5 | 4 | - | - | 28 |
| Body condition | |||||||
| ≤2 | 1 | - | - | - | - | - | 1 |
| 2–3 | 10 | - | 6 | - | - | - | 16 |
| ≥3 | 15 | 7 | 8 | 1 | 1 | 2 | 34 |
| Parameters | Mycobacteria | ||
|---|---|---|---|
| Hematology | Presence | Absence | p-Value |
| WBC (109/L) | 10.1392 ± 0.9923 | 10.6708 ± 1.2574 | 0.32 |
| Neutrophils 109/L | 7.7458 ± 1.0682 | 8.3675 ± 1.2127 | 0.31 |
| Lymphocytes 109/L | 1.6000 ± 0.1892 | 1.7100 ± 0.1788 | 0.82 |
| Monocytes 109/L | 0.2958 ± 0.0355 | 0.2592 ± 0.0446 | 0.99 |
| Eosinophils 109/L | 0.4717 ± 0.0527 | 0.2967 ± 0.0458 | 0.05 |
| Basophils 109/L | 0.0267 ± 0.0054 | 0.0225 ± 0.0051 | 0.92 |
| RBC (1012/L) | 8.6825 ± 0.4233 | 8.7117 ± 0.4257 | 0.93 |
| Hemoglobin (g/dL) | 12.100 ± 0.4919 | 12.342 ± 0.4375 | 0.33 |
| Hematocrit%/PCV% | 40.025 ± 1.8812 | 40.300 ± 1.9909 | 0.84 |
| MCV (fL) | 46.192 ± 0.5460 | 46.40 ± 0.7918 | 0.12 |
| MCH (pg) Hb/RBC*10 | 14.0050 ± 0.1598 | 14.2885 ± 0.2604 | 0.15 |
| MCHC (g/dL) | 30.3158 ± 0.4441 | 30.8667 ± 0.5076 | 0.46 |
| Platelets (109/L) | 307.750 ± 29.3330 | 270.50 ± 19.4533 | 0.97 |
| Biochemistry | |||
| Glucose (mg/dL) | 203.9792 ± 19.0453 | 146.1958 ± 17.6241 | 0.29 |
| Urea (mg/dL) | 112.550 ± 7.1684 | 116.350 ± 5.9995 | 0.49 |
| Creatinine (mg/dL) | 1.7700 ± 0.1275 | 1.5517 ± 0.1243 | 0.2 |
| Cholesterol (mg/dL) | 118.667 ± 13.5503 | 139.167 ± 12.9468 | 0.63 |
| Triglyceride (mg/dL) | 53.250 ± 11.4290 | 51.417 ± 12.0519 | 0.24 |
| Total protein (g/dL) | 6.992 ± 0.1406 | 7.092 ± 0.1097 | 0.68 |
| Albumin (g/dL) | 3.5283 ± 0.0902 | 3.5992 ± 0.0626 | 0.85 |
| Globulin (g/dL) | 3.4675 ± 0.1286 | 3.4975 ± 0.1283 | 0.32 |
| Alb/Glob | 1.0325 ± 0.0497 | 1.0500 ± 0.0526 | 0.63 |
| Total bilirubin (mg/dL) | 0.4517 ± 0.0626 | 0.4225 ± 0.0533 | 0.53 |
| Direct bilirubin (mg/dL) | 0.2525 ± 0.0835 | 0.2166 ± 0.4827 | 0.29 |
| Indirect bilirubin (mg/dL) | 0.1992 ± 0.0325 | 0.2058 ± 0.0166 | 0.89 |
| Calcium (mg/dL) | 10.9417 ± 0.4733 | 10.3425 ± 0.4585 | 0.78 |
| Phosphorus (mg/dL) | 5.2033 ± 0.3861 | 5.3817 ± 0.4418 | |
| Sodium (mEq/L) | 156.133 ± 1.3982 | 151.392 ± 2.0453 | 0.85 |
| Potassium (mEq/L) | 4.2583 ± 0.0940 | 4.3925 ± 0.9834 | 0.841 |
| Na/K | 36.8167 ± 0.7154 | 34.6508 ± 0.8854 | 0.07 |
| Chloride (mEq/L) | 112.958 ± 1.0244 | 117.575 ± 1.7664 | 0.09 |
| Plasma electrophoresis | |||
| Albumin (g/dL) | 3.8967 ± 0.1816 | 4.0842 ± 0.0815 | 0.17 |
| Alfa 1 globulin (g/dL) | 0.3467 ± 0.0811 | 0.2625± 0.0588 | 0.89 |
| Alfa 2 globulin (g/dL) | 0.4375 ± 0.0355 | 0.3325 ± 0.0374 | 0.59 |
| Beta 1 globulin (g/dL) | 0.6875 ± 0.0500 | 0.7242 ± 0.0408 | 0.67 |
| Beta 2 globulin (g/dL) | 0.5558 ± 0.0353 | 0.5317 ± 0.0316 | 0.69 |
| Gamma globulin (g/dL) | 1.1583 ± 0.0901 | 1.1492 ± 0.0959 | 0.77 |
| Alb/Glob | 1.5175 ± 0.1131 | 1.5625 ± 0.0728 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Pizarro, N.; Serrano, B.; Peña, J.; Barrera, R.; Gil-Molino, M.; Risco, D.; Hermoso-de-Mendoza, J. Identification of Non-Tuberculous Mycobacteria in Iberian Lynx (Lynx pardinus) and Their Impact on Its Health. Vet. Sci. 2025, 12, 527. https://doi.org/10.3390/vetsci12060527
Jiménez-Pizarro N, Serrano B, Peña J, Barrera R, Gil-Molino M, Risco D, Hermoso-de-Mendoza J. Identification of Non-Tuberculous Mycobacteria in Iberian Lynx (Lynx pardinus) and Their Impact on Its Health. Veterinary Sciences. 2025; 12(6):527. https://doi.org/10.3390/vetsci12060527
Chicago/Turabian StyleJiménez-Pizarro, Natalia, Beatriz Serrano, Jorge Peña, Rafael Barrera, María Gil-Molino, David Risco, and Javier Hermoso-de-Mendoza. 2025. "Identification of Non-Tuberculous Mycobacteria in Iberian Lynx (Lynx pardinus) and Their Impact on Its Health" Veterinary Sciences 12, no. 6: 527. https://doi.org/10.3390/vetsci12060527
APA StyleJiménez-Pizarro, N., Serrano, B., Peña, J., Barrera, R., Gil-Molino, M., Risco, D., & Hermoso-de-Mendoza, J. (2025). Identification of Non-Tuberculous Mycobacteria in Iberian Lynx (Lynx pardinus) and Their Impact on Its Health. Veterinary Sciences, 12(6), 527. https://doi.org/10.3390/vetsci12060527





