Assessing Q Fever Exposure in Veterinary Professionals: A Study on Seroprevalence and Awareness in Portugal, 2024
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honarmand, H. Q Fever: An Old but Still a Poorly Understood Disease. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 131932. [Google Scholar] [CrossRef] [PubMed]
- Redden, P.; Parker, K.; Henderson, S.; Fourie, P.; Agnew, L.; Stenos, J.; Graves, S.R.; Govan, B.; Norton, R.; Ketheesan, N. Q Fever-Immune Responses and Novel Vaccine Strategies. Future Microbiol. 2023, 18, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Alemneh, T.; Ayelign, M. Q Fever (Coxiellosis) in Animals and Humans. Approaches Poult. Dairy Vet. Sci. 2018, 5, 458–465. [Google Scholar] [CrossRef]
- Kazar, J. Coxiella burnetii Infection. Ann. N. Y. Acad. Sci. 2005, 1063, 105–114. [Google Scholar] [CrossRef]
- Eldin, C.; Melenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.; Maurin, M.; Raoult, D. From Q Fever to Coxiella burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2016, 30, 115–190. [Google Scholar] [CrossRef]
- Duron, O.; Sidi-Boumedine, K.; Rousset, E.; Moutailler, S.; Jourdain, E. The Importance of Ticks in Q Fever Transmission: What Has (and Has Not) Been Demonstrated? Trends Parasitol. 2015, 31, 536–552. [Google Scholar] [CrossRef]
- Roest, H.; Bossers, A.; van Zijderveld, F.V.; Rebel, J. Clinical Microbiology of Coxiella burnetii and Relevant Aspects for the Diagnosis and Control of the Zoonotic Disease Q Fever. Vet. Q. 2013, 33, 148–160. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Q Fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef]
- Suputtamongkol, Y.; Rolain, J.; Losuwanaruk, K.; Niwatayakul, K.; Suthinont, C.; Chierakul, W.; Pimda, K.; Raoult, D. Q Fever in Thailand. Emerg. Infect. Dis. 2003, 9, 1186–1188. [Google Scholar] [CrossRef]
- Zendoia, I.I.; Cevidanes, A.; Hurtado, A.; Vázquez, P.; Barral, M.; Barandika, J.; García-Pérez, A. Stable Prevalence of Coxiella burnetii in Wildlife after a Decade of Surveillance in Northern Spain. Vet. Microbiol. 2022, 268, 109422. [Google Scholar] [CrossRef]
- Gisbert, P.; Garcia-Ispierto, I.; Quintela, L.; Guatteo, R. Coxiella burnetii and Reproductive Disorders in Cattle: A Systematic Review. Animals 2024, 14, 1313. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.; Cleaveland, S.; Denwood, M.; Brown, J.; Shaw, D. Coxiella burnetii (Q-Fever) Seroprevalence in Prey and Predators in the United Kingdom: Evaluation of Infection in Wild Rodents, Foxes and Domestic Cats Using a Modified ELISA. Transbound. Emerg. Dis. 2015, 62, 639–649. [Google Scholar] [CrossRef]
- Voss, L.; Huaman, J.; Pacioni, C.; Tolpinrud, A.; Helbig, K.; Carvalho, T.G.; Firestone, S.M. Seroprevalence of Coxiella burnetii Antibodies in Wild Deer Populations in Eastern Australia. Aust. Vet. J. 2022, 101, 106–114. [Google Scholar] [CrossRef]
- Krzysiak, M.; Puchalska, M.; Olech, W.; Anusz, K. A Freedom of Coxiella burnetii Infection Survey in European Bison (Bison bonasus) in Poland. Animals 2021, 11, 651. [Google Scholar] [CrossRef]
- Fenga, C.; Gangemi, S.; Luca, A.D.; Calimeri, S.; Giudice, D.L.; Pugliese, M.; Licitra, F.; Alibrandi, A.; Costa, C. Seroprevalence and Occupational Risk Survey for Coxiella burnetii among Exposed Workers in Sicily, Southern Italy. Int. J. Occup. Med. Environ. Health 2015, 28, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Woldeyohannes, S.; Gilks, C.; Baker, P.; Perkins, N.; Reid, S. Seroprevalance of Coxiella burnetii among Abattoir and Slaughterhouse Workers: A Meta-Analysis. One Health 2018, 6, 23–28. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Hebbar, B.K.; Mitra, P.; Shinde, S.; Chaudhari, S.; Barbuddhe, S. Seroprevalence and Risk Factors of Toxoplasma gondii Infection among Veterinary Personnel and Abattoir Workers in Central India. Parasitol. Int. 2021, 84, 102402. [Google Scholar] [CrossRef]
- Riccò, M.; Baldassarre, A.; Corrado, S.; Marchesi, F. Seroprevalence of Coxiella burnetii in Occupational Settings: A Meta-Analysis of Italian Studies. Zoonotic Dis. 2023, 3, 38–51. [Google Scholar] [CrossRef]
- De França, D.A.; Kmetiuk, L.B.; Rodrigues, O.J.D.; Panazzolo, G.A.K.; Morikawa, V.; Duré, A.Í.d.L.; Langoni, H.; Fávero, G.M.; Biondo, A.W. Coxiella burnetii (Q Fever) Exposure in Wildlife Professionals. Front. Public Health 2024, 12, 1466981. [Google Scholar] [CrossRef]
- Scott, G.H.; Williams, J.C. Susceptibility of Coxiella burnetii to Chemical Disinfectants. Ann. N. Y. Acad. Sci. 1990, 590, 291–296. [Google Scholar] [CrossRef]
- Celina, S.S.; Černý, J. Coxiella burnetii in Ticks, Livestock, Pets and Wildlife: A Mini-Review. Front. Vet. Sci. 2022, 9, 1068129. [Google Scholar] [CrossRef]
- Plummer, P.J.; McClure, J.T.; Menzies, P.; Morley, P.S.; Van den Brom, R.; Van Metre, D.C. Management of Coxiella burnetii Infection in Livestock Populations and the Associated Zoonotic Risk: A Consensus Statement. J. Vet. Intern. Med. 2018, 32, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Cumbassá, A.; Barahona, M.; Cunha, M.V.; Azórin, B.; Fonseca, C.; Rosalino, L.; Tilburg, J.; Hagen, F.; Santos, A.; Botelho, A. Coxiella burnetii DNA Detected in Domestic Ruminants and Wildlife from Portugal. Vet. Microbiol. 2015, 180, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Tilburg, J.; Botelho, A.; Barahona, M.; Núncio, M.; Nabuurs-Franssen, M.; Klaassen, C. Genotypic Diversity of Clinical Coxiella burnetii Isolates from Portugal Based on MST and MLVA Typing. Int. J. Med. Microbiol. IJMM 2012, 302, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Afonso, A.; Neubauer, H.; Needham, H.; Thiéry, R.; Rodolakis, A.; Roest, H.; Stark, K.; Stegeman, J.; Vellema, P.; et al. Q Fever in Humans and Farm Animals in Four European Countries, 1982 to 2010. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2013, 18, 20407. [Google Scholar] [CrossRef]
- Bernard, H.; Brockmann, S.; Kleinkauf, N.; Klinc, C.; Wagner-Wiening, C.; Stark, K.; Jansen, A. High Seroprevalence of Coxiella burnetii Antibodies in Veterinarians Associated with Cattle Obstetrics, Bavaria, 2009. Vector Borne Zoonotic Dis. 2012, 12, 552–557. [Google Scholar] [CrossRef]
- Sellens, E.; Bosward, K.; Norris, J.; Wood, N.; Heller, J.; Graves, S.; Gidding, H. Coxiella burnetii Seroprevalence in Unvaccinated Veterinary Workers in Australia: Evidence to Support Q Fever Vaccination. Zoonoses Public Health 2020, 67, 79–88. [Google Scholar] [CrossRef]
- Conan, A.; Gallagher, C.; Erskine, N.; Howland, M.; Smith-Anthony, M.; Marchi, S.; Magouras, I.; Müller, A.; Becker, A.A.M.J. Is There a Higher Risk of Exposure to Coxiella burnetii for Pre-Clinical Veterinary Students? One Health 2022, 16, 100485. [Google Scholar] [CrossRef]
- Meadows, S.; Jones-Bitton, A.; McEwen, S.; Jansen, J.; Patel, S.N.; Filejski, C.; Menzies, P. Coxiella burnetii (Q Fever) Seropositivity and Associated Risk Factors in Sheep and Goat Farm Workers in Ontario, Canada. Vector Borne Zoonotic Dis. 2016, 16, 643–649. [Google Scholar] [CrossRef]
- Schimmer, B.; Schotten, N.; van Engelen, E.; Hautvast, J.; Schneeberger, P.; van Duijnhoven, Y.V. Coxiella burnetii Seroprevalence and Risk for Humans on Dairy Cattle Farms, the Netherlands, 2010–2011. Emerg. Infect. Dis. 2014, 20, 417–425. [Google Scholar] [CrossRef]
- Sun, W.-W.; Cong, W.; Li, M.; Wang, C.-F.; Shan, X.; Qian, A. Coxiella burnetii Seroprevalence and Risk Factors in Cattle Farmers and Farm Residents in Three Northeastern Provinces and Inner Mongolia Autonomous Region, China. BioMed Res. Int. 2016, 2016, 7059196. [Google Scholar] [CrossRef] [PubMed]
- Kersh, G. Tropical Q Fever. Am. J. Trop. Med. Hyg. 2022, 107, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, B.; Notermans, D.; Harms, M.; Reimerink, J.; Bakker, J.; Schneeberger, P.; Mollema, L.; Teunis, P.; van Pelt, W.; van Duynhoven, Y.V. Low Seroprevalence of Q Fever in The Netherlands Prior to a Series of Large Outbreaks. Epidemiol. Infect. 2011, 140, 27–35. [Google Scholar] [CrossRef]
- Leuken, J.V.; Swart, A.; Brandsma, J.; Terink, W.; Kassteele, J.; Droogers, P.; Sauter, F.; Havelaar, A.H.; Hoek, W.V.D. Human Q Fever Incidence Is Associated to Spatiotemporal Environmental Conditions. One Health 2016, 2, 77–87. [Google Scholar] [CrossRef]
- Tan, T.; Hernandez-Jover, M.; Hayes, L.; Wiethoelter, A.; Firestone, S.; Stevenson, M.; Heller, J. Identifying Scenarios and Risk Factors for Q Fever Outbreaks Using Qualitative Analysis of Expert Opinion. Zoonoses Public Health 2022, 69, 344–358. [Google Scholar] [CrossRef]
- Hackert, V.; Hoebe, C.J.P.A.; Dukers-Muijrers, N.; Krafft, T.; Kauhl, B.; Henning, K.; Karges, W.; Sprague, L.; Neubauer, H.; Dahouk, S.A. Q Fever: Evidence of a Massive yet Undetected Cross-border Outbreak, with Ongoing Risk of Extra Mortality, in a Dutch–German Border Region. Transbound. Emerg. Dis. 2020, 67, 1660–1670. [Google Scholar] [CrossRef]
- Dupuis, G.; Petite, J.; Péter, O.; Vouilloz, M. An Important Outbreak of Human Q Fever in a Swiss Alpine Valley. Int. J. Epidemiol. 1987, 16, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.; Nabuurs-Franssen, M.; Tilburg, J.; Hamans, M.; Horrevorts, A.M. Multigenotype Q Fever Outbreak, the Netherlands. Emerg. Infect. Dis. 2009, 15, 613–614. [Google Scholar] [CrossRef]
- Cardeñosa, N.; Sanfeliú, I.; Font, B.; Muñoz, T.; Nogueras, M.; Segura, F. Short Report: Seroprevalence of Human Infection by Coxiella burnetii in Barcelona (Northeast of Spain). Am. J. Trop. Med. Hyg. 2006, 75, 33–35. [Google Scholar] [CrossRef]
- Meurer, I.R.; Silva, M.R.; Silva, M.V.; de Lima Duré, A.Í.; Adelino, T.É.; da Costa, A.V.; Vanelli, C.P.; de Paula Souza e Guimarães, R.J.; Rozental, T.; de Lemos, E.R.; et al. Seroprevalence Estimate and Risk Factors for Coxiella burnetii Infections among Humans in a Highly Urbanised Brazilian State. Trans. R. Soc. Trop. Med. Hyg. 2021, 116, 261–269. [Google Scholar] [CrossRef]
- Vanderburg, S.; Rubach, M.; Halliday, J.E.B.; Cleaveland, S.; Reddy, E.A.; Crump, J. Epidemiology of Coxiella burnetii Infection in Africa: A OneHealth Systematic Review. PLoS Negl. Trop. Dis. 2014, 8, e2787. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, F.D.; Martinelle, L.; Leonard, P.; Renaville, B.; Renaville, R.; Thys, C.; Smeets, F.; Czaplicki, G.; Esbroeck, M.; Saegerman, C. Q Fever Serological Survey and Associated Risk Factors in Veterinarians, Southern Belgium, 2013. Transbound. Emerg. Dis. 2017, 64, 959–966. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, M.M.T.; Schimmer, B.; Versteeg, B.; Schneeberger, P.; Berends, B.R.; Heederik, D.; van der Hoek, W.; Wouters, I.M. Risk Factors of Coxiella burnetii (Q Fever) Seropositivity in Veterinary Medicine Students. PLoS ONE 2012, 7, e32108. [Google Scholar] [CrossRef][Green Version]
- Hellenbrand, W.; Breuer, T.; Petersen, L. Changing epidemiology of Q fever in Germany, 1947–1999. Emerg. Infect. Dis. 2001, 7, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Long, C.M.; Beare, P.; Cockrell, D.; Larson, C.L.; Heinzen, R. Comparative Virulence of Diverse Coxiella burnetii Strains. Virulence 2019, 10, 133–150. [Google Scholar] [CrossRef]
- Kuley, R.; Kuijt, E.; Smits, M.; Roest, H.; Smith, H.E.; Bossers, A. Genome Plasticity and Polymorphisms in Critical Genes Correlate with Increased Virulence of Dutch Outbreak-Related Coxiella burnetii Strains. Front. Microbiol. 2017, 8, 1526. [Google Scholar] [CrossRef]
- Metters, G.; Norville, I.; Titball, R.; Hemsley, C. From Cell Culture to Cynomolgus Macaque: Infection Models Show Lineage-Specific Virulence Potential of Coxiella burnetii. J. Med. Microbiol. 2019, 68, 1419. [Google Scholar] [CrossRef]
- Beare, P.; Samuel, J.; Howe, D.; Virtaneva, K.; Porcella, S.; Heinzen, R. Genetic Diversity of the Q Fever Agent, Coxiella burnetii, Assessed by Microarray-Based Whole-Genome Comparisons. J. Bacteriol. 2006, 188, 2309–2324. [Google Scholar] [CrossRef]
- Toledo-Perona, R.; Contreras, A.; Gomis, J.; Quereda, J.; García-Galán, A.; Sánchez, A.; Gómez-Martín, Á. Controlling Coxiella burnetii in Naturally Infected Sheep, Goats and Cows, and Public Health Implications: A Scoping Review. Front. Vet. Sci. 2024, 11, 1321553. [Google Scholar] [CrossRef]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic Identification of Rickettsiae and Estimation of Intraspecies Sequence Divergence for Portions of Two Rickettsial Genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef]
- Taurel, A.; Guatteo, R.; Joly, A.; Beaudeau, F. Effectiveness of Vaccination and Antibiotics to Control Coxiella burnetii Shedding around Calving in Dairy Cows. Vet. Microbiol. 2012, 159, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Galganski, L.; Keller, B.; Long, C.; Yamashiro, K.J.; Hegazi, M.S.; Pivetti, C.; Talken, L.; Raff, G.; Farmer, D.; Chomel, B.; et al. Minimizing the Risk of Occupational Q Fever Exposure: A Protocol for Ensuring Coxiella burnetii–Negative Pregnant Ewes Are Used for Medical Research. Lab. Anim. 2020, 55, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Brom, R.; Engelen, E.; Roest, H.; van der Hoek, W.; Vellema, P. Coxiella Burnetii infections in Sheep or Goats: An Opinionated Review. Vet. Microbiol. 2015, 181, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Vidić, B.; Šeguljev, Z.; Savić, S.; Prica, N. Q Fever Epidemiology and Control in Domestic Animals. Arch. Vet. Med. 2013, 6, 15–26. [Google Scholar] [CrossRef]
- Idoko, D.O.; Agaba, J.A.; Ijeoma, N.; Badu, S.G.; Ijiga, A.C.; Okereke, E.K. The Role of HSE Risk Assessments in Mitigating Occupational Hazards and Infectious Disease Spread: A Public Health Review. Open Access Res. J. Biol. Pharm. 2024, 11, 29. [Google Scholar] [CrossRef]
| Group | Positive Cases Absolute Frequency (n) | Positive Cases Relative Frequency (%) | Negative Cases Absolute Frequency (n) | Negative Cases Relative Frequency (%) | Total (n) |
|---|---|---|---|---|---|
| Test Group | 31 | 33.70% | 61 | 66.30% | 92 |
| Control Group | 32 | 17.39% | 152 | 82.61% | 184 |
| Total | 276 |
| Variable | OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
|---|---|---|---|---|
| Age Group | - | |||
| 20–29 Years | 1.00 (Ref) | - | 1.00 (Ref) | |
| 30–39 Years | 1.20 (0.45–3.20) | 0.72 | 1.10 (0.40–3.00) | 0.86 |
| 40–49 Years | 1.10 (0.40–3.00) | 0.85 | 0.95 (0.30–3.00) | 0.93 |
| 50–59 Years | 0.90 (0.30–2.70) | 0.86 | 0.80 (0.25–2.60) | 0.71 |
| ≥60 Years | 1.50 (0.50–4.50) | 0.47 | 1.30 (0.40–4.20) | 0.66 |
| Gender (Male vs. Female) | 1.12 (0.52–2.41) | 0.72 | 1.08 (0.45–2.60) | 0.86 |
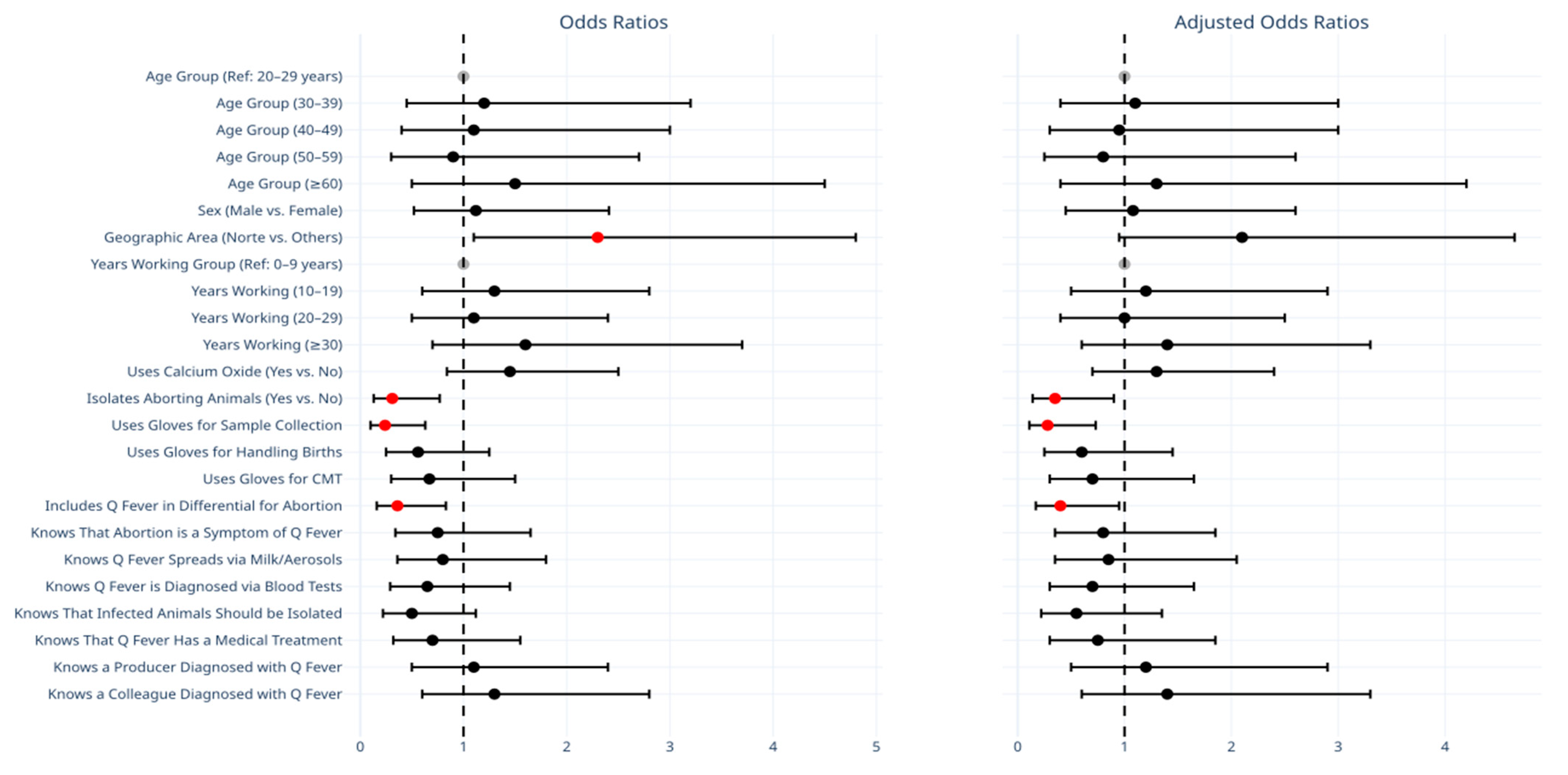
| Geographic Area (Norte vs. Others) | 2.30 (1.10–4.80) | 0.03 | 2.10 (0.95–4.65) | 0.07 |
| Years Working Group (Ref: 0–9 Years) | 1.00 (Ref) | - | 1.00 (Ref) | - |
| - 10–19 Years | 1.30 (0.60–2.80) | 0.51 | 1.20 (0.50–2.90) | 0.68 |
| - 20–29 Years | 1.10 (0.50–2.40) | 0.83 | 1.00 (0.40–2.50) | 0.99 |
| - ≥30 Years | 1.60 (0.70–3.70) | 0.28 | 1.40 (0.60–3.30) | 0.45 |
| Uses Calcium Oxide (Yes vs. No) | 1.45 (0.84–2.50) | 0.18 | 1.30 (0.70–2.40) | 0.41 |
| Isolates Aborting Animals (Yes vs. No) | 0.31 (0.13–0.77) | 0.01 | 0.35 (0.14–0.90) | 0.03 |
| Uses Gloves for Sample Collection | 0.24 (0.10–0.63) | 0.003 | 0.28 (0.11–0.73) | 0.009 |
| Uses Gloves for Handling Parturitions | 0.56 (0.25–1.25) | 0.15 | 0.60 (0.25–1.45) | 0.26 |
| Uses Gloves for CMT | 0.67 (0.30–1.50) | 0.33 | 0.70 (0.30–1.65) | 0.42 |
| Includes Q Fever in Differential for Abortion | 0.36 (0.16–0.83) | 0.02 | 0.40 (0.17–0.95) | 0.04 |
| Knows Abortion as Clinical Sign of Q Fever | 0.75 (0.34–1.65) | 0.47 | 0.80 (0.35–1.85) | 0.61 |
| Knows Q Fever is Spread via Milk or Aerosols | 0.80 (0.36–1.80) | 0.59 | 0.85 (0.35–2.05) | 0.72 |
| Knows that Q Fever is Diagnosed via Blood Tests | 0.65 (0.29–1.45) | 0.29 | 0.70 (0.30–1.65) | 0.42 |
| Knows that Infected Animals Should Be Isolated | 0.50 (0.22–1.12) | 0.09 | 0.55 (0.22–1.35) | 0.19 |
| Knows that Q Fever Has Medical Treatment | 0.70 (0.32–1.55) | 0.38 | 0.75 (0.30–1.85) | 0.53 |
| Knows a Producer Diagnosed with Q Fever | 1.10 (0.50–2.40) | 0.81 | 1.20 (0.50–2.90) | 0.68 |
| Knows a Colleague Diagnosed with Q Fever | 1.30 (0.60–2.80) | 0.51 | 1.40 (0.60–3.30) | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, G.; Ribeiro, M.; Martins, M.; Cardoso, J.M.; Esteves, F.; Anastácio, S.; Duarte, S.; Vala, H.; Cruz, R.; Mesquita, J.R. Assessing Q Fever Exposure in Veterinary Professionals: A Study on Seroprevalence and Awareness in Portugal, 2024. Vet. Sci. 2025, 12, 512. https://doi.org/10.3390/vetsci12060512
Moreira G, Ribeiro M, Martins M, Cardoso JM, Esteves F, Anastácio S, Duarte S, Vala H, Cruz R, Mesquita JR. Assessing Q Fever Exposure in Veterinary Professionals: A Study on Seroprevalence and Awareness in Portugal, 2024. Veterinary Sciences. 2025; 12(6):512. https://doi.org/10.3390/vetsci12060512
Chicago/Turabian StyleMoreira, Guilherme, Mário Ribeiro, Miguel Martins, José Maria Cardoso, Fernando Esteves, Sofia Anastácio, Sofia Duarte, Helena Vala, Rita Cruz, and João R. Mesquita. 2025. "Assessing Q Fever Exposure in Veterinary Professionals: A Study on Seroprevalence and Awareness in Portugal, 2024" Veterinary Sciences 12, no. 6: 512. https://doi.org/10.3390/vetsci12060512
APA StyleMoreira, G., Ribeiro, M., Martins, M., Cardoso, J. M., Esteves, F., Anastácio, S., Duarte, S., Vala, H., Cruz, R., & Mesquita, J. R. (2025). Assessing Q Fever Exposure in Veterinary Professionals: A Study on Seroprevalence and Awareness in Portugal, 2024. Veterinary Sciences, 12(6), 512. https://doi.org/10.3390/vetsci12060512






