Simple Summary
Veterinarians have a higher risk of Coxiella burnetii exposure due to contact with livestock. This study examines its prevalence and risk factors in Portuguese veterinarians. C. burnetii antibody titers in veterinarians and in a matched control group were compared. Logistic regression analyzed demographic, occupational, and biosecurity factors. The seroprevalence was 33.7% in veterinarians, which was higher (p = 0.0023) than that in the controls (17.4%). Northern veterinarians had higher seropositivity (p = 0.03), but this was not significant after adjustment (p = 0.07). Isolating aborting animals from the rest of the herd (OR: 0.35, p = 0.03) and wearing gloves during sampling (OR: 0.28, p = 0.009) reduced the risk. Veterinarians have higher C. burnetii exposure rates, but proper biosecurity lowers this risk. Personal protective equipment (PPE) use and training should be reinforced. Further research on the vaccination and epidemiology of this agent is needed.
Abstract
Due to their frequent contact with animals, veterinarians may be at preferential risk of Coxiella burnetii exposure due to occupational contact with livestock. This study assesses the seroprevalence and risk factors associated with C. burnetii seropositivity in Portuguese veterinarians. A cross-sectional study compared IgG anti-C. burnetii in veterinarians’ sera to a demographically matched control group. Univariate and multivariate logistic regression analyses evaluated associations between the demographic, occupational, and biosecurity factors and seropositivity. Seroprevalence among veterinarians was 33.7%, significantly higher (p = 0.0023) than in the controls (17.39%). Univariate analysis identified higher seropositivity in the northern region (p = 0.03), though this association was not significant after adjustment (p = 0.07). Protective measures, including isolating aborting animals from the rest of the herd (adjusted OR [aOR]: 0.35, p = 0.03) and wearing gloves during sample collection (OR: 0.28, p = 0.009), were significantly associated with lower infection risk. Veterinarians face increased C. burnetii exposure, but specific biosecurity practices reduce risk. Strengthening preventive measures, including personal protective equipment (PPE) use and biosecurity training, is essential to mitigate occupational and public health risks. Further research should explore vaccination strategies and molecular epidemiology to improve risk reduction efforts.
1. Introduction
Coxiella burnetii is an obligate intracellular, Gram-negative coccobacillus and the causative agent of Q fever, a zoonotic disease that presents a wide range of clinical manifestations in humans and animals [1]. Horizontal transmission primarily occurs via inhalation of aerosolized particles contaminated with the bacterium’s environmentally resistant small cell variant, often from parturient fluids or other excreta of infected animals [2]. Direct contact and consumption of unpasteurized dairy products are less common routes, while tick bites, though relatively infrequent, represent a recognized mode of transmission [3].
In arthropod vectors, particularly ticks, C. burnetii populations are maintained through both transstadial and transovarial transmission. These vertical transmission pathways contribute to the persistence of the pathogen in natural cycles and may play a role in both animal and human infections [1,3,4].
Q fever in humans can present as either an acute or chronic illness. Acute Q fever most commonly manifests as a non-specific flu-like syndrome, atypical pneumonia, or hepatitis. Rarely, more severe complications such as acute endocarditis or meningoencephalitis may occur. Chronic Q fever typically develops in individuals with underlying risk factors such as immunosuppression, prosthetic implants, or preexisting valvular heart disease. It is most frequently associated with infective endocarditis and vascular infections, including infected aneurysms and vascular grafts. Less commonly, it may involve pulmonary, hepatic, or osteoarticular sites [5,6]. In pregnant women, C. burnetii infection may lead to obstetric complications such as miscarriage, premature delivery, or fetal growth restriction. Despite its pathogenic potential, C. burnetii infections are frequently asymptomatic, particularly in healthy individuals and in a variety of domestic and wild animal reservoirs [7,8,9].
According to [1,2,3,4,5,6,7,8,9], ruminants, particularly small ruminants such as sheep and goats, are considered primary reservoirs of C. burnetii [10]. Infected animals often do not exhibit clinical signs, but when they do, reproductive disorders, including abortions, stillbirths, weak offspring, mastitis, metritis, and infertility are the most frequently associated signs. Infected animals shed C. burnetii in their feces, urine, milk, and particularly in birth products, with abortive tissues such as the placenta and amniotic fluid containing markedly higher bacterial loads [11]. This shedding significantly contributes to environmental contamination and the ongoing transmission cycle of the pathogen. Apart from domestic animals, wildlife also plays a role in the epidemiology of Q fever. Rodents, deer, and other mammals can harbor C. burnetii, serving as additional sources of infection for humans and livestock [12,13,14].
Humans become infected primarily by inhaling contaminated aerosols or dust particles from environments contaminated with C. burnetii. This mode of transmission makes occupational exposure a significant risk factor. Individuals who frequently work with animals, particularly small ruminants, are at an elevated risk of infection. High-risk groups include veterinarians [15], farmers, livestock handlers, abattoir and slaughterhouse workers, laboratory personnel handling infected samples, and individuals living in rural areas with high levels of livestock farming [15,16,17,18]. Additionally, foresters and those involved in wildlife management are also susceptible due to potential contact with infected wild animal populations. Infections can also occur through the consumption of unpasteurized dairy products from infected animals, though this is a less common route [19].
One of the most concerning aspects of C. burnetii is its exceptional resistance to environmental influences. The bacterium can survive desiccation, high temperatures, and exposure to common disinfectants, which contributes to its persistence in the environment. In its spore-like form, C. burnetii can remain viable for extended periods—up to 10 months on sheep wool and up to 40 months in powdered milk stored at room temperature. This remarkable resilience underscores the importance of stringent biosecurity measures to prevent the spread of Q fever, particularly in livestock farming and veterinary practices [20].
Coxiellosis poses a significant threat to both public and animal health, with economic repercussions due to reproductive losses and decreased productivity in affected livestock. The disease can lead to increased culling rates, reduced milk production, and higher veterinary costs, making it a considerable concern for the agricultural sector and food industry. Effective surveillance, vaccination, and management strategies are crucial for controlling the spread of C. burnetii and minimizing its impact on animal and human populations [21,22].
Vaccination remains a cornerstone in C. burnetii infection control by significantly reducing the incidence of reproductive losses and minimizing environmental contamination, thereby limiting onward transmission to susceptible animals and humans. Currently, two veterinary vaccines are commercially available in various regions worldwide. Coxevac® (CEVA Santé Animale, Libourne, France) is a non-adjuvanted, inactivated vaccine formulated with phase I C. burnetii (Nine Mile strain) which is licensed for use in goats and cattle. Chlamyvax® (Mérial, Lyon, France) is a bivalent inactivated vaccine composed of Chlamydia abortus and phase II C. burnetii which is intended for immunization of sheep and goats [21].
Despite its relevance as an occupational zoonosis, Q fever remains an understudied disease in many regions, including Portugal. In contrast to the prevalence of C. burnetii in livestock populations [23,24], limited research has been conducted on the awareness and seroprevalence of C. burnetti among veterinarians who work closely with farm animals. This gap in knowledge is particularly concerning, given the high-risk nature of veterinary work, which involves regular exposure to potentially infected animals and contaminated environments.
The objective of this study is to address this knowledge gap by evaluating the awareness levels of Portuguese farm animal veterinarians regarding Q fever as well as assessing their perceived risk of exposure and past infection history. By conducting a comprehensive survey and serological testing among veterinarians in Portugal, this study aims to determine the prevalence of Q fever antibodies in this professional group, shedding light on the extent of occupational exposure and informing future public health strategies. Ultimately, the findings from this research could contribute to improved preventive measures, including vaccination campaigns, enhanced biosecurity protocols, and greater educational outreach efforts targeting veterinary professionals.
Given the growing recognition of Q fever as a major public health concern worldwide [21,22], it is essential to improve surveillance and prevention strategies to mitigate its impact. Veterinary professionals play a crucial role in controlling the spread of C. burnetii, not only by diagnosing and managing infections in livestock but also by implementing measures to protect themselves and their colleagues. By increasing awareness and promoting proactive risk management, the veterinary community can help reduce the burden of Q fever and safeguard both animal and human health.
2. Materials and Methods
This cross-sectional study was designed to assess the seroprevalence of infection in two distinct human population cohorts.
For the study group, participants were selected through a convenience sampling method during scientific seminars focused on Q fever which were specifically organized for veterinarians. These seminars were held across mainland Portugal between February and March 2024 and targeted active members of the Portuguese Veterinary Medical Association (OMV) who provide veterinary services to livestock farms. Informed consent was obtained from all participants after a comprehensive explanation of the objectives and procedures of the study. Additionally, a structured questionnaire was administered to collect sociodemographic and occupational information. The questionnaire was divided into two sections. The first section aimed to gather sociodemographic data on the veterinarians, including the main geographic region of professional activity (Figure 1), while the second focused on disease-related aspects, including biosecurity practices on farms, animal handling methods during peripartum procedures, the use of personal PPE, diagnostic practices, and knowledge of the epidemiology of Q fever and of previous confirmed cases in both farmers and veterinarians.
Figure 1.
Geographical division of Portugal into three macro-regions: Norte (North), Centro (Centre), and Sul (South).
For the control group, sera from blood donors with no direct or indirect contact with farm animals or agricultural environments that could expose them to C. burnetii or other zoonotic pathogens associated with farm animals were used. These samples were obtained from an unrelated research project, and their utilization in studies investigating zoonotic agents was approved by the Ethics Committee of EUVG (CE-EUVG decision nº 01/2024). The control sera were selected at a 1:2 ratio, meaning two control samples were matched with each participant from the study group. Matching was performed based on age group, geographical location, and gender, ensuring demographic comparability between the study and control groups.
This study involved the analysis of a total of 276 serum samples, of which 92 samples corresponded to the study group and 184 corresponded to the control group.
Blood samples were collected by nurses with active registration within the respective professional associations, following good practice guidelines and other applicable regulations. The samples were collected in tubes containing a coagulation activator using a tube holder and 23Gx1 ½″ needles, all of which were part of the Vacumed® (FL Medical S.R.L., Torreglia, Italy) system. The samples were refrigerated at 4 °C during transport to the laboratory. The samples were centrifuged at 3000 rpm for 15 min (ZIP-IQ® centrifuge, LW Scientific, Lawrenceville, GA, USA). The resulting serum was transferred to sterile and properly labeled 2 mL microtubes. After this process, the serum samples were immediately frozen at −20 °C, and this temperature was maintained until the laboratory analysis was performed. For the control group, aliquots of serum samples previously stored at −20 °C were thawed.
The sera were tested for the presence of anti-C. burnetii IgG antibodies using an indirect commercial enzyme-linked immunosorbent assay (ELISA) test, COXIELLA BURNETII ELISA FASE II IgG® (Vircell Microbiologists, Granada, Spain), according to the instructions of the manufacturer. The sensitivity and specificity of this assay were reported to be 95% and 97%, respectively (Vircell Microbiologists, as per the internal validation report of the manufacturer). Manufacturer-provided positive and negative controls were included in each run. Samples with an index lower than 9 were considered negative, samples with an index equal to or between 9 and 11 were considered equivocal, and samples with indexes greater than 11 were considered positive. Equivocal samples were retested, and if still equivocal, then they were considered negative. Exact 95% binomial confidence intervals (CIs) were calculated for proportions.
Data processing was performed using Excel® software and organized into three sections according to the structure of the questionnaire. Responses to the questions were categorized, and in the absence of a response, the blank space was considered as a missing value during data processing. The categorization was based on veterinarians’ sociodemographic information, biosecurity practices on farms, animal handling methods during peripartum procedures, the use of personal protective equipment (PPE), diagnostic practices, and knowledge of Q fever epidemiology, including confirmed cases among both farmers and veterinarians.
Statistical analysis and data processing were performed using a combination of Microsoft Excel (Version 2410, Build 18129.20116), R (version 4.2.3), and custom Python (version 3.12.10) scripts. Only values with p < 0.05 were considered statistically significant. Values with p < 0.1 were described as borderline significant.
3. Results
Within the veterinarians group (n = 92), 31 individuals (33.7%) tested seropositive, whereas 61 (66.3%) were seronegative. In the control group (n = 184), seropositivity was detected in 32 individuals (17.39%), with the remaining 152 (82.61%) testing negative (Table 1).

Table 1.
Seropositivity results for IgG anti-C. burnetii in veterinarians and controls.
Statistical analysis revealed a strong association between veterinary occupation and seropositivity. The calculated odds ratio (OR) of 2.41 (95% CI: 1.37–4.26) indicates that veterinarians had more than twice the odds of exhibiting serological evidence of previous C. burnetii exposure compared with the control group. The chi-square test for independence yielded a statistically significant result (X2 = 9.256, df = 1, p = 0.0023), confirming a non-random distribution of seropositivity across groups and confirming that the seroprevalence of C burnetii IgG antibodies was significantly higher among veterinarians compared with the control population.
A univariate logistic regression analysis was conducted to evaluate the association between demographic, occupational, and knowledge-related variables and seropositivity for C. burnetii. The results are summarized in Figure 2 and Table 2.
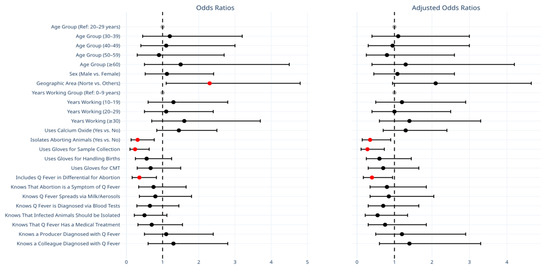
Figure 2.
Comparison of odds ratios (ORs) and adjusted odds ratios (AORs) for demographic, occupational, and knowledge-related variables associated with C. burnetii seropositivity. Black circles represent unadjusted OR, while red circles indicate AOR after adjusting for counfounding variables. Error bars denote 95% confidence intervals. Reference categories are depicted as greyed-out markers positioned at the null value (e.g., OR = 1 or log OR = 0) and do not display confidence intervals.

Table 2.
Univariate and multivariate risk factor analysis. Statistically significant values (p < 0.05) are shown in bold.
Geographic location was significantly associated with seropositivity, with individuals from the Northern region exhibiting higher odds of testing positive compared with other regions (OR: 2.30, 95% CI: 1.10–4.80, p = 0.03). Certain protective behaviors were also significantly associated with reduced odds of seropositivity. Specifically, individuals who isolated aborting animals had significantly lower odds of testing positive (OR: 0.31, 95% CI: 0.13–0.77, p = 0.01). Similarly, wearing gloves during sample collection was associated with a significantly lower likelihood of C. burnetii seropositivity (OR: 0.24, 95% CI: 0.10–0.63, p = 0.003). Including Q fever in the differential diagnosis of abortion cases was also associated with lower seropositivity (OR: 0.36, 95% CI: 0.16–0.83, p = 0.02). Conversely, the variables of age, gender, years of experience, and knowledge of Q fever transmission were not significantly associated with seropositivity.
A multivariate logistic regression model was constructed to adjust for potential confounders (Table 2). After adjusting for covariates, the association between geographic location and seropositivity was no longer statistically significant (adjusted OR: 2.10, 95% CI: 0.95–4.65, p = 0.07). However, the protective effect of isolating aborting animals remained significant (adjusted OR: 0.35, 95% CI: 0.14–0.90, p = 0.03). Wearing gloves during sample collection also continued to be significantly associated with lower odds of seropositivity (adjusted OR: 0.28, 95% CI: 0.11–0.73, p = 0.009). Including Q fever in the differential diagnosis for abortion cases retained their protective effect (adjusted OR: 0.40, 95% CI: 0.17–0.95, p = 0.04). Other variables, including years of experience, gender, and glove use for other tasks, did not show statistically significant associations.
4. Discussion
This study provides robust evidence of a higher seroprevalence of C. burnetii antibodies among veterinarians compared with a demographically matched control population. The overall seropositivity rate in the study group was 33.7%, which is significantly higher than the 17.39% observed in the control group. Statistical analysis demonstrated a strong association between the veterinary profession and prior exposure to C. burnetii, with an odds ratio (OR) of 2.41 (95% CI: 1.37–4.26, p = 0.0023), indicating that veterinarians were more than twice as likely to exhibit serological evidence of infection.
Both univariate and multivariate analyses highlighted key factors influencing C. burnetii seropositivity. Geographic location was initially associated with higher seropositivity, particularly in the Northern region (p = 0.03), where more intensive and clustered livestock production systems are common compared with the typically smaller-scale, extensive systems of the south, but this association lost significance after adjusting for confounders (adjusted OR: 2.10, 95% CI: 0.95–4.65, p = 0.07). In contrast, specific biosecurity measures consistently showed a protective effect. Isolating aborting animals (adjusted OR: 0.35, 95% CI: 0.14–0.90, p = 0.03) and using gloves during sample collection (adjusted OR: 0.28, 95% CI: 0.11–0.73, p = 0.009) were significantly associated with lower seropositivity, emphasizing the importance of these practices in reducing occupational exposure. These findings reinforce the need for targeted interventions to mitigate the risk of C. burnetii infection among veterinarians.
Our findings align with prior research demonstrating an elevated prevalence of C. burnetii exposure among individuals with occupational contact with livestock [17,25,26,27]. Studies conducted in various regions have consistently reported that veterinarians [26,27,28], farmers [29,30,31], abattoir workers [17], and laboratory personnel working with animal specimens [15] face a heightened risk of infection. Although reports from the Netherlands, Australia, and France have documented similar seroprevalence rates [32], variations in prevalence also exist across different studies, influenced by factors such as geographic location, livestock density, climate, biosecurity measures, and study design [33,34,35].
Our results indicate that isolating aborting animals and using gloves during sample collection had a protective effect against C. burnetii infection. The practice of isolating aborting animals might substantially reduce the risk of Q fever in veterinarians by limiting exposure to highly infectious birth materials, which are a major source of C. burnetii-containing aerosols. Likewise, the use of gloves during sample collection may help prevent direct contact with contaminated fluids and tissues, thereby decreasing the likelihood of infection. Together, these measures might play an important role in minimizing occupational exposure and may contribute to lower seropositivity rates among veterinarians.
Compared with studies conducted in regions with known Q fever outbreaks, the observed seroprevalence in the current study was relatively low [36,37,38]. This may reflect differences in exposure intensity, the effectiveness of biosecurity protocols, or the presence of vaccination programs for high-risk groups. In contrast, studies from endemic areas—such as the large Q fever outbreak in the Netherlands (2007–2010)—have reported significantly higher seroprevalence rates in exposed populations [30,33,38]. Such differences suggest that local epidemiological factors, including environmental persistence of C. burnetii, host–pathogen interactions, and public health interventions, play a crucial role in determining infection risk [39,40,41].
Currently, there is no human vaccine for Q fever authorized for routine use in Europe. The only licensed human vaccine, Q-Vax®—an inactivated whole-cell vaccine derived from the Henzerling phase I strain—is approved exclusively in Australia for high-risk populations. Its broader adoption has been limited due to concerns about adverse reactions and the requirement for pre-vaccination screening, including serological and skin tests [22].
Another important point of comparison is the relationship between seropositivity and clinical manifestations of Q fever. While some reports have identified strong correlations between seroprevalence and symptomatic individuals, others—including findings in the current study—suggest that exposure associated with asymptomatic or subclinical disease manifestations is common [42,43]. This highlights the potential for underdiagnosis, as individuals may acquire a C. burnetii infection without developing noticeable clinical signs, thereby remaining undetected in clinical settings. A study from Germany, for example, found that a substantial proportion of seropositive individuals had no recollection of experiencing Q fever-related symptoms [44], reinforcing the need for targeted screening in high-risk populations.
Molecular epidemiology studies conducted in various settings have further revealed strain-specific differences in virulence, transmission dynamics, and host preference, which may contribute to the observed variations in seroprevalence [45,46,47]. For instance, research utilizing whole-genome sequencing of C. burnetii strains has demonstrated genetic diversity among isolates from different hosts and geographic regions [48]. Understanding these genetic differences could provide insights into the potential for zoonotic spillover and inform control strategies. Expanding the present study to include molecular characterization of circulating strains in both human and animal populations would enhance the understanding of C. burnetii epidemiology and improve risk assessment strategies [49,50,51].
Frequent and direct contact with livestock, particularly during peripartum events, is a key risk factor for C. burnetii exposure. This organism is highly infectious and can be transmitted via aerosolized particles from birth products, urine, feces, and milk [28,29,41]. Inconsistent adherence to biosecurity measures in high-risk scenarios may further elevate exposure levels.
This study highlights the protective effect of specific biosecurity measures, particularly the use of gloves during sample collection and the isolation of aborting animals, both of which were associated with significantly lower odds of seropositivity. However, the persistently high seroprevalence suggests gaps in implementation, influenced by factors such as limited PPE access, variability in compliance, and time constraints during emergency interventions. Addressing these barriers is essential, as even minor lapses in protective measures can increase occupational exposure. Aditionally, considering Q fever as a potential cause of abortion seemed to protect veterinarians from C. burnetii infection, suggesting that increased awareness leads to better protective measures [15,50,52].
From a public health perspective, the elevated seroprevalence among veterinarians raises concerns about potential transmission risks, particularly for professionals working closely with infected animals or in contaminated environments. Veterinarians play a critical role in zoonotic disease surveillance and control, and their awareness of Q fever as a differential diagnosis for abortion cases is vital for early detection and intervention. Our findings suggest that routinely considering C. burnetii in diagnostic evaluations may reduce exposure risk by facilitating timely case identification and management.
The environmental resilience of C. burnetii presents additional challenges for farm-level biosecurity. This bacterium can persist for extended periods in dust, soil, and animal housing, leading to ongoing transmission risks [20,22]. Stricter sanitation protocols, proper disposal of birth materials, and quarantine measures for infected animals may help mitigate exposure. Additionally, exploring vaccination as a preventive strategy, particularly for veterinarians and livestock workers in endemic regions, could further reduce infection risk [49,53,54].
Overall, these findings emphasize the necessity of a multi-faceted approach to mitigating both occupational and public health risks associated with C. burnetii. Strengthening educational programs, reinforcing PPE usage, and improving adherence to farm-level biosecurity protocols are key strategies for reducing exposure among veterinarians. Furthermore, enhanced surveillance efforts and improved risk communication between veterinary professionals, farmers, and public health authorities will be critical in limiting zoonotic transmission and improving disease control efforts [16,18,25,44,55].
This study has several limitations. For one, the use of convenience sampling for veterinarians may have introduced selection bias, potentially limiting the generalizability of our findings. Additionally, seroprevalence data reflect past exposure rather than active infection, making it difficult to determine the timing or source of exposure. The control group, while matched by demographic factors, may not fully account for unmeasured confounders influencing seropositivity.
Future research should focus on several key areas to enhance our understanding of C. exposure in occupational settings. Longitudinal studies assessing seroconversion over time would provide valuable insight into infection dynamics among veterinarians and other high-risk groups, helping to identify early markers of infection and evaluate preventive measures.
Further investigation into the effectiveness of targeted biosecurity interventions, such as improved PPE, enhanced disinfection protocols, and vaccination strategies, is essential. These studies could optimize existing safety measures and establish evidence-based guidelines to reduce exposure risk in veterinary settings.
Expanding research to include other high-risk occupational groups, such as farmers, livestock handlers, and public health workers, would provide a broader perspective on risk factors and seroprevalence across professions. Comparing data across multiple high-risk groups could help identify common exposure pathways and inform comprehensive public health strategies.
Finally, conducting molecular epidemiology studies to characterize the genetic diversity of C. burnetii strains in both human and animal populations is of the utmost importance. Understanding transmission pathways, strain variations, and zoonotic risks could enhance diagnostic and preventive efforts, leading to more targeted interventions and a reduced burden of Q fever.
5. Conclusions
This study underscores the occupational risk veterinarians face regarding exposure to C. burnetii. The significantly higher seroprevalence in veterinarians highlights the need for enhanced biosecurity measures, particularly during sample collection and peripartum handling. Preventive strategies such as isolating aborting animals, using gloves, and incorporating Q fever into differential diagnoses for abortion cases can significantly reduce exposure risk. These findings emphasize the importance of targeted training, improved biosecurity protocols, and increased awareness to mitigate both occupational and public health risks associated with C. burnetii.
Author Contributions
Conceptualization, G.M. and J.R.M.; methodology, G.M.; software, G.M.; validation, G.M., M.R. and M.M.; formal analysis, G.M.; investigation, G.M.; resources, S.D. and H.V.; data curation, G.M.; writing—original draft preparation, G.M.; writing—review and editing, G.M., M.R., M.M., J.M.C., F.E., S.A., S.D., H.V., R.C. and J.R.M.; visualization, G.M.; supervision, J.R.M.; project administration, H.V.; funding acquisition, F.E., S.A. and H.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCT/MCTES (UIDB/50006/2020). Additionally, this work was supported by National Funds through the FCT—Portuguese Foundation for Science and Technology under projects UIDB/00681/2020 (CERNAS; https://doi.org/10.54499/UIDB/00681/2020) (accessed on 19 May 2025), UI/04033, and LA/P/0126/2020 (CITAB; https://doi.org/10.54499/LA/P/0126/2020) (accessed on 19 May 2025).
Institutional Review Board Statement
The study protocol was approved by the Ethics Committee of EUVG (CE-EUVG parecer nº 01/2024), date 2025-2-28. All participants provided informed consent before inclusion in the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We sincerely thank the veterinary professionals who participated in this study, as well as the veterinary nurses whose support was essential for the collection of samples. We also acknowledge the organizers of the seminars, whose coordination efforts greatly contributed to the engagement and mobilization of participants.
Conflicts of Interest
José Maria Cardoso was employed by the company José Maria Cardoso Ceva Saúde Animal. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Honarmand, H. Q Fever: An Old but Still a Poorly Understood Disease. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 131932. [Google Scholar] [CrossRef] [PubMed]
- Redden, P.; Parker, K.; Henderson, S.; Fourie, P.; Agnew, L.; Stenos, J.; Graves, S.R.; Govan, B.; Norton, R.; Ketheesan, N. Q Fever-Immune Responses and Novel Vaccine Strategies. Future Microbiol. 2023, 18, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Alemneh, T.; Ayelign, M. Q Fever (Coxiellosis) in Animals and Humans. Approaches Poult. Dairy Vet. Sci. 2018, 5, 458–465. [Google Scholar] [CrossRef]
- Kazar, J. Coxiella burnetii Infection. Ann. N. Y. Acad. Sci. 2005, 1063, 105–114. [Google Scholar] [CrossRef]
- Eldin, C.; Melenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.; Maurin, M.; Raoult, D. From Q Fever to Coxiella burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2016, 30, 115–190. [Google Scholar] [CrossRef]
- Duron, O.; Sidi-Boumedine, K.; Rousset, E.; Moutailler, S.; Jourdain, E. The Importance of Ticks in Q Fever Transmission: What Has (and Has Not) Been Demonstrated? Trends Parasitol. 2015, 31, 536–552. [Google Scholar] [CrossRef]
- Roest, H.; Bossers, A.; van Zijderveld, F.V.; Rebel, J. Clinical Microbiology of Coxiella burnetii and Relevant Aspects for the Diagnosis and Control of the Zoonotic Disease Q Fever. Vet. Q. 2013, 33, 148–160. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Q Fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef]
- Suputtamongkol, Y.; Rolain, J.; Losuwanaruk, K.; Niwatayakul, K.; Suthinont, C.; Chierakul, W.; Pimda, K.; Raoult, D. Q Fever in Thailand. Emerg. Infect. Dis. 2003, 9, 1186–1188. [Google Scholar] [CrossRef]
- Zendoia, I.I.; Cevidanes, A.; Hurtado, A.; Vázquez, P.; Barral, M.; Barandika, J.; García-Pérez, A. Stable Prevalence of Coxiella burnetii in Wildlife after a Decade of Surveillance in Northern Spain. Vet. Microbiol. 2022, 268, 109422. [Google Scholar] [CrossRef]
- Gisbert, P.; Garcia-Ispierto, I.; Quintela, L.; Guatteo, R. Coxiella burnetii and Reproductive Disorders in Cattle: A Systematic Review. Animals 2024, 14, 1313. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.; Cleaveland, S.; Denwood, M.; Brown, J.; Shaw, D. Coxiella burnetii (Q-Fever) Seroprevalence in Prey and Predators in the United Kingdom: Evaluation of Infection in Wild Rodents, Foxes and Domestic Cats Using a Modified ELISA. Transbound. Emerg. Dis. 2015, 62, 639–649. [Google Scholar] [CrossRef]
- Voss, L.; Huaman, J.; Pacioni, C.; Tolpinrud, A.; Helbig, K.; Carvalho, T.G.; Firestone, S.M. Seroprevalence of Coxiella burnetii Antibodies in Wild Deer Populations in Eastern Australia. Aust. Vet. J. 2022, 101, 106–114. [Google Scholar] [CrossRef]
- Krzysiak, M.; Puchalska, M.; Olech, W.; Anusz, K. A Freedom of Coxiella burnetii Infection Survey in European Bison (Bison bonasus) in Poland. Animals 2021, 11, 651. [Google Scholar] [CrossRef]
- Fenga, C.; Gangemi, S.; Luca, A.D.; Calimeri, S.; Giudice, D.L.; Pugliese, M.; Licitra, F.; Alibrandi, A.; Costa, C. Seroprevalence and Occupational Risk Survey for Coxiella burnetii among Exposed Workers in Sicily, Southern Italy. Int. J. Occup. Med. Environ. Health 2015, 28, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Woldeyohannes, S.; Gilks, C.; Baker, P.; Perkins, N.; Reid, S. Seroprevalance of Coxiella burnetii among Abattoir and Slaughterhouse Workers: A Meta-Analysis. One Health 2018, 6, 23–28. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Hebbar, B.K.; Mitra, P.; Shinde, S.; Chaudhari, S.; Barbuddhe, S. Seroprevalence and Risk Factors of Toxoplasma gondii Infection among Veterinary Personnel and Abattoir Workers in Central India. Parasitol. Int. 2021, 84, 102402. [Google Scholar] [CrossRef]
- Riccò, M.; Baldassarre, A.; Corrado, S.; Marchesi, F. Seroprevalence of Coxiella burnetii in Occupational Settings: A Meta-Analysis of Italian Studies. Zoonotic Dis. 2023, 3, 38–51. [Google Scholar] [CrossRef]
- De França, D.A.; Kmetiuk, L.B.; Rodrigues, O.J.D.; Panazzolo, G.A.K.; Morikawa, V.; Duré, A.Í.d.L.; Langoni, H.; Fávero, G.M.; Biondo, A.W. Coxiella burnetii (Q Fever) Exposure in Wildlife Professionals. Front. Public Health 2024, 12, 1466981. [Google Scholar] [CrossRef]
- Scott, G.H.; Williams, J.C. Susceptibility of Coxiella burnetii to Chemical Disinfectants. Ann. N. Y. Acad. Sci. 1990, 590, 291–296. [Google Scholar] [CrossRef]
- Celina, S.S.; Černý, J. Coxiella burnetii in Ticks, Livestock, Pets and Wildlife: A Mini-Review. Front. Vet. Sci. 2022, 9, 1068129. [Google Scholar] [CrossRef]
- Plummer, P.J.; McClure, J.T.; Menzies, P.; Morley, P.S.; Van den Brom, R.; Van Metre, D.C. Management of Coxiella burnetii Infection in Livestock Populations and the Associated Zoonotic Risk: A Consensus Statement. J. Vet. Intern. Med. 2018, 32, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Cumbassá, A.; Barahona, M.; Cunha, M.V.; Azórin, B.; Fonseca, C.; Rosalino, L.; Tilburg, J.; Hagen, F.; Santos, A.; Botelho, A. Coxiella burnetii DNA Detected in Domestic Ruminants and Wildlife from Portugal. Vet. Microbiol. 2015, 180, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Tilburg, J.; Botelho, A.; Barahona, M.; Núncio, M.; Nabuurs-Franssen, M.; Klaassen, C. Genotypic Diversity of Clinical Coxiella burnetii Isolates from Portugal Based on MST and MLVA Typing. Int. J. Med. Microbiol. IJMM 2012, 302, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Afonso, A.; Neubauer, H.; Needham, H.; Thiéry, R.; Rodolakis, A.; Roest, H.; Stark, K.; Stegeman, J.; Vellema, P.; et al. Q Fever in Humans and Farm Animals in Four European Countries, 1982 to 2010. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2013, 18, 20407. [Google Scholar] [CrossRef]
- Bernard, H.; Brockmann, S.; Kleinkauf, N.; Klinc, C.; Wagner-Wiening, C.; Stark, K.; Jansen, A. High Seroprevalence of Coxiella burnetii Antibodies in Veterinarians Associated with Cattle Obstetrics, Bavaria, 2009. Vector Borne Zoonotic Dis. 2012, 12, 552–557. [Google Scholar] [CrossRef]
- Sellens, E.; Bosward, K.; Norris, J.; Wood, N.; Heller, J.; Graves, S.; Gidding, H. Coxiella burnetii Seroprevalence in Unvaccinated Veterinary Workers in Australia: Evidence to Support Q Fever Vaccination. Zoonoses Public Health 2020, 67, 79–88. [Google Scholar] [CrossRef]
- Conan, A.; Gallagher, C.; Erskine, N.; Howland, M.; Smith-Anthony, M.; Marchi, S.; Magouras, I.; Müller, A.; Becker, A.A.M.J. Is There a Higher Risk of Exposure to Coxiella burnetii for Pre-Clinical Veterinary Students? One Health 2022, 16, 100485. [Google Scholar] [CrossRef]
- Meadows, S.; Jones-Bitton, A.; McEwen, S.; Jansen, J.; Patel, S.N.; Filejski, C.; Menzies, P. Coxiella burnetii (Q Fever) Seropositivity and Associated Risk Factors in Sheep and Goat Farm Workers in Ontario, Canada. Vector Borne Zoonotic Dis. 2016, 16, 643–649. [Google Scholar] [CrossRef]
- Schimmer, B.; Schotten, N.; van Engelen, E.; Hautvast, J.; Schneeberger, P.; van Duijnhoven, Y.V. Coxiella burnetii Seroprevalence and Risk for Humans on Dairy Cattle Farms, the Netherlands, 2010–2011. Emerg. Infect. Dis. 2014, 20, 417–425. [Google Scholar] [CrossRef]
- Sun, W.-W.; Cong, W.; Li, M.; Wang, C.-F.; Shan, X.; Qian, A. Coxiella burnetii Seroprevalence and Risk Factors in Cattle Farmers and Farm Residents in Three Northeastern Provinces and Inner Mongolia Autonomous Region, China. BioMed Res. Int. 2016, 2016, 7059196. [Google Scholar] [CrossRef] [PubMed]
- Kersh, G. Tropical Q Fever. Am. J. Trop. Med. Hyg. 2022, 107, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, B.; Notermans, D.; Harms, M.; Reimerink, J.; Bakker, J.; Schneeberger, P.; Mollema, L.; Teunis, P.; van Pelt, W.; van Duynhoven, Y.V. Low Seroprevalence of Q Fever in The Netherlands Prior to a Series of Large Outbreaks. Epidemiol. Infect. 2011, 140, 27–35. [Google Scholar] [CrossRef]
- Leuken, J.V.; Swart, A.; Brandsma, J.; Terink, W.; Kassteele, J.; Droogers, P.; Sauter, F.; Havelaar, A.H.; Hoek, W.V.D. Human Q Fever Incidence Is Associated to Spatiotemporal Environmental Conditions. One Health 2016, 2, 77–87. [Google Scholar] [CrossRef]
- Tan, T.; Hernandez-Jover, M.; Hayes, L.; Wiethoelter, A.; Firestone, S.; Stevenson, M.; Heller, J. Identifying Scenarios and Risk Factors for Q Fever Outbreaks Using Qualitative Analysis of Expert Opinion. Zoonoses Public Health 2022, 69, 344–358. [Google Scholar] [CrossRef]
- Hackert, V.; Hoebe, C.J.P.A.; Dukers-Muijrers, N.; Krafft, T.; Kauhl, B.; Henning, K.; Karges, W.; Sprague, L.; Neubauer, H.; Dahouk, S.A. Q Fever: Evidence of a Massive yet Undetected Cross-border Outbreak, with Ongoing Risk of Extra Mortality, in a Dutch–German Border Region. Transbound. Emerg. Dis. 2020, 67, 1660–1670. [Google Scholar] [CrossRef]
- Dupuis, G.; Petite, J.; Péter, O.; Vouilloz, M. An Important Outbreak of Human Q Fever in a Swiss Alpine Valley. Int. J. Epidemiol. 1987, 16, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.; Nabuurs-Franssen, M.; Tilburg, J.; Hamans, M.; Horrevorts, A.M. Multigenotype Q Fever Outbreak, the Netherlands. Emerg. Infect. Dis. 2009, 15, 613–614. [Google Scholar] [CrossRef]
- Cardeñosa, N.; Sanfeliú, I.; Font, B.; Muñoz, T.; Nogueras, M.; Segura, F. Short Report: Seroprevalence of Human Infection by Coxiella burnetii in Barcelona (Northeast of Spain). Am. J. Trop. Med. Hyg. 2006, 75, 33–35. [Google Scholar] [CrossRef]
- Meurer, I.R.; Silva, M.R.; Silva, M.V.; de Lima Duré, A.Í.; Adelino, T.É.; da Costa, A.V.; Vanelli, C.P.; de Paula Souza e Guimarães, R.J.; Rozental, T.; de Lemos, E.R.; et al. Seroprevalence Estimate and Risk Factors for Coxiella burnetii Infections among Humans in a Highly Urbanised Brazilian State. Trans. R. Soc. Trop. Med. Hyg. 2021, 116, 261–269. [Google Scholar] [CrossRef]
- Vanderburg, S.; Rubach, M.; Halliday, J.E.B.; Cleaveland, S.; Reddy, E.A.; Crump, J. Epidemiology of Coxiella burnetii Infection in Africa: A OneHealth Systematic Review. PLoS Negl. Trop. Dis. 2014, 8, e2787. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, F.D.; Martinelle, L.; Leonard, P.; Renaville, B.; Renaville, R.; Thys, C.; Smeets, F.; Czaplicki, G.; Esbroeck, M.; Saegerman, C. Q Fever Serological Survey and Associated Risk Factors in Veterinarians, Southern Belgium, 2013. Transbound. Emerg. Dis. 2017, 64, 959–966. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, M.M.T.; Schimmer, B.; Versteeg, B.; Schneeberger, P.; Berends, B.R.; Heederik, D.; van der Hoek, W.; Wouters, I.M. Risk Factors of Coxiella burnetii (Q Fever) Seropositivity in Veterinary Medicine Students. PLoS ONE 2012, 7, e32108. [Google Scholar] [CrossRef][Green Version]
- Hellenbrand, W.; Breuer, T.; Petersen, L. Changing epidemiology of Q fever in Germany, 1947–1999. Emerg. Infect. Dis. 2001, 7, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Long, C.M.; Beare, P.; Cockrell, D.; Larson, C.L.; Heinzen, R. Comparative Virulence of Diverse Coxiella burnetii Strains. Virulence 2019, 10, 133–150. [Google Scholar] [CrossRef]
- Kuley, R.; Kuijt, E.; Smits, M.; Roest, H.; Smith, H.E.; Bossers, A. Genome Plasticity and Polymorphisms in Critical Genes Correlate with Increased Virulence of Dutch Outbreak-Related Coxiella burnetii Strains. Front. Microbiol. 2017, 8, 1526. [Google Scholar] [CrossRef]
- Metters, G.; Norville, I.; Titball, R.; Hemsley, C. From Cell Culture to Cynomolgus Macaque: Infection Models Show Lineage-Specific Virulence Potential of Coxiella burnetii. J. Med. Microbiol. 2019, 68, 1419. [Google Scholar] [CrossRef]
- Beare, P.; Samuel, J.; Howe, D.; Virtaneva, K.; Porcella, S.; Heinzen, R. Genetic Diversity of the Q Fever Agent, Coxiella burnetii, Assessed by Microarray-Based Whole-Genome Comparisons. J. Bacteriol. 2006, 188, 2309–2324. [Google Scholar] [CrossRef]
- Toledo-Perona, R.; Contreras, A.; Gomis, J.; Quereda, J.; García-Galán, A.; Sánchez, A.; Gómez-Martín, Á. Controlling Coxiella burnetii in Naturally Infected Sheep, Goats and Cows, and Public Health Implications: A Scoping Review. Front. Vet. Sci. 2024, 11, 1321553. [Google Scholar] [CrossRef]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic Identification of Rickettsiae and Estimation of Intraspecies Sequence Divergence for Portions of Two Rickettsial Genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef]
- Taurel, A.; Guatteo, R.; Joly, A.; Beaudeau, F. Effectiveness of Vaccination and Antibiotics to Control Coxiella burnetii Shedding around Calving in Dairy Cows. Vet. Microbiol. 2012, 159, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Galganski, L.; Keller, B.; Long, C.; Yamashiro, K.J.; Hegazi, M.S.; Pivetti, C.; Talken, L.; Raff, G.; Farmer, D.; Chomel, B.; et al. Minimizing the Risk of Occupational Q Fever Exposure: A Protocol for Ensuring Coxiella burnetii–Negative Pregnant Ewes Are Used for Medical Research. Lab. Anim. 2020, 55, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Brom, R.; Engelen, E.; Roest, H.; van der Hoek, W.; Vellema, P. Coxiella Burnetii infections in Sheep or Goats: An Opinionated Review. Vet. Microbiol. 2015, 181, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Vidić, B.; Šeguljev, Z.; Savić, S.; Prica, N. Q Fever Epidemiology and Control in Domestic Animals. Arch. Vet. Med. 2013, 6, 15–26. [Google Scholar] [CrossRef]
- Idoko, D.O.; Agaba, J.A.; Ijeoma, N.; Badu, S.G.; Ijiga, A.C.; Okereke, E.K. The Role of HSE Risk Assessments in Mitigating Occupational Hazards and Infectious Disease Spread: A Public Health Review. Open Access Res. J. Biol. Pharm. 2024, 11, 29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).