Development of an Immunochromatographic Test with Recombinant MIC2-MIC3 Fusion Protein for Serological Detection of Toxoplasma gondii
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
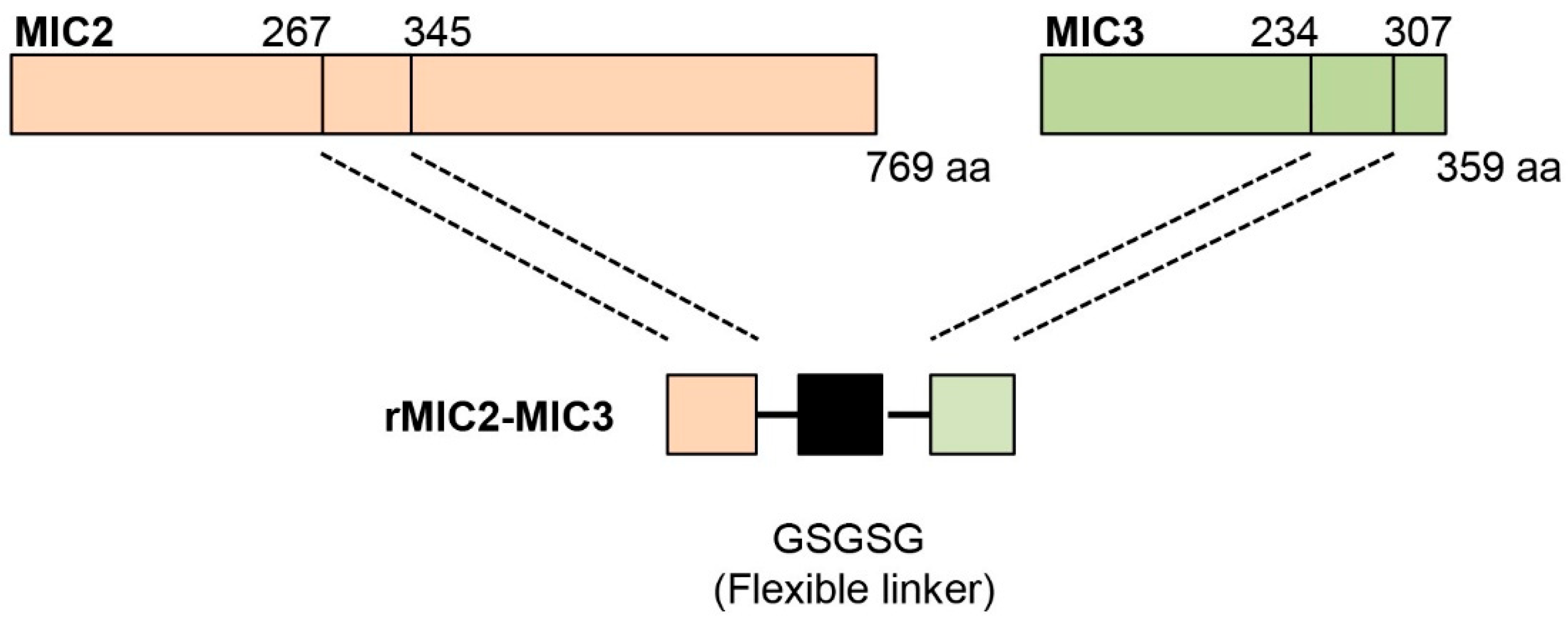
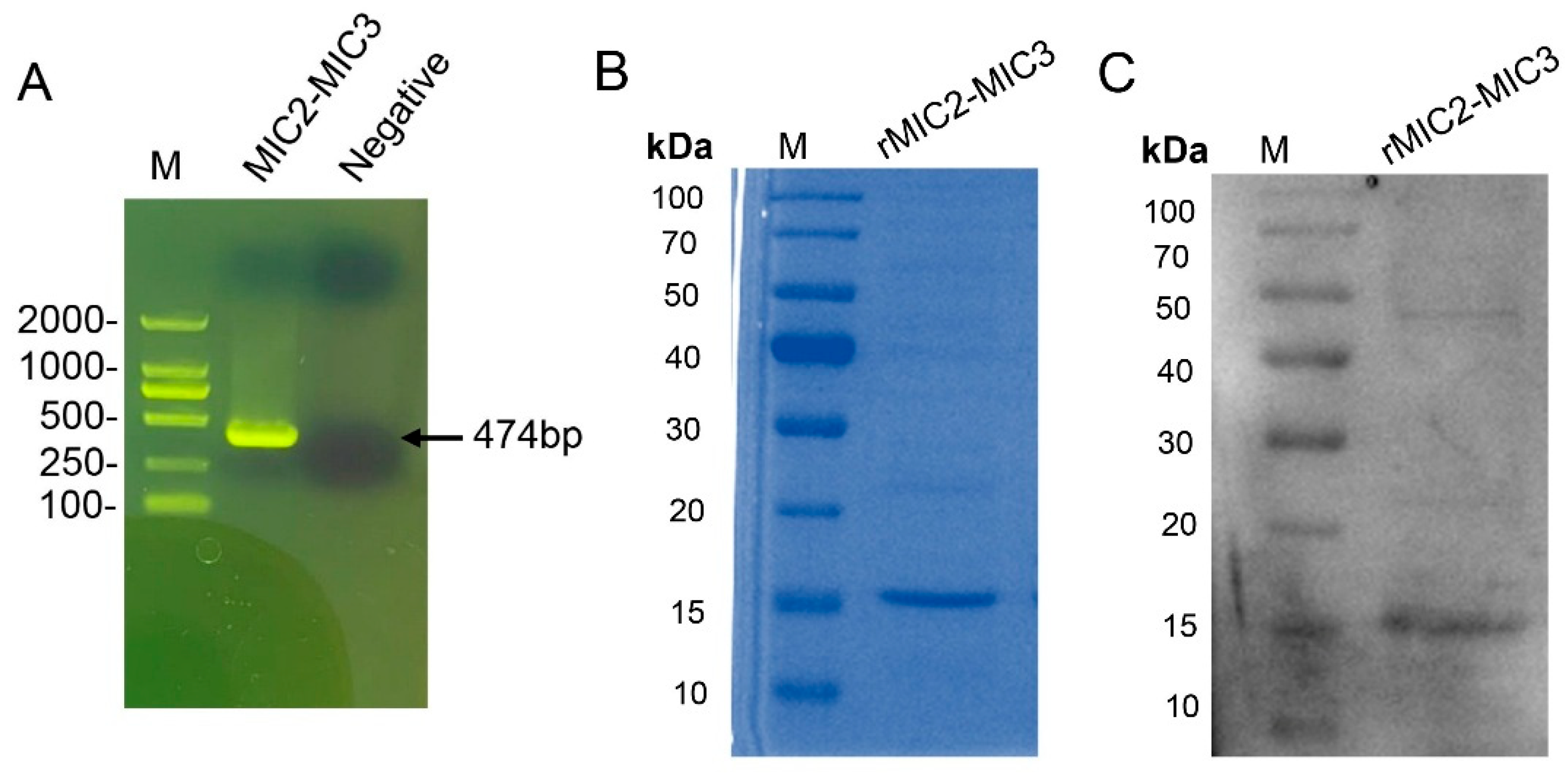
2.1. Preparation of the Recombinant Proteins
2.2. Preparation of Gold Conjugated Protein A
2.3. Preparation of the Immunochromatographic Strip
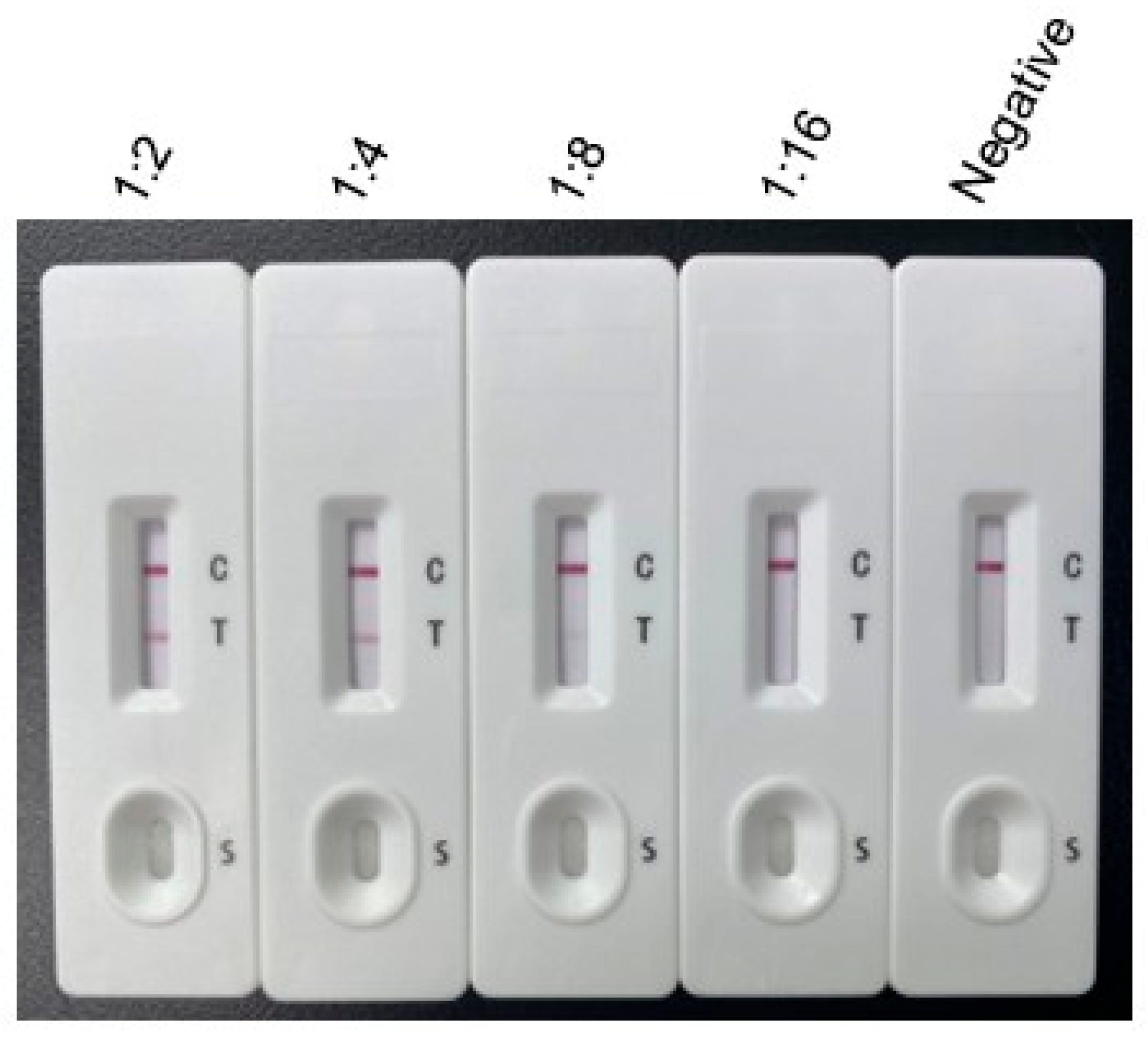
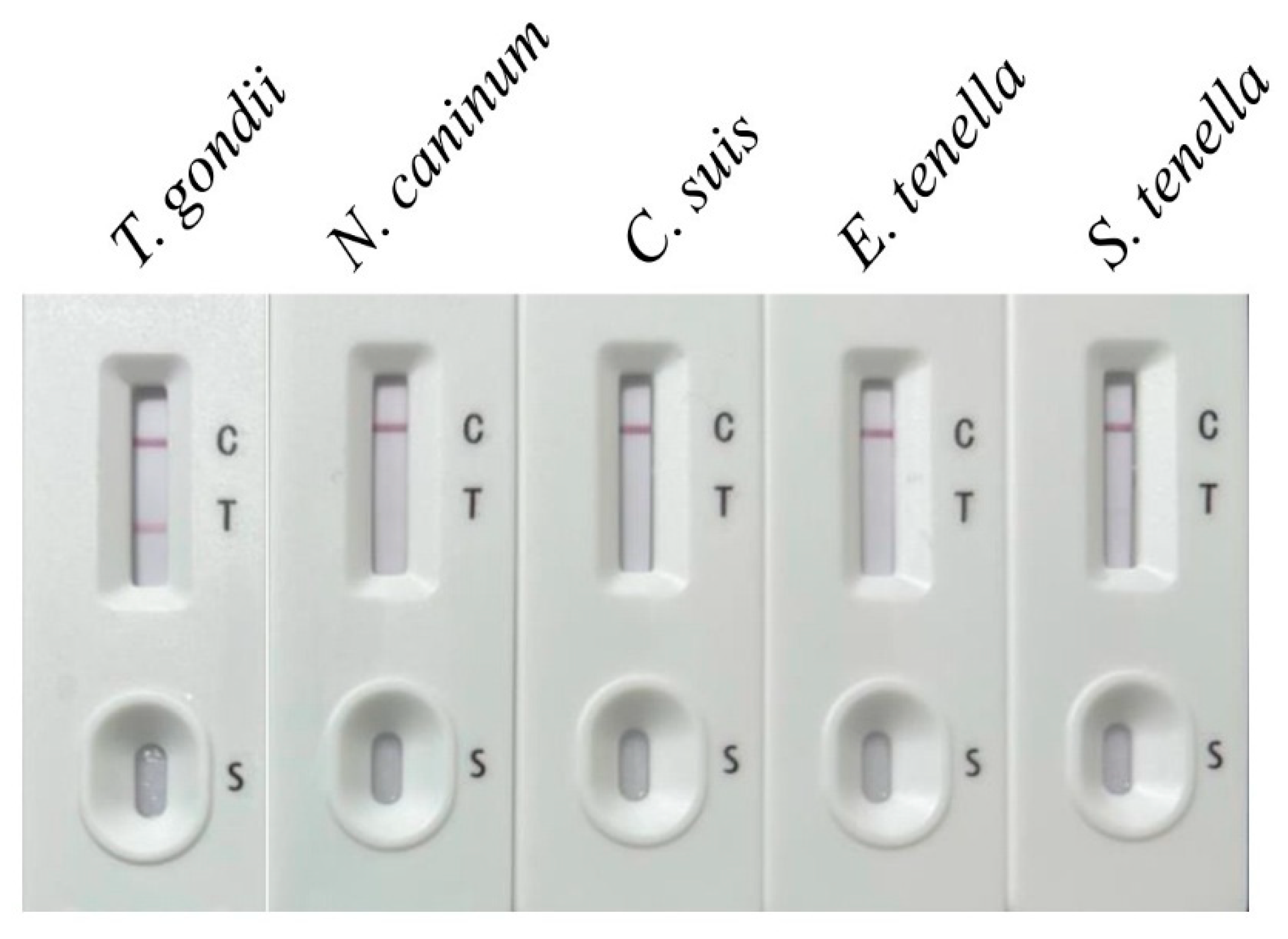
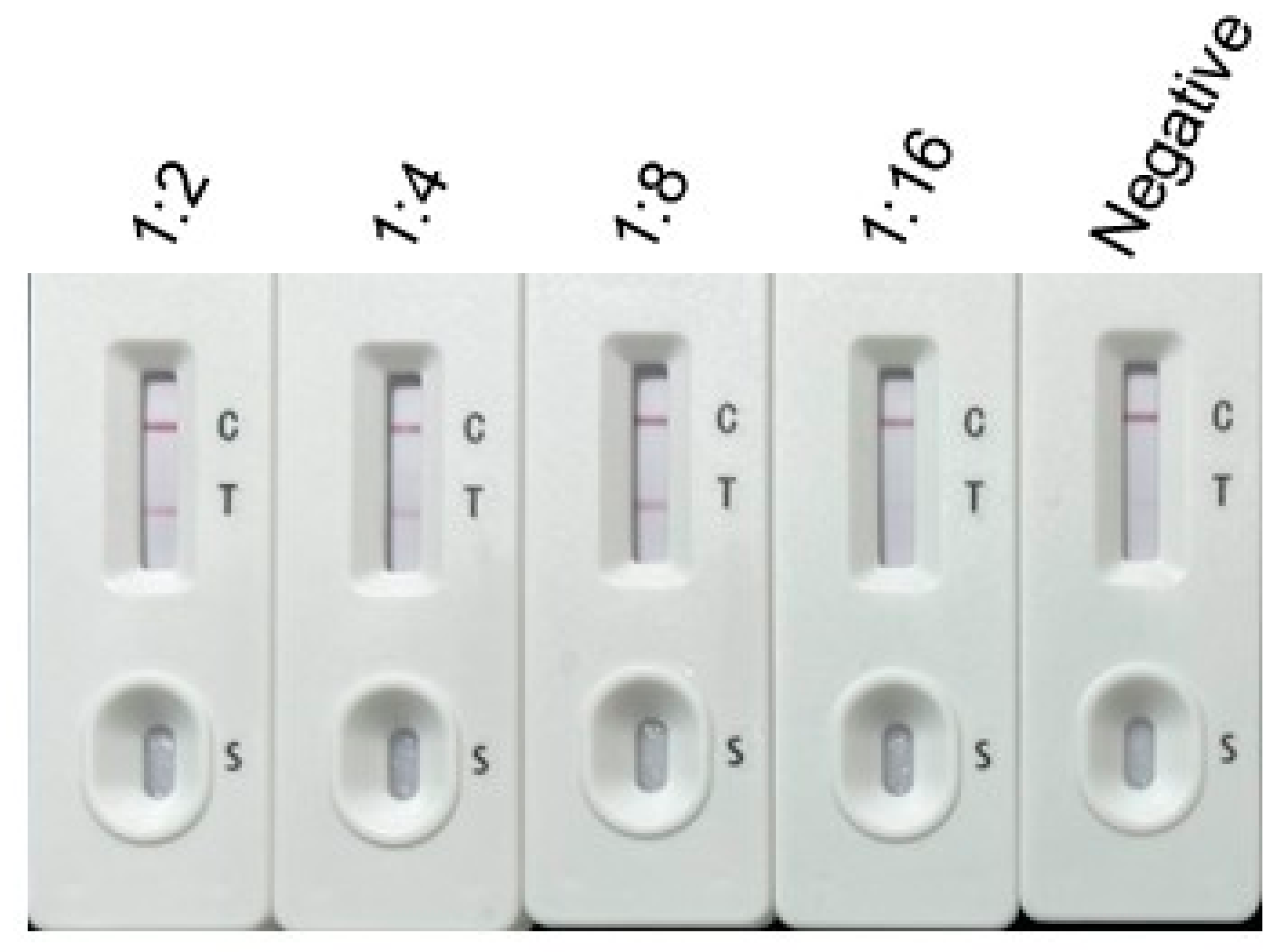
2.4. Sensitivity, Specificity, and Stability of the Immunochromatographic Test
2.5. Clinical Evaluation of the Colloidal Gold Test Strip
2.6. Statistical Analysis
3. Results
3.1. Production of MIC2-MIC3 Fusion Protein
3.2. Sensitivity, Specificity, and Stability of the Immunochromatographic Test
3.3. Comparative Analysis with Commercial ELISA Kit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lourido, S. Toxoplasma gondii. Trends Parasitol. 2019, 35, 944–945. [Google Scholar] [CrossRef]
- Molan, A.; Nosaka, K.; Wang, W.; Hunter, M.L.J.T.B. Global Status of Toxoplasma gondii Infection: Systematic Review and Prevalence Snapshots. Trop. Biomed. 2019, 36, 947–960. [Google Scholar]
- Pappas, G.; Roussos, N.; Falagas, M.E. Toxoplasmosis Snapshots: Global Status of Toxoplasma gondii Seroprevalence and Implications for Pregnancy and Congenital Toxoplasmosis. Int. J. Parasitol. 2009, 39, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii Infection in Humans and Animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.-C.; Zhou, C.-X.; Zhu, X.-Q. Research Advances in Interactions Related to Toxoplasma gondii Microneme Proteins. Exp. Parasitol. 2017, 176, 89–98. [Google Scholar] [CrossRef]
- Rezaei, F.; Sarvi, S.; Sharif, M.; Hejazi, S.H.; Pagheh, A.S.; Aghayan, S.A.; Daryani, A. A Systematic Review of Toxoplasma gondii Antigens to Find the Best Vaccine Candidates for Immunization. Microb. Pathog. 2019, 126, 172–184. [Google Scholar] [CrossRef]
- Wang, J.-L.; Zhang, N.-Z.; Li, T.-T.; He, J.-J.; Elsheikha, H.M.; Zhu, X.-Q. Advances in the Development of Anti-Toxoplasma gondii Vaccines: Challenges, Opportunities, and Perspectives. Trends Parasitol. 2019, 35, 239–253. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, H. Research Advances in Microneme Protein 3 of Toxoplasma gondii. Parasites Vectors 2015, 8, 384. [Google Scholar] [CrossRef]
- Zhang, K.; Lin, G.; Han, Y.; Li, J. Serological Diagnosis of Toxoplasmosis and Standardization. Clin. Chim. Acta 2016, 461, 83–89. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Dardé, M.L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Nakayama, T.; Zhao, J.; Takeuchi, D.; Kerdsin, A.; Chiranairadul, P.; Areeratana, P.; Loetthong, P.; Pienpringam, A.; Akeda, Y.; Oishi, K. Colloidal Gold-Based Immunochromatographic Strip Test Comprising Optimised Combinations of Anti-Streptococcus suis Capsular Polysaccharide Polyclonal Antibodies for Detection of Streptococcus suis. Biosens. Bioelectron. 2014, 60, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Sun, W.; Zhao, P.; Zhang, L.; Cai, D.; Cheng, Z.; Guo, H.; Liu, J.; Yang, D.; Wang, S.; et al. Development of Colloidal Gold-Based Immunochromatographic Assay for Rapid Detection of Mycoplasma suis in Porcine Plasma. Biosens. Bioelectron. 2014, 55, 396–399. [Google Scholar] [CrossRef]
- Huang, X.; Xuan, X.; Hirata, H.; Yokoyama, N.; Xu, L.; Suzuki, N.; Igarashi, I. Rapid Immunochromatographic Test Using Recombinant SAG2 for Detection of Antibodies against Toxoplasma gondii in Cats. J. Clin. Microbiol. 2004, 42, 351–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, J.; Sun, H.; Zhao, X.; Wang, S.; Zhuo, X.; Yang, Y.; Chen, X.; Yao, C.; Du, A. Development of an Immunochromatographic Test Based on Monoclonal Antibodies against Surface Antigen 3 (TgSAG3) for Rapid Detection of Toxoplasma gondii. Vet. Parasitol. 2018, 252, 52–57. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Kameyama, K.; Rasul, N.H.; Xuan, X.; Nishikawa, Y. Development of an Immunochromatographic Assay Based on Dense Granule Protein 7 for Serological Detection of Toxoplasma gondii Infection. Clin. Vaccine Immunol. 2013, 20, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Ybañez, R.H.D.; Kyan, H.; Nishikawa, Y. Detection of Antibodies against Toxoplasma gondii in Cats Using an Immunochromatographic Test Based on GRA7 Antigen. J. Vet. Med. Sci. 2020, 82, 441–445. [Google Scholar] [CrossRef]
- Minot, S.; Melo, M.B.; Li, F.; Lu, D.; Niedelman, W.; Levine, S.S.; Saeij, J.P. Admixture and Recombination among Toxoplasma gondii Lineages Explain Global Genome Diversity. Proc. Natl. Acad. Sci. USA 2012, 109, 13458–13463. [Google Scholar] [CrossRef]
- Ramakrishnan, C.; Maier, S.; Walker, R.A.; Rehrauer, H.; Joekel, D.E.; Winiger, R.R.; Basso, W.U.; Grigg, M.E.; Hehl, A.B.; Deplazes, P.; et al. An Experimental Genetically Attenuated Live Vaccine to Prevent Transmission of Toxoplasma gondii by Cats. Sci. Rep. 2019, 9, 1474. [Google Scholar] [CrossRef]
- Beghetto, E.; Nielsen, H.V.; Del Porto, P.; Buffolano, W.; Guglietta, S.; Felici, F.; Petersen, E.; Gargano, N. A Combination of Antigenic Regions of Toxoplasma gondii Microneme Proteins Induces Protective Immunity against Oral Infection with Parasite Cysts. J. Infect. Dis. 2005, 191, 637–645. [Google Scholar] [CrossRef]
- Liu, X.; Xiang, J.J.; Tang, Y.; Zhang, X.L.; Fu, Q.Q.; Zou, J.H.; Lin, Y. Colloidal Gold Nanoparticle Probe-Based Immunochromatographic Assay for the Rapid Detection of Chromium Ions in Water and Serum Samples. Anal. Chim. Acta 2012, 745, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Dakroub, H.; Sgroi, G.; D’Alessio, N.; Russo, D.; Serra, F.; Veneziano, V.; Rea, S.; Pucciarelli, A.; Lucibelli, M.G.; De Carlo, E.; et al. Molecular Survey of Toxoplasma gondii in Wild Mammals of Southern Italy. Pathogens 2023, 12, 471. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Karanis, P.; Fallahi, S. Advances in Serological, Imaging Techniques and Molecular Diagnosis of Toxoplasma gondii Infection. Infection 2018, 46, 303–315. [Google Scholar] [CrossRef]
- Holec-Gasior, L. Toxoplasma gondii Recombinant Antigens as Tools for Serodiagnosis of Human Toxoplasmosis: Current Status of Studies. Clin. Vaccine Immunol. 2013, 20, 1343–1351. [Google Scholar] [CrossRef]
- Gamble, H.R.; Andrews, C.D.; Dubey, J.P.; Webert, D.W.; Parmley, S.F. Use of Recombinant Antigens for Detection of Toxoplasma gondii Infection in Swine. J. Parasitol. 2000, 86, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Gong, D.; Ma, L.A.; Nie, H.; Zhou, Y.; Yao, B.; Zhao, J. Evaluation of a Recombinant MIC3-Based Latex Agglutination Test for the Rapid Serodiagnosis of Toxoplasma gondii Infection in Swine. Vet. Parasitol. 2008, 158, 51–56. [Google Scholar] [CrossRef]
- Ismael, A.B.; Sekkai, D.; Collin, C.; Bout, D.; Mévélec, M.N. The MIC3 Gene of Toxoplasma gondii Is a Novel Potent Vaccine Candidate against Toxoplasmosis. Infect. Immun. 2003, 71, 6222–6228. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.; Zhao, X.; Lin, M.; Chen, L.; Zhao, M.; Chen, X.; Yang, Y.; Ma, G.; Yao, C.; et al. Development of an Immunochromatographic Test Based on Rhoptry Protein 14 for Serological Detection of Toxoplasma gondii Infection in Swine. Animals 2022, 12, 1929. [Google Scholar] [CrossRef]
- Ferra, B.; Holec-Gąsior, L.; Kur, J. Serodiagnosis of Toxoplasma gondii Infection in Farm Animals (Horses, Swine, and Sheep) by Enzyme-Linked Immunosorbent Assay Using Chimeric Antigens. Parasitol. Int. 2015, 64, 288–294. [Google Scholar] [CrossRef]
- Fan, J.; Sun, H.; Fang, J.; Gao, Y.; Ding, H.; Zheng, B.; Kong, Q.; Zhuo, X.; Lu, S. Application of Gold Immunochromatographic Assay Strip Combined with Digital Evaluation for Early Detection of Toxoplasma gondii Infection in Multiple Species. Parasites Vectors 2024, 17, 81. [Google Scholar] [CrossRef]
- Song, K.J.; Yang, Z.; Chong, C.K.; Kim, J.S.; Lee, K.C.; Kim, T.S.; Nam, H.W. A Rapid Diagnostic Test for Toxoplasmosis Using Recombinant Antigenic N-Terminal Half of SAG1 Linked with Intrinsically Unstructured Domain of GRA2 Protein. Korean J. Parasitol. 2013, 51, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, Diagnosis and Prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.M.; Dubey, J.P. Toxoplasmosis: A History of Clinical Observations. Int. J. Parasitol. 2009, 39, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, E.; Bernardes, E.; Silva, N.; Mineo, J.; Panunto-Castelo, A.; Roque-Barreira, M. Immunization with MIC1 and MIC4 Induces Protective Immunity against Toxoplasma gondii. Microbes Infect. 2006, 8, 1244–1251. [Google Scholar] [CrossRef]

| ICT Strips | Positive | Negative | Total |
|---|---|---|---|
| Test Positive | 23 (a) | 2 (b) | 25 |
| Test Negative | 0 (c) | 12 (d) | 12 |
| Total | 23 | 14 | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, Y.; Qiu, J.; Liu, J.; Zhou, R.; Ma, X.; Wu, X.; Li, X.; Mao, W.; Liu, Y.; et al. Development of an Immunochromatographic Test with Recombinant MIC2-MIC3 Fusion Protein for Serological Detection of Toxoplasma gondii. Vet. Sci. 2025, 12, 509. https://doi.org/10.3390/vetsci12060509
Wang J, Zhao Y, Qiu J, Liu J, Zhou R, Ma X, Wu X, Li X, Mao W, Liu Y, et al. Development of an Immunochromatographic Test with Recombinant MIC2-MIC3 Fusion Protein for Serological Detection of Toxoplasma gondii. Veterinary Sciences. 2025; 12(6):509. https://doi.org/10.3390/vetsci12060509
Chicago/Turabian StyleWang, Jianzhong, Yi Zhao, Jicheng Qiu, Jing Liu, Rui Zhou, Xialin Ma, Xiaojie Wu, Xiaoguang Li, Wei Mao, Yiduo Liu, and et al. 2025. "Development of an Immunochromatographic Test with Recombinant MIC2-MIC3 Fusion Protein for Serological Detection of Toxoplasma gondii" Veterinary Sciences 12, no. 6: 509. https://doi.org/10.3390/vetsci12060509
APA StyleWang, J., Zhao, Y., Qiu, J., Liu, J., Zhou, R., Ma, X., Wu, X., Li, X., Mao, W., Liu, Y., & Zhang, H. (2025). Development of an Immunochromatographic Test with Recombinant MIC2-MIC3 Fusion Protein for Serological Detection of Toxoplasma gondii. Veterinary Sciences, 12(6), 509. https://doi.org/10.3390/vetsci12060509





