Evaluation of Plasma Nitric Oxide and Serum Endothelial Nitric Oxide Synthase in Pulmonary Hypertensive Dogs: A Clinical and Echocardiography Investigation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Animal Population
3.2. Hematological and Biochemical Data
3.3. Echocardiographic Data
3.3.1. Left Cardiac Parameters
3.3.2. Right Cardiac Parameters
- Right Cardiac Chamber Size and MPA: The right cardiac size, including the right atrial size (RAA index) and right ventricular size (RVEDA index), was significantly larger in dogs with PH (12.59 ± 6.21 cm2/m2 and 13.87 ± 5.50 cm2/m2, respectively) than in healthy dogs (4.86 ± 0.91 cm2/m2 and 5.07 ± 2.02 cm2/m2, respectively) (p < 0.001). Additionally, the main pulmonary artery diameter (MPA/Ao) in dogs with PH was greater than in healthy dogs (1.36 ± 0.33 vs. 0.90 ± 0.08) (p < 0.001).
- Estimated Systolic Pulmonary Artery Pressure (PAP) Parameters: The average TRmaxPG was significantly higher in dogs with PH (54.33 ± 18.93 mmHg) compared with healthy dogs (p < 0.001). However, tricuspid regurgitation was not observed in healthy dogs, and, therefore, TRmaxPG could not be assessed in this group using echocardiography. In contrast, the AT/ET ratio was significantly lower in dogs with PH (0.24 ± 0.07) than in healthy dogs (0.45 ± 0.03) (p < 0.001).
- Right Ventricular Function Parameters: Right ventricular systolic function, as assessed by the RV FAC, was significantly reduced in dogs with PH (46.86 ± 23.43%) compared with healthy dogs (64.77 ± 12.34%) (p < 0.05). Additionally, dogs with PH exhibited significantly lower pulmonary artery compliance, as indicated by the RPAD index, compared with healthy dogs (19.15 ± 10.05% vs. 47.66 ± 7.84%, respectively) (p < 0.001). Furthermore, the right ventricular afterload, assessed using PVR, was significantly increased in dogs with PH (2.05 ± 0.76) compared with healthy dogs (p < 0.001). In healthy dogs, PVR assessment was not possible due to the absence of measurable tricuspid regurgitation velocity.
3.3.3. Echocardiographic Parameters in Dogs with PH, with and Without Ascites
3.4. Plasma NO and Serum eNOS Levels
3.5. Correlation Between Plasma NO and Serum eNOS and Echocardiographic Parameters
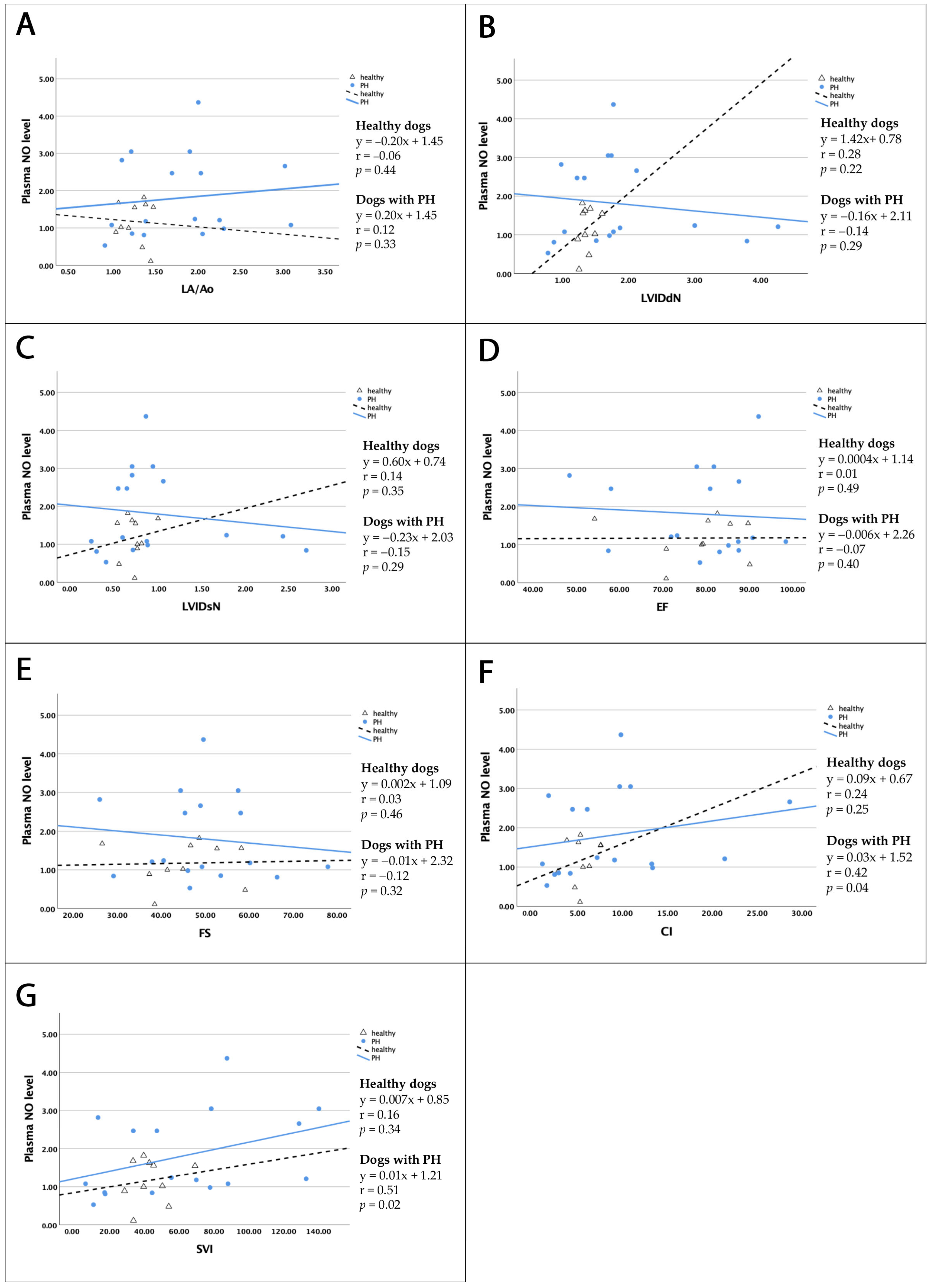
3.5.1. Correlation Between Plasma NO and Serum eNOS and Left Cardiac Parameters
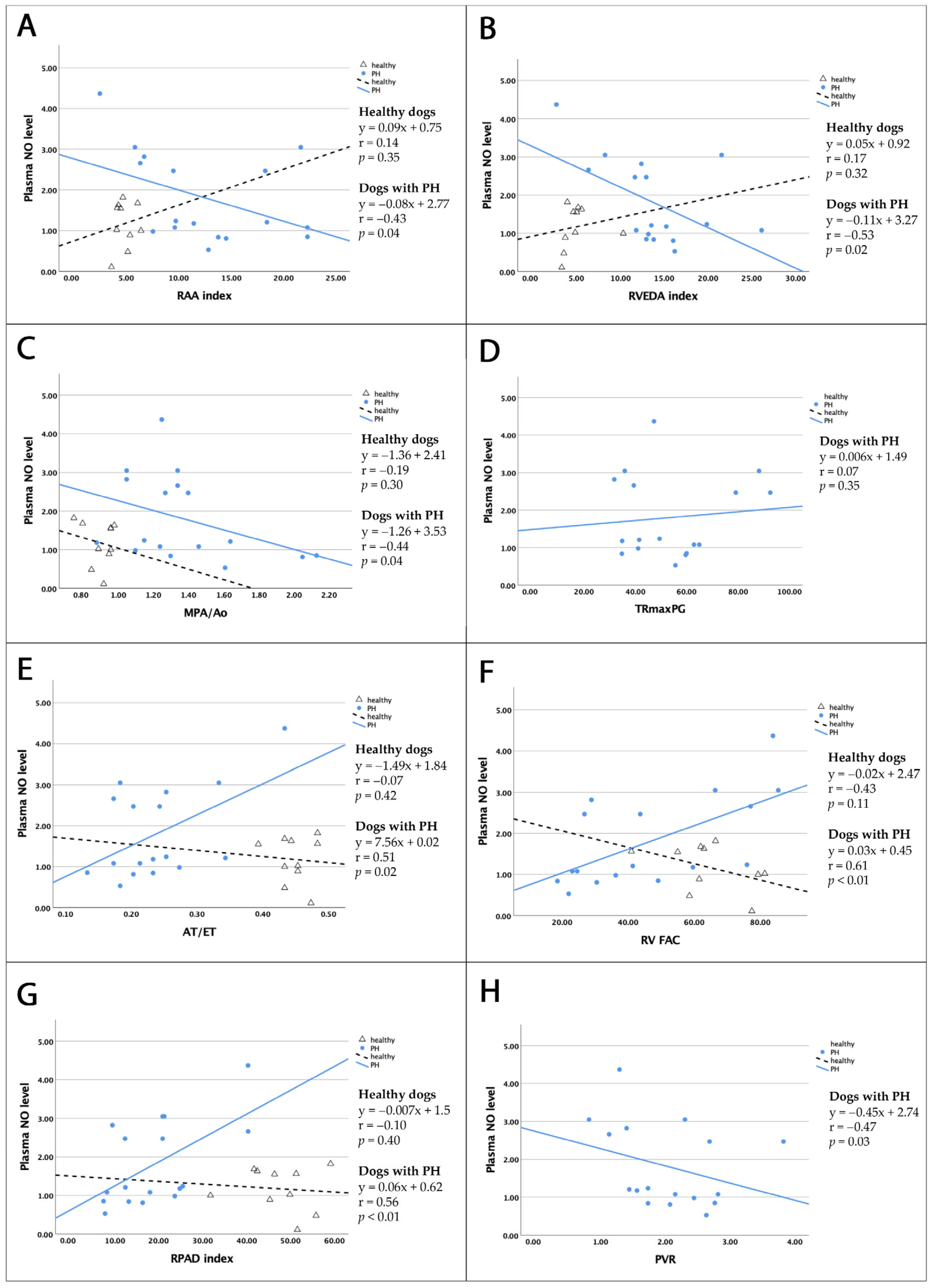
3.5.2. Correlation Between Plasma NO and Serum eNOS Levels and Right Cardiac Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM Consensus Statement Guidelines for the Diagnosis, Classification, Treatment, and Monitoring of Pulmonary Hypertension in Dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Boon, J.; Orton, E.C. Clinical Characteristics of 53 Dogs with Doppler-Derived Evidence of Pulmonary Hypertension: 1992–1996. J. Vet. Intern. Med. Am. Coll. Vet. Intern. Med. 1999, 13, 440–447. [Google Scholar] [CrossRef]
- Akabane, R.; Shimano, S.; Sakatani, A.; Ogawa, M.; Nagakawa, M.; Miyakawa, H.; Miyagawa, Y.; Takemura, N. Relationship between Right Heart Echocardiographic Parameters and Invasive Pulmonary Artery Pressures in Canine Models of Chronic Embolic Pulmonary Hypertension. J. Vet. Med. Sci. 2019, 81, 1485–1491. [Google Scholar] [CrossRef]
- Vezzosi, T.; Domenech, O.; Costa, G.; Marchesotti, F.; Venco, L.; Zini, E.; del Palacio, M.J.F.; Tognetti, R. Echocardiographic Evaluation of the Right Ventricular Dimension and Systolic Function in Dogs with Pulmonary Hypertension. J. Vet. Intern. Med. 2018, 32, 1541–1548. [Google Scholar] [CrossRef]
- Visser, L.C.; Scansen, B.A.; Schober, K.E.; Bonagura, J.D. Echocardiographic Assessment of Right Ventricular Systolic Function in Conscious Healthy Dogs: Repeatability and Reference Intervals. J. Vet. Cardiol. 2015, 17, 83–96. [Google Scholar] [CrossRef]
- Pariaut, R.; Saelinger, C.; Strickland, K.N.; Beaufrère, H.; Reynolds, C.A.; Vila, J. Tricuspid Annular Plane Systolic Excursion (TAPSE) in Dogs: Reference Values and Impact of Pulmonary Hypertension. J. Vet. Intern. Med. 2012, 26, 1083–1286. [Google Scholar] [CrossRef]
- Visser, L.C.; Im, M.K.; Johnson, L.R.; Stern, J.A. Diagnostic Value of Right Pulmonary Artery Distensibility Index in Dogs with Pulmonary Hypertension: Comparison with Doppler Echocardiographic Estimates of Pulmonary Arterial Pressure. J. Vet. Intern. Med. 2016, 30, 543–552. [Google Scholar] [CrossRef]
- Schober, K.E.; Baade, H. Doppler Echocardiographic Prediction of Pulmonary Hypertension in West Highland White Terriers with Chronic Pulmonary Disease. J. Vet. Intern. Med. 2006, 20, 912–920. [Google Scholar] [CrossRef]
- Vezzosi, T.; Domenech, O.; Iacona, M.; Marchesotti, F.; Zini, E.; Venco, L.; Tognetti, R. Echocardiographic Evaluation of the Right Atrial Area Index in Dogs with Pulmonary Hypertension. J. Vet. Intern. Med. 2018, 32, 42–47. [Google Scholar] [CrossRef]
- Gentile-Solomon, J.M.; Abbott, J.A. Conventional Echocardiographic Assessment of the Canine Right Heart: Reference Intervals and Repeatability. J. Vet. Cardiol. 2016, 18, 234–247. [Google Scholar] [CrossRef]
- Chen, K.; Pittman, R.N.; Popel, A.S. Nitric Oxide in the Vasculature: Where Does It Come from and Where Does It Go? A Quantitative Perspective. Antioxid. Redox Signal. 2008, 10, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Andrew, P.J.; Mayer, B. Enzymatic Function of Nitric Oxide Synthases. Cardiovasc. Res. 1999, 43, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.P.; Mittal, C.K.; Katsuki, S.; Murad, F. Nitric Oxide Activates Guanylate Cyclase and Increases Guanosine 3′:5′-Cyclic Monophosphate Levels in Various Tissue Preparations. Proc. Natl. Acad. Sci. USA 1977, 74, 3203–3207. [Google Scholar] [CrossRef]
- Kone, B.C.; Kuncewicz, T.; Zhang, W.; Yu, Z.Y. Protein Interactions with Nitric Oxide Synthases: Controlling the Right Time, the Right Place, and the Right Amount of Nitric Oxide. Am. J. Physiol. Renal Physiol. 2003, 285, F178–F190. [Google Scholar] [CrossRef]
- Klinger, J.R.; Kadowitz, P.J. The Nitric Oxide Pathway in Pulmonary Vascular Disease. Am. J. Cardiol. 2017, 120, S71–S79. [Google Scholar] [CrossRef]
- Sibal, L.; Agarwal, S.C.; Home, P.D.; Boger, R.H. The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dysfunction and Cardiovascular Disease. Curr. Cardiol. Rev. 2010, 6, 82–90. [Google Scholar] [CrossRef]
- Warwick, G.; Thomas, P.S.; Yates, D.H. Biomarkers in Pulmonary Hypertension. Eur. Respir. J. 2008, 32, 503–512. [Google Scholar] [CrossRef]
- Schäfer, M.; Kheyfets, V.O.; Schroeder, J.D.; Dunning, J.; Shandas, R.; Buckner, J.K.; Browning, J.; Hertzberg, J.; Hunter, K.S.; Fenster, B.E. Main Pulmonary Arterial Wall Shear Stress Correlates with Invasive Hemodynamics and Stiffness in Pulmonary Hypertension. Pulm. Circ. 2016, 6, 37–45. [Google Scholar] [CrossRef]
- Glaus, T.M.; Tomsa, K.; Hässig, M.; Reusch, C. Echocardiographic Changes Induced by Moderate to Marked Hypobaric Hypoxia in Dogs. Vet. Radiol. Ultrasound 2004, 45, 233–237. [Google Scholar] [CrossRef]
- Jaffey, J.A.; Wiggen, K.; Leach, S.B.; Masseau, I.; Girens, R.E.; Reinero, C.R. Pulmonary Hypertension Secondary to Respiratory Disease and/or Hypoxia in Dogs: Clinical Features, Diagnostic Testing and Survival. Vet. J. 2019, 251, 105347. [Google Scholar] [CrossRef]
- Kylhammar, D.; Rådegran, G. The Principal Pathways Involved in the in Vivo Modulation of Hypoxic Pulmonary Vasoconstriction, Pulmonary Arterial Remodelling and Pulmonary Hypertension. Acta Physiol. 2017, 219, 728–756. [Google Scholar] [CrossRef] [PubMed]
- Carretón, E.; Cerón, J.J.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Caro-Vadillo, A.; Montoya-Alonso, J.A. Acute Phase Proteins and Markers of Oxidative Stress to Assess the Severity of the Pulmonary Hypertension in Heartworm-Infected Dogs. Parasites Vectors 2017, 10, 477. [Google Scholar] [CrossRef]
- Price, L.C.; Wort, S.J.; Perros, F.; Dorfmüller, P.; Huertas, A.; Montani, D.; Cohen-Kaminsky, S.; Humbert, M. Inflammation in Pulmonary Arterial Hypertension. Chest 2012, 141, 210–221. [Google Scholar] [CrossRef]
- Klinger, J.R.; Abman, S.H.; Gladwin, M.T. Nitric Oxide Deficiency and Endothelial Dysfunction in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2013, 188, 639–646. [Google Scholar] [CrossRef]
- Tuder, R.M.; Cool, C.D.; Geraci, M.W.; Wang, J.; Abman, S.H.; Wright, L.; Badesch, D.; Voelkel, N.F. Prostacyclin Synthase Expression Is Decreased in Lungs from Patients with Severe Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 1999, 159, 1925–1932. [Google Scholar] [CrossRef]
- Freitas, C.F.; Faro, R.; Dragosavac, D.; Clozel, M.; De Nucci, G.; Antunes, E. Role of Endothelin-1 and Thromboxane A2 in the Pulmonary Hypertension Induced by Heparin-Protamine Interaction in Anesthetized Dogs. J. Cardiovasc. Pharmacol. 2004, 43, 106–112. [Google Scholar] [CrossRef]
- Kim, H.; Yung, G.L.; Marsh, J.J.; Konopka, R.G.; Pedersen, C.A.; Chiles, P.G.; Morris, T.A.; Channick, R.N. Endothelin Mediates Pulmonary Vascular Remodelling in a Canine Model of Chronic Embolic Pulmonary Hypertension. Eur. Respir. J. 2000, 15, 640–648. [Google Scholar] [CrossRef]
- Jonigk, D.; Golpon, H.; Bockmeyer, C.L.; Maegel, L.; Hoeper, M.M.; Gottlieb, J.; Nickel, N.; Hussein, K.; Maus, U.; Lehmann, U.; et al. Plexiform Lesions in Pulmonary Arterial Hypertension: Composition, Architecture, and Microenvironment. Am. J. Pathol. 2011, 179, 167–179. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, S.; Yan, H.; Cao, Y.; Zhang, X.; Wang, L.; Zhang, Z.; Lin, S.; Wang, X.; Mao, J. Pulmonary Vascular Remodeling in Pulmonary Hypertension. J. Pers. Med. 2023, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, K.; Smolders, V.F.E.D.; Tura-Ceide, O.; Wouter Jukema, J.; Quax, P.H.A.; Goumans, M.J. Endothelial Dysfunction in Pulmonary Hypertension: Cause or Consequence? Biomedicines 2021, 9, 57. [Google Scholar] [CrossRef]
- Stamler, J.S.; Loh, E.; Roddy, M.A.; Currie, K.E.; Creager, M.A. Nitric Oxide Regulates Basal Systemic and Pulmonary Vascular Resistance in Healthy Humans. Circulation 1994, 89, 2035–2040. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, F.T.; Arroliga, A.C.; Dweik, R.A.; Comhair, S.A.; Laskowski, D.; Oppedisano, R.; Thomassen, M.J.; Erzurum, S.C. Biochemical Reaction Products of Nitric Oxide as Quantitative Markers of Primary Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 1998, 158, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Giaid, A.; Saleh, D. Reduced Expression of Endothelial Nitric Oxide Synthase in the Lungs of Patients with Pulmonary Hypertension. N. Engl. J. Med. 1995, 333, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, S.; Lu, X.; Wang, M.; Liu, M. Gene Transfer of Endothelial Nitric Oxide Synthase Attenuates Flow-Induced Pulmonary Hypertension in Rabbits. Ann. Thorac. Surg. 2008, 85, 581–585. [Google Scholar] [CrossRef]
- Steudel, W.; Ichinose, F.; Huang, P.L.; Hurford, W.E.; Jones, R.C.; Bevan, J.A.; Fishman, M.C.; Zapol, W.M. Pulmonary Vasoconstriction and Hypertension in Mice with Targeted Disruption of the Endothelial Nitric Oxide Synthase (NOS 3) Gene. Circ. Res. 1997, 81, 34–41. [Google Scholar] [CrossRef]
- Resta, T.C.; Gonzales, R.J.; Dail, W.G.; Sanders, T.C.; Walker, B.R. Selective Upregulation of Arterial Endothelial Nitric Oxide Synthase in Pulmonary Hypertension. Am. J. Physiol. Heart Circ. Physiol. 1997, 41, H806–H813. [Google Scholar] [CrossRef]
- Fagan, K.A.; Morrissey, B.; Fouty, B.W.; Sato, K.; Harral, J.W.; Morris, K.G.; Hoedt-Miller, M.; Vidmar, S.; McMurtry, I.F.; Rodman, D.M. Upregulation of Nitric Oxide Synthase in Mice with Severe Hypoxia-Induced Pulmonary Hypertension. Respir. Res. 2001, 2, 306. [Google Scholar] [CrossRef]
- Santos-Gomes, J.; Gandra, I.; Adão, R.; Perros, F.; Brás-Silva, C. An Overview of Circulating Pulmonary Arterial Hypertension Biomarkers. Front. Cardiovasc. Med. 2022, 9, 924873. [Google Scholar] [CrossRef]
- Pedersen, H.D.; Schütt, T.; Søndergaard, R.; Qvortrup, K.; Olsen, L.H.; Kristensen, A.T. Decreased Plasma Concentration of Nitric Oxide Metabolites in Dogs with Untreated Mitral Regurgitation. J. Vet. Intern. Med. 2003, 17, 178–184. [Google Scholar] [CrossRef]
- Vail, D.M.; Thamm, D.H.; Liptak, J.M. Withrow and MacEwen’s Small Animal Clinical Oncology; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Haskins, S.; Pascoe, P.J.; Ilkiw, J.E.; Fudge, J.; Hopper, K.; Aldrich, J. Reference Cardiopulmonary Values in Normal Dogs. Comp. Med. 2005, 55, 156–161. [Google Scholar]
- Yuchi, Y.; Suzuki, R.; Yasumura, Y.; Saito, T.; Teshima, T.; Matsumoto, H.; Koyama, H. Prognostic Value of Pulmonary Vascular Resistance Estimated by Echocardiography in Dogs with Myxomatous Mitral Valve Disease and Pulmonary Hypertension. J. Vet. Intern. Med. 2023, 37, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Girgis, R.E.; Champion, H.C.; Diette, G.B.; Johns, R.A.; Permutt, S.; Sylvester, J.T. Decreased Exhaled Nitric Oxide in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2005, 172, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, X.J.; Zhang, H.D.; Sun, X.Q.; Zhao, Q.H.; Wang, L.; He, J.; Jiang, X.; Liu, J.M.; Jing, Z.C. Profiling Nitric Oxide Metabolites in Patients with Idiopathic Pulmonary Arterial Hypertension. Eur. Respir. J. 2016, 48, 1386–1395. [Google Scholar] [CrossRef]
- Tyler, R.C.; Muramatsu, M.; Abman, S.H.; Stelzner, T.J.; Rodman, D.M.; Bloch, K.D.; McMurtry, I.F. Variable Expression of Endothelial No Synthase in Three Forms of Rat Pulmonary Hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 1999, 276, L297–L303. [Google Scholar] [CrossRef]
- Le Cras, T.D.; Tyler, R.C.; Horan, M.P.; Morris, K.G.; Tuder, R.M.; McMurtry, I.F.; Johns, R.A.; Abman, S.H. Effects of Chronic Hypoxia and Altered Hemodynamics on Endothelial Nitric Oxide Synthase Expression in the Adult Rat Lung. J. Clin. Investig. 1998, 101, 795–801. [Google Scholar] [CrossRef]
- Hoffmann, A.; Gloe, T.; Pohl, U. Hypoxia-Induced Upregulation of ENOS Gene Expression Is Redox-Sensitive: A Comparison between Hypoxia and Inhibitors of Cell Metabolism. J. Cell Physiol. 2001, 188, 33–44. [Google Scholar] [CrossRef]
- Ellison, L.T.; Hall, D.P.; Yeh, T.J.; Mobarhan, H.; Rossi, J.H.; Ellison, R.G. Physiological Alterations in Increased Pulmonary Blood Flow with and without Pulmonary Hypertension. J. Appl. Physiol. 1961, 16, 305–308. [Google Scholar] [CrossRef]
- Deshmukh, D.R.; Ghole, V.S.; Marescau, B.; De Deyn, P.P. Effect of Endotoxemia on Plasma and Tissue Levels of Nitric Oxide Metabolites and Guanidino Compounds. Arch. Physiol. Biochem. 1997, 105, 32–37. [Google Scholar] [CrossRef]
- Dillioglugil, M.O.; Mekık, H.; Muezzinoglu, B.; Ozkan, T.A.; Demir, C.G.; Dillioglugil, O. Blood and Tissue Nitric Oxide and Malondialdehyde Are Prognostic Indicators of Localized Prostate Cancer. Int. Urol. Nephrol. 2012, 44, 1691–1696. [Google Scholar] [CrossRef]
- Huang, J.; Wolk, J.H.; Gewitz, M.H.; Mathew, R. Progressive Endothelial Cell Damage in an Inflammatory Model of Pulmonary Hypertension. Exp. Lung Res. 2010, 36, 57–66. [Google Scholar] [CrossRef]
- Gaynor, S.L.; Maniar, H.S.; Bloch, J.B.; Steendijk, P.; Moon, M.R. Right Atrial and Ventricular Adaptation to Chronic Right Ventricular Pressure Overload. Circulation 2005, 112, I212–I218. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Tognetti, R.; Domenech, O.; Della Pina, A.; Marchesotti, F.; Patata, V.; Vezzosi, T. Echocardiographic Evaluation of the Size of the Main Pulmonary Artery and Right Pulmonary Artery in Dogs with Pulmonary Hypertension. J. Vet. Intern. Med. 2025, 39, e17241. [Google Scholar] [CrossRef]
- Greenfield, J.C.; Griggs, D.M. Relation between Pressure and Diameter in Main Pulmonary Artery of Man. J. Appl. Physiol. 1963, 18, 557–559. [Google Scholar] [CrossRef]
- Kassem, E.; Humpl, T.; Friedberg, M.K. Pediatrics: Prognostic Significance of 2-Dimensional, m-Mode, and Doppler Echo Indices of Right Ventricular Function in Children with Pulmonary Arterial Hypertension. Am. Heart J. 2013, 165, 1024–1031. [Google Scholar] [CrossRef]
- Reuben, S.R. Compliance of the Human Pulmonary Arterial System in Disease. Circ. Res. 1971, 29, 40–50. [Google Scholar] [CrossRef]
- Dai, Z.K.; Tan, M.S.; Chai, C.Y.; Yeh, J.L.; Chou, S.H.; Chiu, C.C.; Jeng, A.Y.; Chen, I.J.; Wu, J.R. Upregulation of Endothelial Nitric Oxide Synthase and Endothelin-1 in Pulmonary Hypertension Secondary to Heart Failure in Aorta-Banded Rats. Pediatr. Pulmonol. 2004, 37, 249–256. [Google Scholar] [CrossRef]
- Khadour, F.H.; O’Brien, D.W.; Fu, Y.; Armstrong, P.W.; Schulz, R. Endothelial Nitric Oxide Synthase Increases in Left Atria of Dogs with Pacing-Induced Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 1998, 275, H1971–H1978. [Google Scholar] [CrossRef]
- Agnoletti, L.; Curello, S.; Bachetti, T.; Malacarne, F.; Gaia, G.; Comini, L.; Volterrani, M.; Bonetti, P.; Parrinello, G.; Cadei, M.; et al. Serum from Patients with Severe Heart Failure Downregulates ENOS and Is Proapoptotic. Role of Tumor Necrosis Factor-α. Circulation 1999, 100, 1983–1991. [Google Scholar] [CrossRef]
- Loh, E.; Lankford, E.B.; Polidori, D.J.; Doering-Lubit, E.B.; Hanson, C.W.; Acker, M.A. Cardiovascular Effects of Inhaled Nitric Oxide in a Canine Model of Cardiomyopathy. Ann. Thorac. Surg. 1999, 67, P1380–P1385. [Google Scholar] [CrossRef]
- Celant, L.R.; Wessels, J.N.; Kianzad, A.; Marcus, J.T.; Meijboom, L.J.; Bogaard, H.J.; De Man, F.S.; Vonk Noordegraaf, A. Restoration of Right Ventricular Function in the Treatment of Pulmonary Arterial Hypertension. Heart 2023, 109, 1844–1850. [Google Scholar] [CrossRef]
- Roberts, J.D.; Roberts, C.T.; Jones, R.C.; Zapol, W.M.; Bloch, K.D. Continuous Nitric Oxide Inhalation Reduces Pulmonary Arterial Structural Changes, Right Ventricular Hypertrophy, and Growth Retardation in the Hypoxic Newborn Rat. Circ. Res. 1995, 76, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.P.; Bittner, H.B.; Tull, F.; Craig, D.; Davis, R.D.; Van Trigt, P. Nitric Oxide Improves Pulmonary Vascular Impedance, Transpulmonary Efficiency, and Left Ventricular Filling in Chronic Pulmonary Hypertension. J. Thorac. Cardiovasc. Surg. 1997, 113, 849–857. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jardim, C.; Rochitte, C.E.; Humbert, M.; Rubenfeld, G.; Jasinowodolinski, D.; Carvalho, C.R.R.; Souza, R. Pulmonary Artery Distensibility in Pulmonary Arterial Hypertension: An MRI Pilot Study. Eur. Respir. J. 2007, 29, 476–481. [Google Scholar] [CrossRef]
- Tidholm, A.; Höglund, K.; Häggström, J.; Ljungvall, I. Diagnostic Value of Selected Echocardiographic Variables to Identify Pulmonary Hypertension in Dogs with Myxomatous Mitral Valve Disease. J. Vet. Intern. Med. 2015, 29, 1510–1517. [Google Scholar] [CrossRef]
- Pyle, R.L.; Abbott, J.A.; MacLean, H. Pulmonary Hypertension and Cardiovascular Sequelae in 54 Dogs. Int. J. Appl. Res. Vet. Med. 2004, 2, 99–109. [Google Scholar]
- Yuchi, Y.; Suzuki, R.; Saito, T.; Yasumura, Y.; Teshima, T.; Matsumoto, H.; Koyama, H. Echocardiographic Characteristics of Dogs with Pulmonary Hypertension Secondary to Respiratory Diseases. J. Vet. Intern. Med. 2023, 37, 1656–1666. [Google Scholar] [CrossRef]
- Mazzotta, E.; Guglielmini, C.; Menciotti, G.; Contiero, B.; Baron Toaldo, M.; Berlanda, M.; Poser, H. Red Blood Cell Distribution Width, Hematology, and Serum Biochemistry in Dogs with Echocardiographically Estimated Precapillary and Postcapillary Pulmonary Arterial Hypertension. J. Vet. Intern. Med. 2016, 30, 1806–1815. [Google Scholar] [CrossRef]
| Cardiac Evaluation | Echocardiographic Parameters |
|---|---|
| (1) Left cardiac dimension | LA/Ao LV parameter (IVS, LVID, LVPW) during diastole and systole, normalized using Cornell’s formula |
| (2) Right cardiac dimension | RAA and RVEDA index MPA/Ao |
| (3) LV systolic function | FS, EF |
| (4) Hemodynamic parameters | HR, CO, CI, SV, SVI |
| (5) Right cardiac function | Estimated sPAP: AT/ET and TRmaxPG RV contraction: RV FAC PA compliance: RPAD index RV afterload: PVR |
| Clinical Data | Dogs with PH Number of Dogs; (%) | ||
|---|---|---|---|
| Ascites PH (n = 11; 61.11%) | Non-Ascites PH (n = 6; 38.89%) | Total (n = 17; 100%) | |
| PH probability | |||
| (1) Intermediate | 1; (5.88%) | 3; (17.64%) | 4; (23.53%) |
| (2) High | 10; (58.82%) | 3; (17.64%) | 13; (76.47%) |
| Classification | |||
| (1) Idiopathic | 0 | 0 | 0 |
| (2) Left-sided heart disease | 5; (29.41%) | 3; (17.64%) | 8; (47.06%) |
| (3) Chronic respiratory diseases | 1; (5.88%) | 1; (5.88%) | 2; (11.76%) |
| (4) Pulmonary thromboembolism | 0 | 0 | 0 |
| (5) Parasitic diseases | 1; (5.88%) | 0 | 1; (5.88%) |
| (6) Multifactorial or unclear mechanisms | 4; (23.53%) | 2; (11.76%) | 6; (35.29%) |
| Clinical signs | |||
| (1) Decreased appetite | 11; (64.71%) | 3; (17.64%) | 14; (82.35%) |
| (2) Cough | 6; (35.29%) | 6; (35.29%) | 12; (70.59%) |
| (3) Dyspnea | 8; (47.06%) | 3; (17.64%) | 11; (64.71%) |
| (4) Exercise intolerance | 11; (64.71%) | 6; (35.29%) | 17; (100%) |
| (5) Weight loss | 5; (29.41%) | 2; (11.76%) | 7; (41.18%) |
| (6) Syncope | 3; (17.64%) | 1; (5.88%) | 4; (23.53%) |
| (7) Right-sided congestive heart failure | 11; (64.71%) | 0 | 11; (64.71%) |
| (8) Left-sided congestive heart failure | 3; (17.64%) | 2; (11.76%) | 5; (29.41%) |
| Echocardiographic Parameters | Healthy Dogs (n = 10) | Dog with PH (n = 17) |
|---|---|---|
| LA/Ao | 1.27 ± 0.16 | 1.80 ± 0.65 * |
| IVSdN (cm) | 0.39 ± 0.06 | 0.47 ± 0.13 |
| LVIDdN (cm) | 1.38 ± 0.11 | 1.86 ± 0.98 * |
| LVPWdN (cm) | 0.37 ± 0.05 | 0.49 ± 0.14 * |
| IVSsN (cm) | 0.60 ± 0.08 | 0.74 ± 0.30 |
| LVIDsN (cm) | 0.72 ± 0.13 | 0.96 ± 0.70 |
| LVPWsN (cm) | 0.61 ± 0.10 | 0.78 ± 0.20 * |
| EF (%) | 78.46 ± 10.75 | 79.06 ± 13.46 |
| FS (%) | 45.60 ± 9.99 | 49.44 ± 12.74 |
| HR (bpm) | 127.48 ± 26.25 | 150.73 ± 28.63 * |
| CO (L/min) | 1.76 ± 0.75 | 2.89 ± 2.15 * |
| CI (L/min/m2) | 5.50 ± 1.49 | 8.79 ± 7.43 * |
| SV (ml) | 13.67 ± 4.66 | 19.26 ± 12.55 |
| SVI (mL/beat/m2) | 44.11 ± 11.84 | 62.16 ± 44.00 |
| Echocardiographic Parameters | Healthy Dogs (n = 10) | Dogs with PH (n = 17) |
|---|---|---|
| RAA index (cm2/m2) | 4.86 ± 0.91 | 12.59 ± 6.21 * |
| RVEDA index (cm2/m2) | 5.07 ± 2.02 | 13.87 ± 5.50 * |
| MPA/Ao | 0.90 ± 0.08 | 1.36 ± 0.33 * |
| TRmaxPG (mmHg) | not measurable | 54.33 ± 18.93 * |
| AT/ET | 0.45 ± 0.03 | 0.24 ± 0.07 * |
| RV FAC (%) | 64.77 ± 12.34 | 46.86 ± 23.43 * |
| RPAD index (%) | 47.66 ± 7.84 | 19.15 ± 10.05 * |
| PVR | not measurable | 2.05 ± 0.76 * |
| Echocardiographic Parameters | Ascites PH (n = 11) | Non-Ascites PH (n = 6) | |
|---|---|---|---|
| LA and LV size | LA/Ao | 1.73 ± 0.68 | 1.92 ± 0.63 |
| LVIDdN (cm) | 1.76 ± 1.17 | 2.04 ± 0.50 | |
| Left cardiac hemodynamic parameter | CI (L/min) | 6.68 ± 6.55 | 12.65 ± 7.96 * |
| SVI (mL/beat/m2) | 44.89 ± 39.98 | 93.81 ± 33.77 * | |
| RA and RV size | RAA index (cm2/m2) | 14.21 ± 5.58 | 9.62 ± 6.70 |
| RVEDA index (cm2/m2) | 14.68 ± 4.10 | 12.40 ± 7.70 | |
| MPA size | MPA/Ao | 1.48 ± 0.35 | 1.17 ± 0.18 * |
| Estimated sPAP | TRmaxPG (mmHg) | 56.99 ± 18.72 | 49.44 ± 20.05 |
| AT/ET | 0.22 ± 0.06 | 0.27 ± 0.10 | |
| Right cardiac function | RV FAC (%) | 31.56 ± 9.91 | 74.92 ± 10.04 * |
| RPAD index (%) | 13.78 ± 5.50 | 29.01 ± 9.08 * | |
| PVR | 2.37 ± 0.70 | 1.48 ± 0.52 * | |
| Healthy Dogs (n = 10) | Dogs with PH (n = 17) | |
|---|---|---|
| Plasma NO (mol/L) | 1.17 ± 0.57 | 1.80 ± 1.10 * |
| eNOS (U/mL) | 265.15 ± 128.65 | 382.55 ± 101.42 * |
| Ascites PH Dogs (n = 11) | Non-Ascites PH Dogs (n = 6) | |
|---|---|---|
| Plasma NO (mol/L) | 1.37 ± 0.80 | 2.59 ± 1.21 * |
| eNOS (U/mL) | 366.15 ± 70.83 | 412.63 ± 145.62 |
| r in Healthy Dogs | p-Value | r in Dogs with PH | p-Value | |
|---|---|---|---|---|
| LA/Ao | −0.06 | 0.44 | 0.12 | 0.33 |
| LVIDdN | 0.28 | 0.22 | −0.14 | 0.29 |
| LVIDsN | 0.14 | 0.35 | −0.15 | 0.29 |
| EF | 0.01 | 0.49 | −0.07 | 0.40 |
| FS | 0.03 | 0.46 | −0.12 | 0.32 |
| CI | 0.24 | 0.25 | 0.42 | 0.04 ** |
| SVI | 0.16 | 0.34 | 0.51 | 0.02 ** |
| r in Healthy Dogs | p-Value | r in Dogs with PH | p-Value | |
|---|---|---|---|---|
| LA/Ao | 0.02 | 0.48 | −0.07 | 0.49 |
| LVIDdN | −0.06 | 0.44 | −0.13 | 0.28 |
| LVIDsN | 0.31 | 0.19 | −0.23 | 0.28 |
| EF | −0.31 | 0.19 | 0.20 | 0.29 |
| FS | −0.36 | 0.16 | 0.33 | 0.25 |
| CI | −0.27 | 0.22 | −0.03 | 0.49 |
| SVI | −0.23 | 0.27 | 0.23 | 0.10 |
| r in Healthy Dogs | p-Value | r in Dogs with PH | p-Value | |
|---|---|---|---|---|
| RAA index | 0.14 | 0.35 | −0.43 | 0.04 * |
| RVEDA index | 0.17 | 0.32 | −0.53 | 0.02 * |
| MPA/Ao | −0.19 | 0.30 | −0.44 | 0.04 ** |
| TRmaxPG | - | - | 0.07 | 0.35 |
| AT/ET | −0.07 | 0.42 | 0.51 | 0.02 * |
| RV FAC | −0.43 | 0.11 | 0.61 | <0.01 * |
| RPAD index | −0.10 | 0.40 | 0.56 | <0.01 * |
| PVR | - | - | −0.47 | 0.03 ** |
| r in Healthy Dogs | p-Value | r in Dogs with PH | p-Value | |
|---|---|---|---|---|
| RAA index | 0.41 | 0.12 | 0.26 | 0.22 |
| RVEDA index | 0.39 | 0.14 | 0.25 | 0.21 |
| MPA/Ao | −0.51 | 0.07 | 0.43 | 0.06 |
| TRmaxPG | - | - | 0.39 | 0.08 |
| AT/ET | −0.07 | 0.43 | −0.13 | 0.35 |
| RV FAC | 0.56 | 0.05 | 0.11 | 0.26 |
| RPAD index | −0.19 | 0.30 | −0.01 | 0.47 |
| PVR | - | - | 0.18 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rattanakanokchai, S.; Fungbun, N.; Senaphan, K.; Jitpean, S.; Ployngam, T. Evaluation of Plasma Nitric Oxide and Serum Endothelial Nitric Oxide Synthase in Pulmonary Hypertensive Dogs: A Clinical and Echocardiography Investigation. Vet. Sci. 2025, 12, 486. https://doi.org/10.3390/vetsci12050486
Rattanakanokchai S, Fungbun N, Senaphan K, Jitpean S, Ployngam T. Evaluation of Plasma Nitric Oxide and Serum Endothelial Nitric Oxide Synthase in Pulmonary Hypertensive Dogs: A Clinical and Echocardiography Investigation. Veterinary Sciences. 2025; 12(5):486. https://doi.org/10.3390/vetsci12050486
Chicago/Turabian StyleRattanakanokchai, Siwayu, Numfa Fungbun, Ketmanee Senaphan, Supranee Jitpean, and Trasida Ployngam. 2025. "Evaluation of Plasma Nitric Oxide and Serum Endothelial Nitric Oxide Synthase in Pulmonary Hypertensive Dogs: A Clinical and Echocardiography Investigation" Veterinary Sciences 12, no. 5: 486. https://doi.org/10.3390/vetsci12050486
APA StyleRattanakanokchai, S., Fungbun, N., Senaphan, K., Jitpean, S., & Ployngam, T. (2025). Evaluation of Plasma Nitric Oxide and Serum Endothelial Nitric Oxide Synthase in Pulmonary Hypertensive Dogs: A Clinical and Echocardiography Investigation. Veterinary Sciences, 12(5), 486. https://doi.org/10.3390/vetsci12050486





