Force Plate Gait Analysis in Dogs After Femoral Head and Neck Excision
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedure
2.3. Study Protocol
2.4. Outcome Measures
2.4.1. Ground Reaction Force Measurement: Peak Vertical Force (PVF)
2.4.2. Hip Joint Range of Motion
2.4.3. Orthopedic Assessment Scores (OAS)
2.5. Statistical Analyses
3. Results
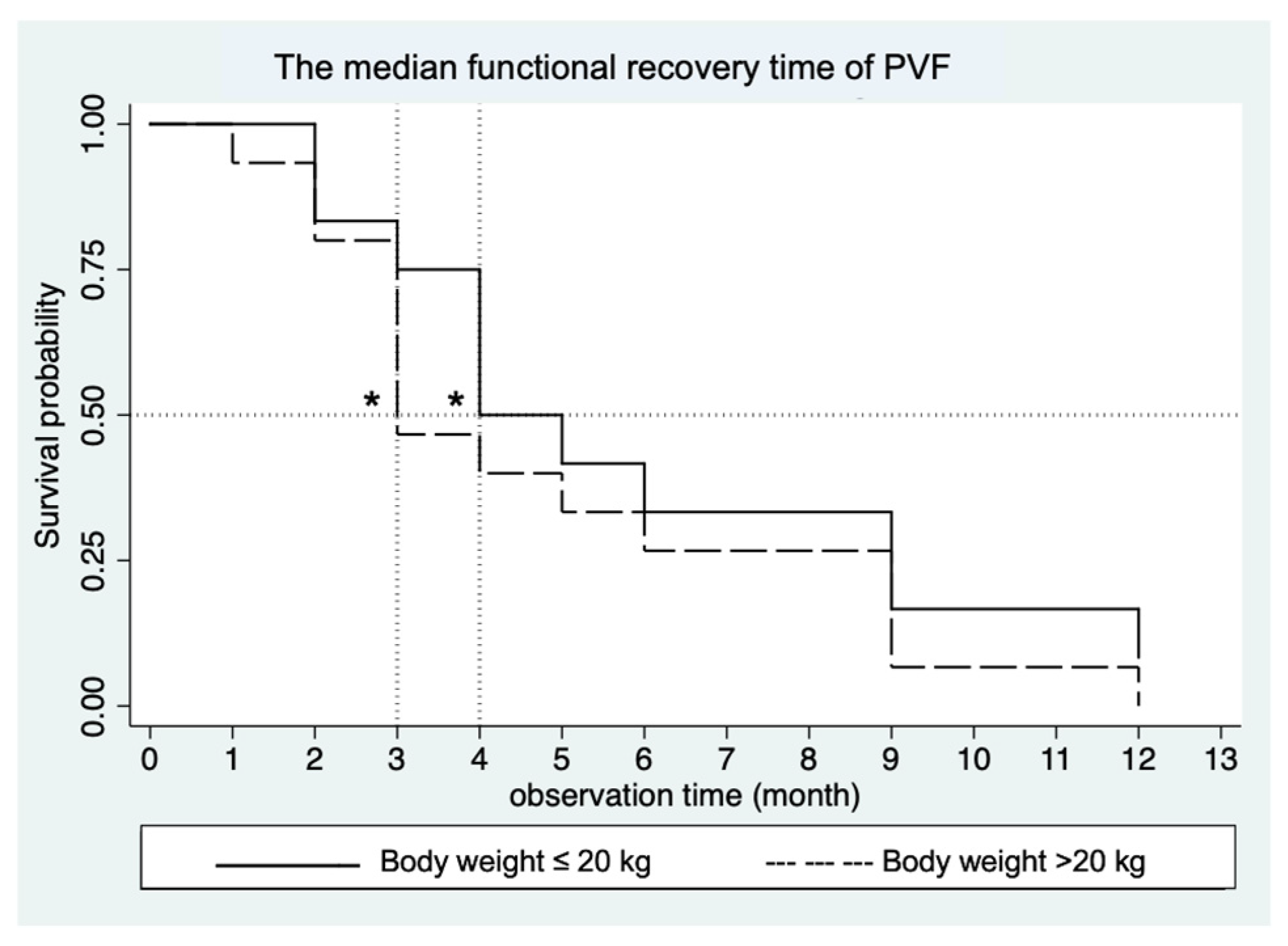
3.1. Force Plate Gait Analysis: Peak Vertical Force (PVF)
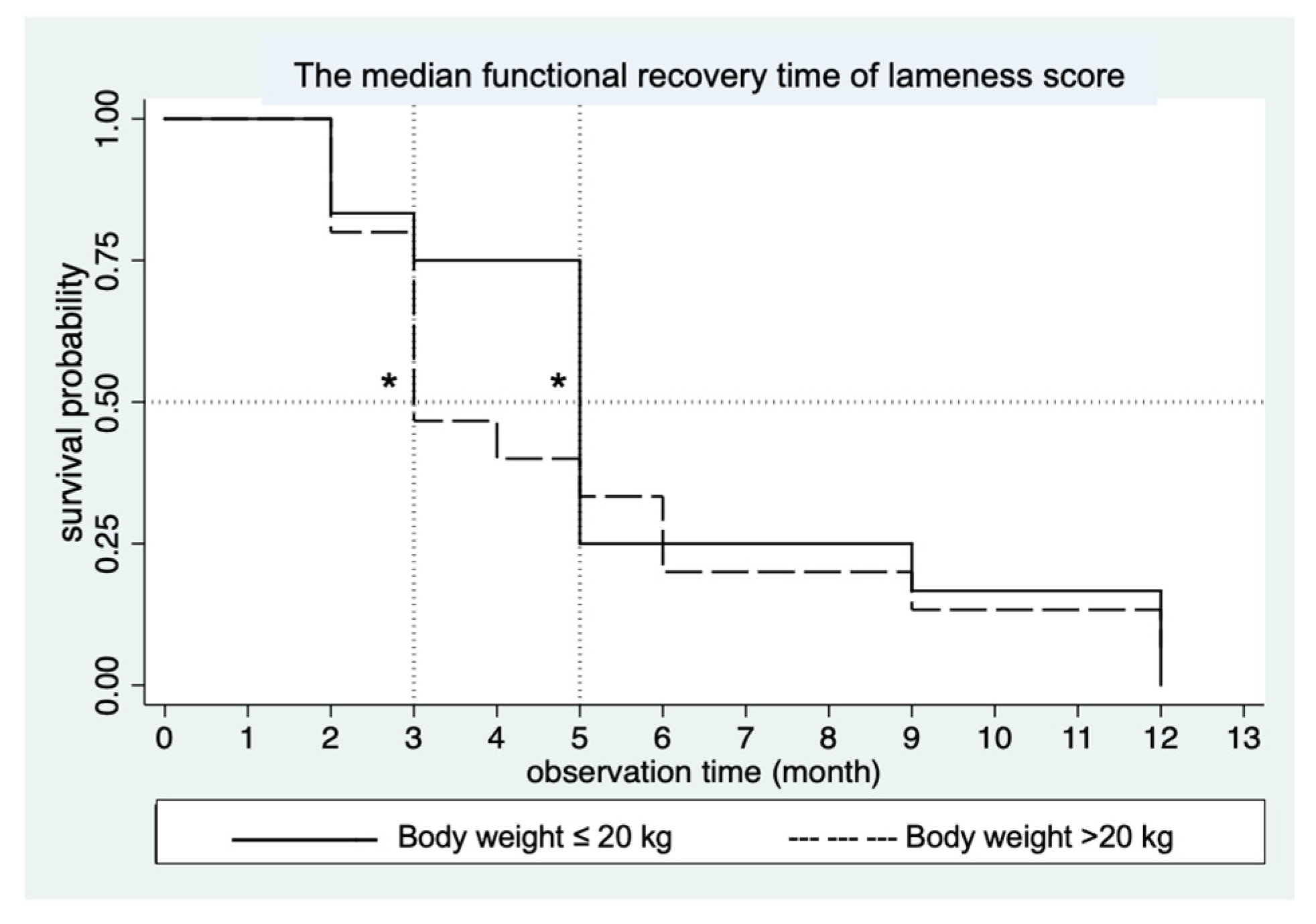
3.2. Orthopedic Assessment Scores (OAS)
3.3. The Range of Motion (ROM) of Hip Joint Flexion and Extension
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCS | Body condition score |
| BW | Body weight |
| CBPI | Canine Brief Pain Inventory |
| CMIs | Clinical metrology instruments |
| FP | Force plate gait analysis |
| FHNE | Femoral head and neck excision |
| FHO | Femoral head and neck osteotomy |
| GRFs | Ground reaction force measurements |
| HD | Hip dysplasia |
| NOL | Non-operated limb |
| OL | Operated limb |
| OAS | Orthopedic assessment scores |
| OA | Osteoarthritis |
| PVF | Peak vertical force |
| ROM | Range of motion |
| SI | Symmetry indices |
| THR | Total hip replacement |
| VTH | Veterinary Teaching Hospital |
| VI | Vertical impulse |
References
- Regina, A.M.d.l.S.; Oronan Rey, B.; Marco, F. Reyes Age, Weight, Breed, and Trauma as Risk Factors for Coxofemoral Luxation in Dogs. Philipp. J. Vet. Med. 2021, 58, 159–166. [Google Scholar]
- Allen, M.J. Advances in Total Joint Replacement in Small Animals. J. Small Anim. Pract. 2012, 53, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Hummel, D. Zurich Cementless Total Hip Replacement. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 917–934. [Google Scholar] [CrossRef]
- Olmstead, M.L.; Hohn, R.B.; Turner, T.M. Technique for Canine Total Hip Replacement. Vet. Surg. 1981, 10, 44–50. [Google Scholar] [CrossRef]
- Schulz, K.S. Application of Arthroplasty Principles to Canine Cemented Total Hip Replacement. Vet. Surg. 2000, 29, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Off, W.; Matis, U. Excision Arthroplasty of the Hip Joint in Dogs and Cats. Vet. Comp. Orthop. Traumatol. 2010, 23, 297–305. [Google Scholar] [CrossRef]
- Manley, P.A.; Vanderby, R.; Dogan, S.; Kohles, S.S.; McBeath, A.A. Ground Reaction Force Comparison of Canine Cemented and Cementless Total Hip Replacement. Clin. Biomech. 1990, 5, 199–204. [Google Scholar] [CrossRef]
- Piermattei, D.L. Excision Arthroplasty of the Hip Joint in Dogs and Cats. Vet. Comp. Orthop. Traumatol. 2011, 24, 89. [Google Scholar] [CrossRef]
- Engstig, M.; Vesterinen, S.; Morelius, M.; Junnila, J.; Hyytiäinen, H.K. Effect of Femoral Head and Neck Osteotomy on Canines’ Functional Pelvic Position and Locomotion. Animals 2022, 12, 1631. [Google Scholar] [CrossRef]
- Goatsang, N.; Niyatiwatchanchai, N.; Thengchaisri, N. Early Report on a Comparison Study between Femoral Head and Neck Excision without or with Single Sling Suture Method in Hip Luxated Dogs. In Proceedings of the 47th Kasetsart University Annual Conference: Veterinary Medicine, Bangkok, Thailand, 17–20 March 2009; KC4703005, 24-30. pp. 24–30. [Google Scholar]
- Olmstead, M.L. Disabling Conditions of Canine Coxofemoral Joint. In Small Animal Orthopedics; Olmstead, M.L., Ed.; Mosby: St Louis, MI, USA, 1995; p. 591. [Google Scholar]
- Ober, C.; Pestean, C.; Bel, L.; Taulescu, M.; Milgram, J.; Todor, A.; Ungur, R.; Leşu, M.; Oana, L. Use of Clinical and Computed Tomography Findings to Assess Long-Term Unsatisfactory Outcome after Femoral Head and Neck Ostectomy in Four Large Breed Dogs. Acta Vet. Scand. 2018, 60, 28. [Google Scholar] [CrossRef]
- Sabiza, S.; Ronagh, A.; Khajeh, A. Effective Medical Management and Physiotherapy Program of Femoral Head and Neck Ostectomy in 24 Dogs and Cats; Clinical Report. Iran. J. Vet. Surg. 2019, 14, 78–84. [Google Scholar]
- Lippincott, C.L. Excision Arthroplasty of the Femoral Head and Neck. Vet. Clin. N. Am. Small Anim. Pract. 1987, 17, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Hicks, D.A.; Millis, D.L. Kinetic and Kinematic Evaluation of Compensatory Movements of the Head, Pelvis and Thoracolumbar Spine Associated with Asymmetric Weight Bearing of the Pelvic Limbs in Trotting Dogs. Vet. Comp. Orthop. Traumatol. 2014, 27, 453–460. [Google Scholar]
- McLaughlin, R.M. Kinetic and Kinematic Gait Analysis in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 193–201. [Google Scholar] [CrossRef]
- Gillette, R.; Angle, T. Recent Developments in Canine Locomotor Analysis: A Review. Vet. J. 2008, 178, 165–176. [Google Scholar] [CrossRef]
- Oosterlinck, M.; Bosmans, T.; Gasthuys, F.; Polis, I.; van Ryssen, B.; Dewulf, J.; Pille, F. Accuracy of Pressure Plate Kinetic Asymmetry Indices and Their Correlation with Visual Gait Assessment Scores in Lame and Nonlame Dogs. Am. J. Vet. Res. 2011, 72, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.M.; Keuler, N.S.; Lu, Y.; Faria, M.L.E.; Muir, P.; Markel, M.D. Evaluation of Agreement between Numerical Rating Scales, Visual Analogue Scoring Scales, and Force Plate Gait Analysis in Dogs. Vet. Surg. 2007, 36, 360–367. [Google Scholar] [CrossRef]
- Waxman, A.; Robinson, D.; Evans, R.; Hulse, D.; Innes, J.; Conzemius, M. Relationship Between Objective and Subjective Assessment of Limb Function in Normal Dogs with an Experimentally Induced Lameness. Vet. Surg. 2008, 37, 241–246. [Google Scholar] [CrossRef]
- Garcia, E.F.V.; Loughin, C.A.; Marino, D.J.; Sackman, J.; Umbaugh, S.E.; Fu, J.; Subedi, S.; Lesser, M.L.; Akerman, M.; Schossler, J.E.W. Medical Infrared Imaging and Orthostatic Analysis to Determine Lameness in the Pelvic Limbs of Dogs. Open Vet. J. 2017, 7, 342–348. [Google Scholar] [CrossRef][Green Version]
- Kampa, N.; Kaenkangploo, D.; Jitpean, S.; Srithunyarat, T.; Seesupa, S.; Hoisang, S.; Yongvanit, K.; Kamlangchai, P.; Tuchpramuk, P.; Lascelles, B.D.X. Study of the Effectiveness of Glucosamine and Chondroitin Sulfate, Marine Based Fatty Acid Compounds (PCSO-524 and EAB-277), and Carprofen for the Treatment of Dogs with Hip Osteoarthritis: A Prospective, Block-Randomized, Double-Blinded, Placebo-Controll. Front. Vet. Sci. 2023, 10, 1033188. [Google Scholar] [CrossRef]
- Piermattei, D.L.; Flo, G.L.; DeCamp, C.E. The Hip Joint. In Brinker, Piermattei, and Flo’s Handbook of Small Animal Orthopedics and Fracture Repair, 4th Edition; W.B. Saunders: Pennsylvania, PA, USA, 2006; pp. 501–506. [Google Scholar]
- Levine, D.; Adamson, C.P. Conceptual Overview of Physical Therapy, Veterinary Medicine, and Canine Physical Rehabilitation. In Canine Rehabilitation and Physical Therapy; Millis, D.L., Levine, D., Taylor, R.A., Eds.; Saunders: Pennsylvania, PA, USA, 2004; p. 25. [Google Scholar]
- Volstad, N.J.; Sandberg, G.; Robb, S.; Budsberg, S.C. The Evaluation of Limb Symmetry Indices Using Ground Reaction Forces Collected with One or Two Force Plates in Healthy Dogs. Vet. Comp. Orthop. Traumatol. 2017, 30, 54–58. [Google Scholar]
- Jaegger, G.; Marcellin-Little, D.J.; Levine, D. Reliability of Goniometry in Labrador Retrievers. Am. J. Vet. Res. 2002, 63, 979–986. [Google Scholar] [CrossRef]
- Nicholson, H.; Osmotherly, P.; Smith, B.A.; Mcgowan, C. Determinants of Passive Hip Range of Motion in Adult Greyhounds. Aust. Vet. J. 2007, 85, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Brebner, N.; Moens, N.; Runciman, J. Evaluation of a Treadmill with Integrated Force Plates for Kinetic Gait Analysis of Sound and Lame Dogs at a Trot. Vet. Comp. Orthop. Traumatol. 2006, 19, 205–212. [Google Scholar] [PubMed]
- Besancon, M.F.; Conzemius, M.; Derrick, T.; Ritter, M.J. Comparison of Vertical Forces in Normal Greyhounds between Force Platform and Pressure Walkway Measurement Systems. Vet. Comp. Orthop. Traumatol. 2003, 16, 153–157. [Google Scholar] [CrossRef]
- Ichinohe, T.; Takahashi, H.; Fujita, Y. Force Plate Analysis of Ground Reaction Forces in Relation to Gait Velocity of Healthy Beagles. Am. J. Vet. Res. 2022, 83. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; Roe, S.C.; Smith, E.; Reynolds, L.; Markham, J.; Marcellin-Little, D.; Bergh, M.S.; Budsberg, S.C. Evaluation of a Pressure Walkway System for Measurement of Vertical Limb Forces in Clinically Normal Dogs. Am. J. Vet. Res. 2006, 67, 277–282. [Google Scholar] [CrossRef]
- McCrory, J.L.; White, S.C.; Lifeso, R.M. Vertical Ground Reaction Forces: Objective Measures of Gait Following Hip Arthroplasty. Gait Posture 2001, 14, 104–109. [Google Scholar] [CrossRef]
- Budsberg, S.C. Long-Term Temporal Evaluation of Ground Reaction Forces during Development of Experimentally Induced Osteoarthritis in Dogs. Am. J. Vet. Res. 2001, 62, 1207–1211. [Google Scholar] [CrossRef]
- Budsberg, S.; Jevens, D.; Brown, J.; Foutz, T.; DeCamp, C.; Reece, L. Evaluation of Limb Symmetry Indices, Using Ground Reaction Forces in Healthy Dogs. Am. J. Vet. Res. 1993, 54, 1569–1574. [Google Scholar] [CrossRef]
- Grisneaux, E.; Dupuis, J.; Pibarot, P.; Bonneau, N.H.; Charette, B.; Blais, D. Effects of Postoperative Administration of Ketoprofen or Carprofen on Short- and Long-Term Results of Femoral Head and Neck Excision in Dogs. J. Am. Vet. Med. Assoc. 2003, 223, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Penwick, R.C. The Variables That Influence the Success of Femoral Head and Neck Excision in Dogs. Vet. Med. 1992, 87, 325–333. [Google Scholar]
- Berzon, J.L.; Howard, P.E.; Covell, S.J.; Trotter, E.J.; Dueland, R. A Retrospective Study of the Efficacy of Femoral Head and Neck Excisions in 94 Dogs and Cats. Vet. Surg. 1980, 9, 88–92. [Google Scholar] [CrossRef]
- Jevens, D.; Hauptman, J.; DeCamp, C.; Budsberg, S.; Soutas-Little, R. Contributions to Variance in Force Plate Analyis of Gait in Dogs. Am. J. Vet. Res. 1993, 54, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.J.; Resident, A.; Dycus, D.L.; Acvs, D.; Animal, S. Canine Gait Analysis. Today’s Vet. Pract. 2016, 6, 93–100. [Google Scholar]
- Charette, B.; Dupuis, J.; Moreau, M.; Daminet, S.; Hébert, P.; Grisneaux, E. Assessing the Efficacy of Long-Term Administration of Tolfenamic Acid in Dogs Undergoing Femoral Head and Neck Excision. Vet. Comp. Orthop. Traumatol. 2003, 16, 232–237. [Google Scholar]
- Vijarnsorn, M.; Kwananocha, I.; Kashemsant, N.; Jarudecha, T.; Lekcharoensuk, C.; Beale, B.; Peirone, B.; Lascelles, B.D.X. The Effectiveness of Marine Based Fatty Acid Compound (PCSO-524) and Firocoxib in the Treatment of Canine Osteoarthritis. BMC Vet. Res. 2019, 15, 349. [Google Scholar] [CrossRef]
- Dycus, D.L.; Levine, D.; Marcellin-Little, D.J. Physical Rehabilitation for the Management of Canine Hip Dysplasia. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 823–850. [Google Scholar] [CrossRef]
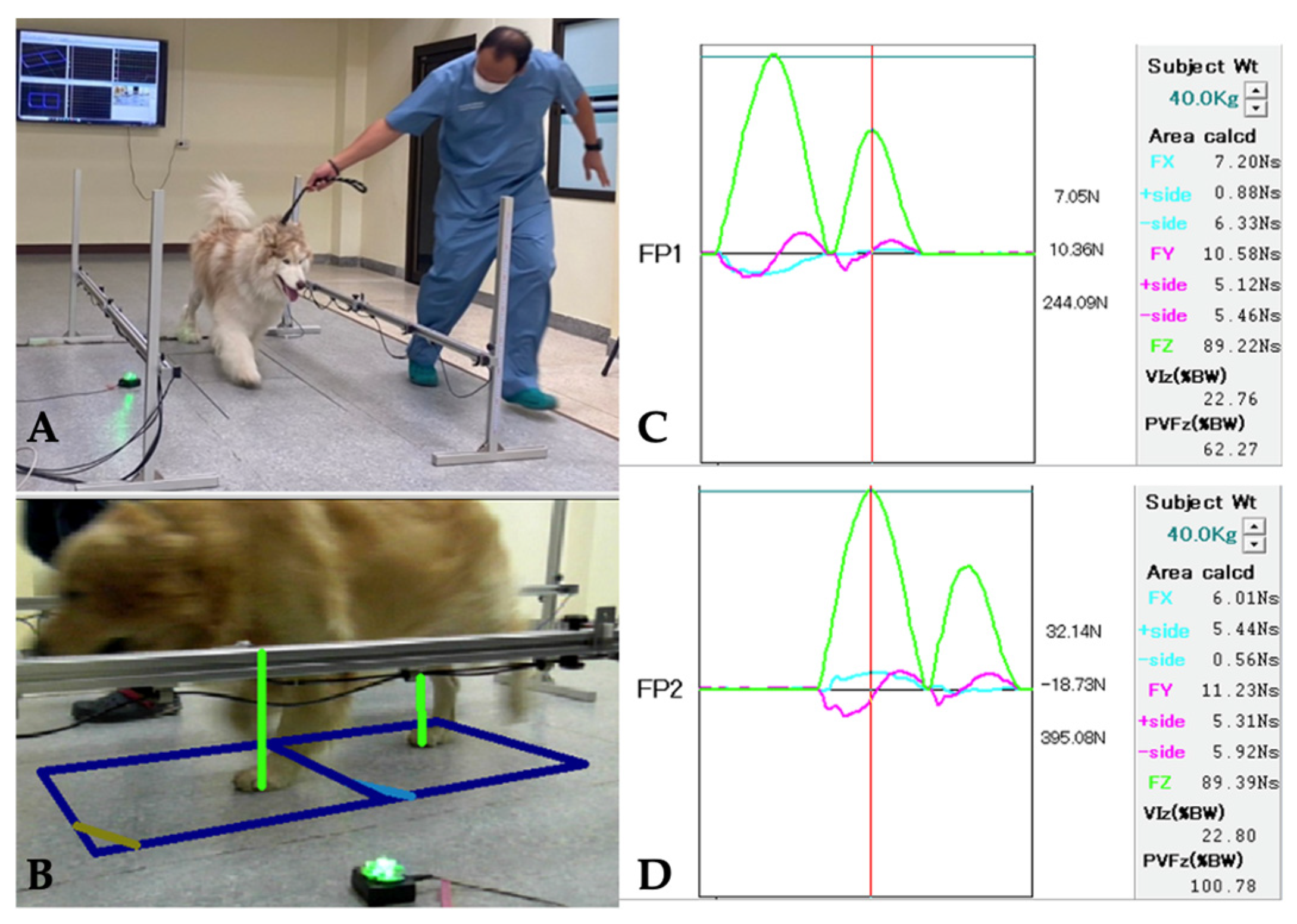

| Criterion | Clinical Evaluation |
|---|---|
| Lameness at trot | 0. No lameness/weight-bearing on all strike observed 1. Mild subtle lameness with partial weight-bearing 2. Obvious lameness with partial weight-bearing 3. Obvious lameness with intermittent weight-bearing 4. Full non weight-bearing |
| Variable | BW ≤ 20 kg n = 12 | BW > 20 kg n = 15 | Total n (%) |
|---|---|---|---|
| Sex | |||
| Female | 6 | 6 | 12 (44.44) |
| Male | 6 | 9 | 15 (55.56) |
| BCS | |||
| 2 | 2 | 3 | 5 (18.52) |
| 3 | 7 | 9 | 16 (59.26) |
| 4 | 3 | 3 | 6 (22.22) |
| Breed | |||
| Mixed breed | 10 | 0 | 10 (37.04) |
| Poodle | 1 | 0 | 1 (3.70) |
| Spitz | 1 | 0 | 1 (3.70) |
| Golden retriever | 0 | 5 | 5 (18.52) |
| Labrador retriever | 0 | 5 | 5 (18.52) |
| Alaskan malamute | 0 | 1 | 1 (3.70) |
| Chao Chao | 0 | 1 | 1 (3.70) |
| Samoyed | 0 | 1 | 1 (3.70) |
| Siberian husky | 0 | 1 | 1 (3.70) |
| Thai ridgeback | 0 | 1 | 1 (3.70) |
| Side of affected limb | |||
| Right | 8 | 6 | 14 (51.85) |
| Left | 4 | 9 | 13 (48.15) |
| Etiology of case | |||
| Vehicular trauma | 11 | 6 | 16 (59.26) |
| Falls from heights | 1 | 0 | 1 (3.70) |
| Hip disease (OA, HD) | 0 | 5 | 5 (18.52) |
| Dog fights | 0 | 3 | 3 (11.12) |
| Pulled the limb | 0 | 1 | 1 (3.70) |
| Slips and falls | 0 | 1 | 1 (3.70) |
| Radiographic finding (operated limb) | |||
| Craniodorsal dislocation | 10 | 11 | 21 (77.78) |
| Femoral head fracture | 1 | 2 | 3 (11.12) |
| Neck of femoral head fracture | 1 | 0 | 1 (3.70) |
| Progressive hip OA | 0 | 2 | 2 (7.40) |
| Variable (Presented as Mean ± SD) | BW ≤ 20 kg n = 12 | BW > 20 kg n = 15 | p-Value |
|---|---|---|---|
| PVF ** | 31.16 ± 16.52 | 33.25 ± 10.71 | 0.71 |
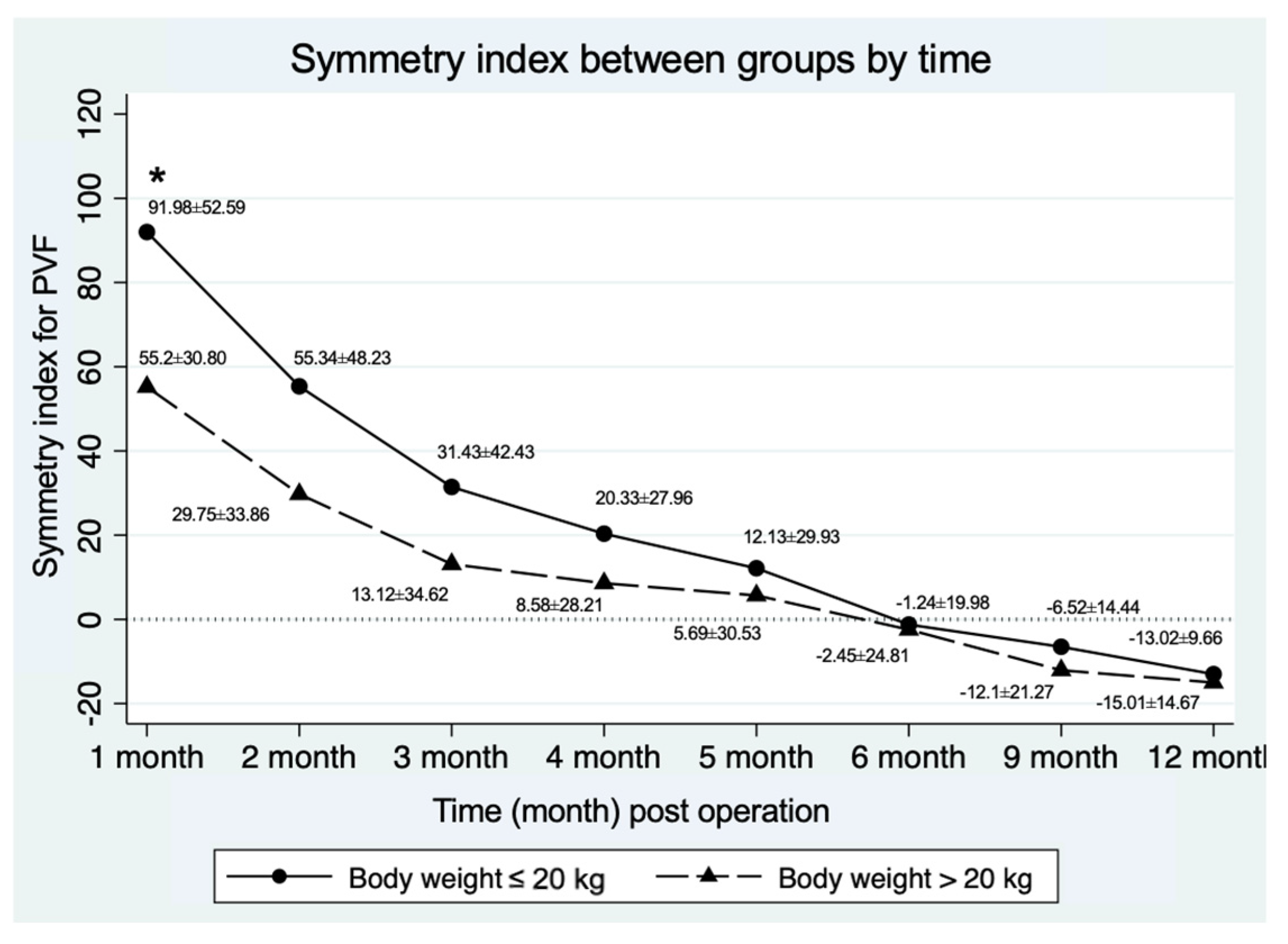
| Symmetry index for PVF ** | 91.98 ± 52.59 | 55.20 ± 30.80 | 0.04 |
| Hip flexion ** | 45.83 ± 6.69 | 44.67 ± 8.96 | 0.71 |
| Hip extension ** | 138.33 ± 17.10 | 145.00 ± 13.76 | 0.27 |
| OAS *** | |||
| Lameness score at trot | 2.67 ± 1.30 | 1.93 ± 0.96 | 0.10 |
| Visited Time (mean ± SD) | PVF | Mean Change PVF | ||||
|---|---|---|---|---|---|---|
| BW ≤ 20 kg | BW > 20 kg | p-Value | BW ≤ 20 kg | BW > 20 kg | p-Value | |
| n = 12 | n = 15 | n = 12 | n = 15 | |||
| 1 month post-operation | 31.16 ± 16.52 | 33.25 ± 10.71 | 0.76 | - | - | - |
| Month 2 | 44.69 ± 19.13 * | 48.28 ± 13.07 * | 0.86 | 13.71 ± 9.39 | 14.78 ± 8.10 | 0.98 |
| Month 3 | 53.28 ± 18.51 * | 52.93 ± 17.87 * | 0.88 | 22.12 ± 12.77 | 18.77 ± 11.25 | 0.43 |
| Month 4 | 56.37 ± 12.02 * | 58.00 ± 15.86 * | 0.88 | 27.57 ± 12.93 | 23.73 ± 11.50 | 0.37 |
| Month 5 | 62.12 ± 16.54 * | 56.19 ± 18.52 * | 0.33 | 30.96 ± 13.70 | 21.83 ± 13.88 | 0.06 |
| Month 6 | 67.31 ± 14.34 * | 62.62 ± 17.71 * | 0.44 | 36.15 ± 13.98 | 28.79 ± 13.44 | 0.15 |
| Month 9 | 68.67 ± 8.92 * | 66.68 ± 17.5 * | 0.40 | 41.045 ± 13.81 | 33.08 ± 11.50 | 0.09 |
| Month 12 | 73.43 ± 10.61 * | 71.86 ± 15.95 * | 0.77 | 42.51 ± 16.52 | 37.19 ± 10.65 | 0.34 |
| Visit Time | Hip Flexion | Hip Extension | ||||||
|---|---|---|---|---|---|---|---|---|
| BW ≤ 20 kg n = 12 | BW > 20 kg n = 15 | BW ≤ 20 kg n = 12 | BW > 20 kg n = 15 | |||||
| OL | NOL | OL | NOL | OL | NOL | OL | NOL | |
| Month 1 | 45.83 ± 6.69 | 47.92 ± 6.56 | 44.67 ± 8.96 | 49.33 ± 7.99 | 138.33 ± 17.10 | 159.17 ± 14.90 | 145.00 ± 13.76 | 157.67 ± 11.16 |
| Month 2 | 45.83 ± 7.02 | 49.17 ± 7.02 | 47.00 ± 8.82 | 50.00 ± 6.27 | 140.00 ± 17.58 | 165.00 ± 11.28 | 149.67 ± 16.20 | 161.33 ± 9.54 |
| Month 3 | 48.75 ± 4.83 | 49.58 ± 4.50 | 48.21 ± 8.23 | 49.64 ± 7.96 | 152.08 ± 14.99 | 166.67 ± 6.15 | 154.64 ± 11.84 | 162.86 ± 8.48 |
| Month 4 | 45.91 ± 5.84 | 50.45 ± 4.72 | 49.67 ± 7.43 | 51.33 ± 8.34 | 153.64 ± 14.51 | 170.00 ± 9.22 | 156.00 ± 9.86 | 164.33 ± 8.21 |
| Month 5 | 50.00 ± 5.22 | 51.67 ± 6.85 | 53.00 ± 5.28 | 51.00 ± 7.37 | 157.5 ± 14.69 | 169.17 ± 5.57 | 158.00 ± 10.66 | 162.67 ± 9.42 |
| Month 6 | 50.83 ± 4.17 | 51.25 ± 5.28 | 52.67 ± 7.04 | 53.00 ± 6.49 | 159.58 ± 9.16 | 167.50 ± 3.99 | 157.33 ± 9.42 | 160.00 ± 9.06 |
| Month 9 | 52.50 ± 2.64 | 51.50 ± 5.30 | 52.86 ± 5.08 | 55.00 ± 3.40 | 159.50 ± 6.85 | 168.50 ± 3.37 | 157.86 ± 8.93 | 160.00 ± 9.20 |
| Month 12 | 54.55 ± 2.70 | 55.00 ± 3.16 | 55.33 ± 3.99 | 55.67 ± 5.30 | 160.00 ± 9.75 | 165.00 ± 4.47 | 159.00 ± 5.73 | 161.67 ± 5.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuchpramuk, P.; Kaenkangploo, D.; Srithunyarat, T.; Seesupa, S.; Hoisang, S.; Lascelles, B.D.X.; Kampa, N. Force Plate Gait Analysis in Dogs After Femoral Head and Neck Excision. Vet. Sci. 2025, 12, 469. https://doi.org/10.3390/vetsci12050469
Tuchpramuk P, Kaenkangploo D, Srithunyarat T, Seesupa S, Hoisang S, Lascelles BDX, Kampa N. Force Plate Gait Analysis in Dogs After Femoral Head and Neck Excision. Veterinary Sciences. 2025; 12(5):469. https://doi.org/10.3390/vetsci12050469
Chicago/Turabian StyleTuchpramuk, Pongsatorn, Duangdaun Kaenkangploo, Thanikul Srithunyarat, Suvaluk Seesupa, Somphong Hoisang, Benedict Duncan X. Lascelles, and Naruepon Kampa. 2025. "Force Plate Gait Analysis in Dogs After Femoral Head and Neck Excision" Veterinary Sciences 12, no. 5: 469. https://doi.org/10.3390/vetsci12050469
APA StyleTuchpramuk, P., Kaenkangploo, D., Srithunyarat, T., Seesupa, S., Hoisang, S., Lascelles, B. D. X., & Kampa, N. (2025). Force Plate Gait Analysis in Dogs After Femoral Head and Neck Excision. Veterinary Sciences, 12(5), 469. https://doi.org/10.3390/vetsci12050469







