Impact of Curcumin on Frozen Bovine Sperm Quality and In Vitro Bovine Oocyte Maturation †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experiment 1
2.2.1. Semen Collection and Cryopreservation
2.2.2. Sperm Viability and Vigor Assay
2.2.3. Assessment of Sperm Plasma Membrane Integrity
2.2.4. Acrosome Integrity Assay
2.2.5. Antioxidant Property Assay
2.2.6. In Vitro Fertilization
2.3. Experiment 2
2.3.1. Oocyte Retrieval and IVM Culture
2.3.2. Oocyte ROS and GSH Assay
2.3.3. Oocyte MMP and MDA Assay
2.3.4. Assessment of In Vitro Fertilization
2.4. Statistics and Analysis
3. Results
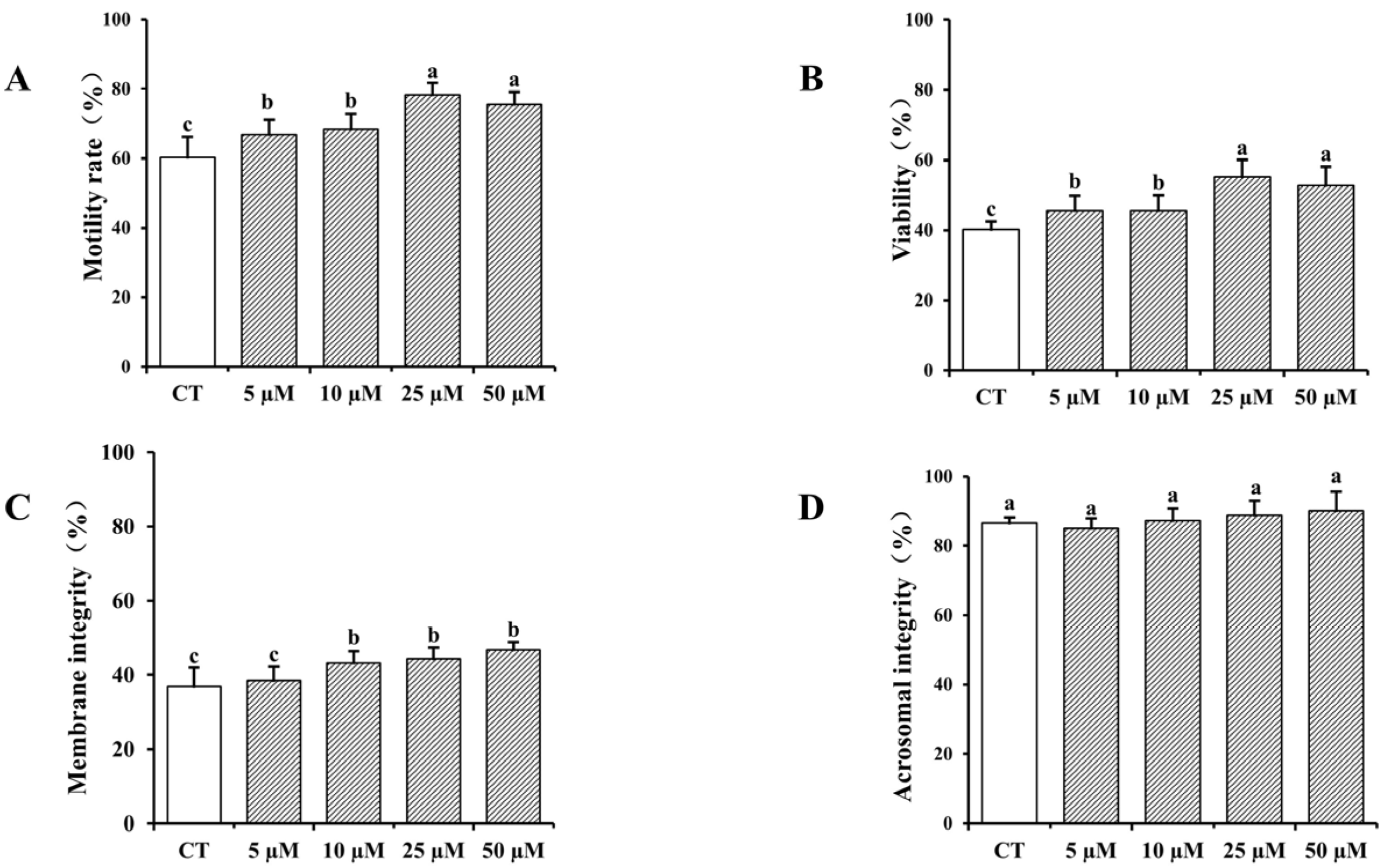
3.1. Impact of Curcumin on Frozen–Thawed Bovine Sperm Quality
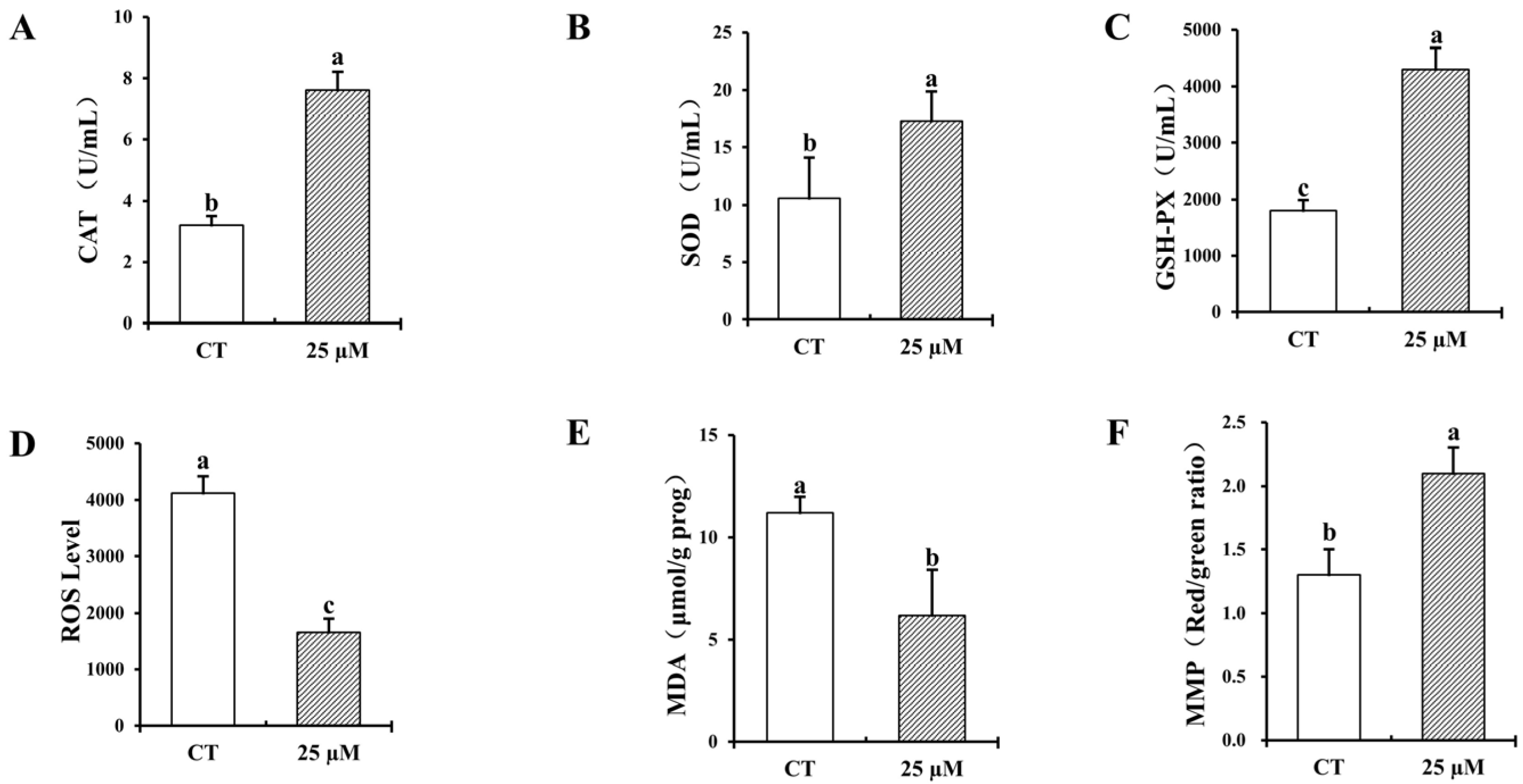
3.2. Effect of Curcumin on Antioxidant Properties of Frozen–Thawed Bovine Sperm
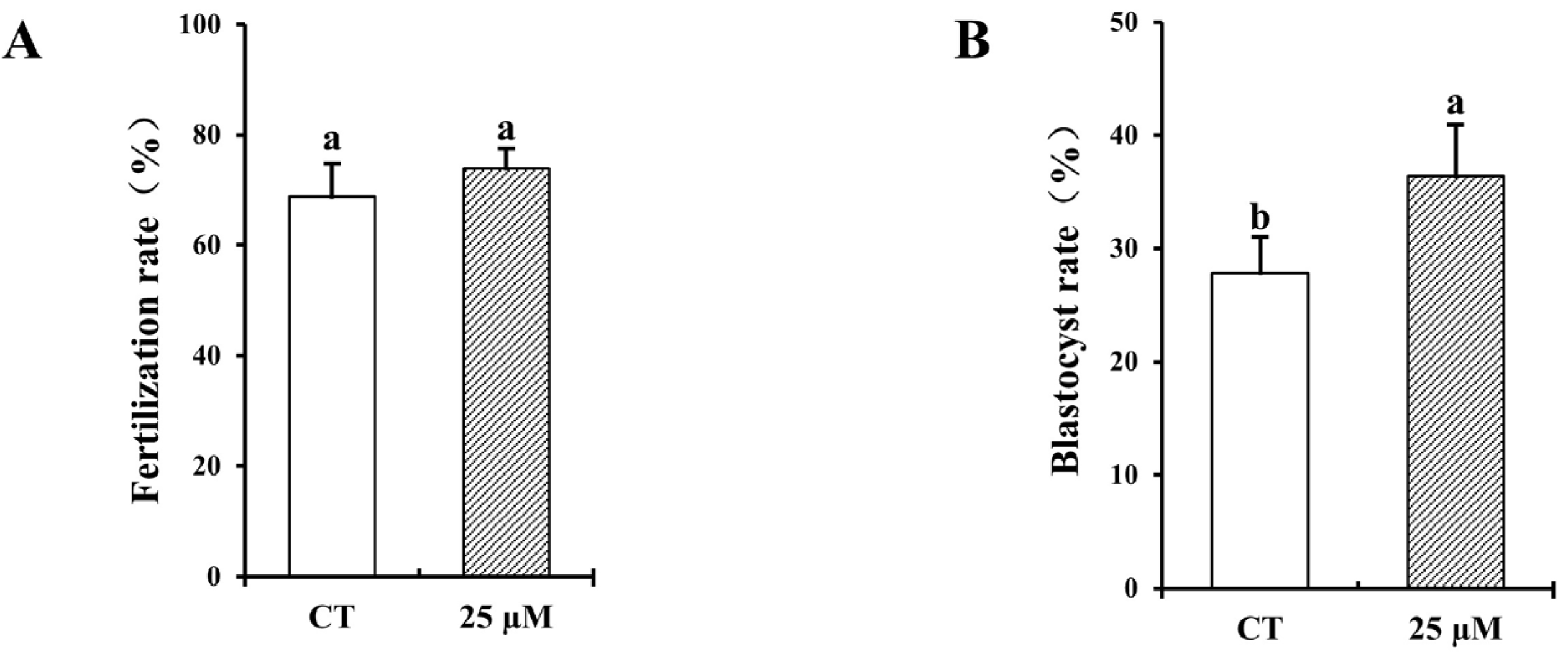
3.3. Effect of Curcumin on Fertilization and Blastocyst Rates with Frozen–Thawed Bovine Sperm
3.4. Impact of Curcumin on Bovine Oocyte Maturation and DNA Fragmented Nuclei During In Vitro Culture
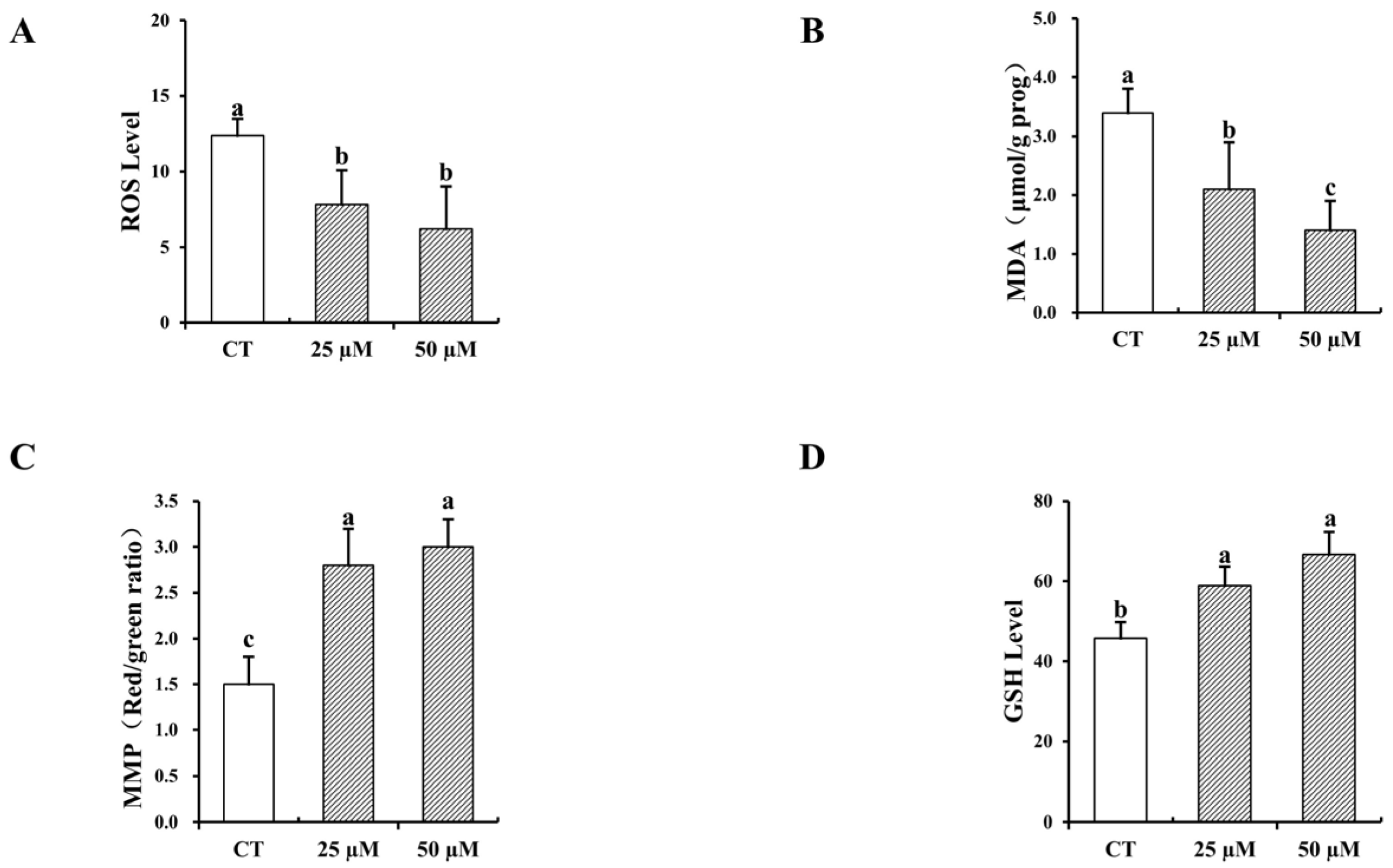
3.5. Impact of Curcumin on the Antioxidative Characteristics of In Vitro-Matured Bovine Oocytes
3.6. Formatting of Mathematical Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAT | catalase |
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| MMP | mitochondrial membrane potential |
| COCs | cumulus oocyte complexes |
| ROS | reactive oxygen species |
| GSH-PX | glutathione peroxidase |
References
- Silva, H.V.R.; Silva, A.R.; da Silvada, L.D.M.; Comizzoli, P. Semen Cryopreservation and Banking for the Conservation of Neotropical Carnivores. Biopreservation Biobanking 2019, 17, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Adelheid, W.; Andrey, K. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Dong, H.; Zhao, P.; Shang, C.; Qi, H.; Ma, Y.; Gao, C.; Zhang, D.; Shen, J.; Lei, Y. Resveratrol and lycium barbarum polysaccharide improve Qinling giant panda (Ailuropoda melanoleuca Qinlingensis) sperm quality during cryopreservation. BMC Vet. Res. 2022, 18, 23. [Google Scholar] [CrossRef]
- Dziekońska, A.; Partyka, A. Current Status and Advances in Semen Preservation. Animals 2022, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.J.; Ortiz-Rodríguez, J.M.; Gaitskell-Phillips, G.L.; Gil, M.C.; Ortega-Ferrusola, C.; Martín-Cano, F.E. An integrated overview on the regulation of sperm metabolism (glycolysis-Krebs cycle-oxidative phosphorylation). Anim. Reprod. Sci. 2022, 246, 106805. [Google Scholar] [CrossRef]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Bouba, I.; Tsirka, G.; Stavros, S.; Vrachnis, D.; Vrachnis, N.; Potiris, A.; Georgiou, I. Sperm Mitochondrial Content and Mitochondrial DNA to Nuclear DNA Ratio Are Associated with Body Mass Index and Progressive Motility. Biomedicines 2023, 11, 3014. [Google Scholar] [CrossRef]
- Sharma, M.; Punetha, M.; Saini, S.; Chaudhary, S.; Jinagal, S.; Thakur, S.; Kumar, P.; Kumar, R.; Sharma, R.K.; Yadav, P.S. Mito-Q supplementation of in vitro maturation or in vitro culture medium improves maturation of buffalo oocytes and developmental competence of cloned embryos by reducing ROS production. Anim. Reprod. Sci. 2024, 260, 107382. [Google Scholar] [CrossRef]
- Khaliq, A.; Hamza, M.A.; Ashraf, T.; Husnain, A.; Yaseen, M.; Rehman, A.; Binyameen, M.; Zahoor, M.Y.; Riaz, A. Effect of supplementing epinephrine in maturation media on in-vitro developmental competence of cattle and buffalo oocytes. Theriogenology 2024, 226, 219–227. [Google Scholar] [CrossRef]
- Gibb, Z.; Blanco-Prieto, O.; Bucci, D. The role of endogenous antioxidants in male animal fertility. Res. Vet. Sci. 2021, 136, 495–502. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Paramio, M.T. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef]
- Ugur, M.R.; Abdelrahman, A.S.; Evans, H.C.; Gilmore, A.A.; Memili, E. Advances in Cryopreservation of Bull Sperm. Front. Vet. Sci. 2019, 6, 268. [Google Scholar] [CrossRef]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The role of resveratrol in mammalian reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, H.; Yuan, C.; Lu, P.; Zhou, Y.; Lu, W.; Zhao, J.; Liu, H.; Wang, J. Epigallocatechin 3-gallate improves the quality of bull semen cryopreservation. Andrologia 2022, 54, e14310. [Google Scholar] [CrossRef] [PubMed]
- Valadez-García, K.M.; Avendaño-Reyes, L.; Meza-Herrera, C.A.; Mellado, M.; Díaz-Molina, R.; González-Ríos, H.; Macías-Cruz, U. Ferulic acid in animal feeding: Mechanisms of action, productive benefits, and future perspectives in meat production. Food Biosci. 2021, 43, 101247. [Google Scholar] [CrossRef]
- Ahmed, H.; Jahan, S.; Ullah, H.; Ullah, F.; Salman, M.M. The addition of resveratrol in tris citric acid extender ameliorates post-thaw quality parameters, antioxidant enzymes levels, and fertilizing capability of buffalo (Bubalus bubalis) bull spermatozoa. Theriogenology 2020, 152, 106–113. [Google Scholar] [CrossRef]
- Kabeer, S.W.; Riaz, A.; Ul-Rahman, A.; Shahbakht, R.M.; Anjum, A.; Khera, H.u.R.A.; Haider, A.; Riaz, F.; Yasin, R.; Yaseen, M. Effect of different concentrations of resveratrol on nuclear maturation and in-vitro development competence of oocytes of Nili Ravi buffalo. Trop. Anim. Health Prod. 2024, 56, 105. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, J.J.; Yin, Y.J.; Jiang, H.; Zhang, J.B.; Liang, S.; Yuan, B. Ferulic Acid Enhances Oocyte Maturation and the Subsequent Development of Bovine Oocytes. Int. J. Mol. Sci. 2023, 24, 14804. [Google Scholar] [CrossRef]
- Slaek, G.; Kotnik, P.; Osmi, A.; Postrunik, V.; Knez, E.; Fingar, M.; Knez Marevci, M. The Extraction Process, Separation, and Identification of Curcuminoids from Turmeric Curcuma longa. Foods 2023, 12, 4000. [Google Scholar] [CrossRef]
- Lin, H.; Zhao, N. Effect of curcumin on the quality of frozen bovine semen and maturation of bovine oocytes in vitro. In Proceedings of the Seventeenth Academic Symposium of the Veterinary Obstetrics Branch of the Chinese Animal Husbandry and Veterinary Medical Association Conference, Zhanjiang, China, 17 November 2024. [Google Scholar]
- Xiang, D.B.; Zhang, K.Q.; Zeng, Y.L.; Yan, Q.Z.; Liao, D.F. Curcumin: From a controversial “panacea” to effective antineoplastic products. Medicine 2020, 99, e18467. [Google Scholar] [CrossRef]
- Namula, Z.; Sato, Y.; Wittayarat, M.; Le, Q.A.; Otoi, T. Curcumin supplementation in the maturation medium improves the maturation, fertilisation and developmental competence of porcine oocytes. Acta Vet. Hung. 2020, 68, 298–304. [Google Scholar] [CrossRef]
- Bahadi, M.A.; Al-Badwi, M.A.; Samara, E.M.; Abdoun, K.A.; Alhidary, I.A.; Al-Haidary, A.A. Group-training of rams at puberty for artificial vagina-mediated semen collection and its influence on semen quality and sexual behavior. Anim. Reprod. 2023, 20, e20220051. [Google Scholar] [CrossRef] [PubMed]
- Namula, Z.; Hirata, M.; Wittayarat, M.; Tanihara, F.; Thi Nguyen, N.; Hirano, T.; Nii, M.; Otoi, T. Effects of chlorogenic acid and caffeic acid on the quality of frozen-thawed boar sperm. Reprod. Domest. Anim. 2018, 53, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Namula, Z.; Kodama, R.; Tanihara, F.; Morita, Y.; Sato, Y.; Wittayarat, M.; Taniguchi, M.; Otoi, T. Effects of skim-milk supplementation on the quality and penetrating ability of boar semen after long-term preservation at 15 C. Acta Vet. Hung. 2014, 62, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Sohail, T.; Kang, Y.; Sun, X.; Li, Y. Chlorogenic acid improves quality of chilled ram sperm by mitigating oxidative stress. Animals 2022, 12, 163. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, Y.; Chen, R.; Tao, Q.; Lu, Q.; Xu, Q.; Yu, H.; Jiang, P.; Zhao, Z. Protective Effects of Chitosan Oligosaccharide Against Lipopolysaccharide-Induced Inflammatory Response and Oxidative Stress in Bovine Mammary Epithelial Cells. Mar. Drugs 2025, 23, 31. [Google Scholar] [CrossRef]
- Namula, Z.; Wittayarat, M.; Hirata, M.; Hirano, T.; Nguyen, N.T.; Le, Q.A.; Fahrudin, M.; Tanihara, F.; Otoi, T. Genome mutation after the introduction of the gene editing by electroporation of Cas9 protein (GEEP) system into bovine putative zygotes. In Vitro Cell. Dev. Biol.-Anim. 2019, 55, 598–603. [Google Scholar] [CrossRef]
- Chanapiwat, P.; Kaeoket, K. The effect of Curcuma longaextracted (curcumin) on the quality of cryopreserved boar semen. Anim. Sci. J. 2015, 86, 863–868. [Google Scholar] [CrossRef]
- Berean, D.I.; Bogdan, L.M.; Cimpean, R. Advancements in Understanding and Enhancing Antioxidant-Mediated Sperm Cryopreservation in Small Ruminants: Challenges and Perspectives. Antioxidants 2024, 13, 624. [Google Scholar] [CrossRef]
- Cojkic, A.; Hansson, I.; Johannisson, A.; Morrell, J.M. Effect of some plant-based substances on microbial content and sperm quality parameters of Bull Semen. Int. J. Mol. Sci. 2023, 24, 3435. [Google Scholar] [CrossRef]
- Kowalczyk, A. The Role of the Natural Antioxidant Mechanism in Sperm Cells. Reprod. Sci. 2021, 29, 1387–1394. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Upadhyay, K.K.; Devkar, R.V.; Khurana, S. Naturally occurring Nrf2 activators: Potential in treatment of liver injury. Oxidative Med. Cell. Longev. 2016, 2016, 3453926. [Google Scholar] [CrossRef]
- Shin, J.W.; Chun, K.-S.; Kim, D.-H.; Kim, S.-J.; Kim, S.H.; Cho, N.-C.; Na, H.-K.; Surh, Y.-J. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem. Pharmacol. 2020, 173, 113820. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones 2020, 25, 395–406. [Google Scholar] [CrossRef]
- Yang, S.; Huang, X.-L.; Chen, J.; Mao, L.-N.; Liu, X.; Yuan, W.-S.; Wu, X.-J.; Luo, G.-W. Curcumin protects BEAS-2B cells from PM 2.5-induced oxidative stress and inflammation by activating NRF2/antioxidant response element pathways. Int. J. Mol. Med. 2021, 47, 45. [Google Scholar] [CrossRef]
- Qiao, Z.; Xun, W.; Yingmin, L.; Xin, W.; Xiufeng, L.; Hongshan, G.; Junqiang, Z. Curcumin improves asthenozoospermia by inhibiting reactive oxygen species reproduction through nuclear factor erythroid 2-related factor 2 activation. Andrologia 2020, 52, e13491. [Google Scholar] [CrossRef]
- Simonik, O.; Sichtar, J.; Krejcarkova, A.; Rajmon, R.; Stadnik, L.; Beran, J.; Dolezalova, M.; Biniova, Z. Computer assisted sperm analysis–the relationship to bull field fertility, possible errors and their impact on outputs: A review. Indian J. Anim. Sci. 2015, 85, 3–11. [Google Scholar] [CrossRef]
- Al-Mutary, M.G. Use of antioxidants to augment semen efficiency during liquid storage and cryopreservation in livestock animals: A review. J. King Saud Univ.-Sci. 2021, 33, 101226. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Z.-T.; Li, Y.; Li, Y.-X.; Xian, M.; Guo, S.-M.; Hu, J.-H. Effect of Astragalus polysaccharides on the cryopreservation of goat semen. Theriogenology 2022, 193, 47–57. [Google Scholar] [CrossRef]
- Medrano, A.; Contreras, C.F.B.; Herrera, F.M.; Alcantar-Rodriguez, A.M. Melatonin as an antioxidant preserving sperm from domestic animals. Asian Pac. J. Reprod. 2017, 6, 241–246. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Ma, J.; Xiong, Y.; Xiong, X.; Lan, D.; Li, J.; Yin, S. Factors influencing the maturation and developmental competence of yak (Bos grunniens) oocytes in vitro. Genes 2023, 14, 1882. [Google Scholar] [CrossRef]
- Saifi, B.; Haftcheshmeh, S.M.; Feligioni, M.; Izadpanah, E.; Rahimi, K.; Hassanzadeh, K.; Mohammadi, A.; Sahebkar, A. An overview of the therapeutic effects of curcumin in reproductive disorders with a focus on the antiinflammatory and immunomodulatory activities. Phytother. Res. 2022, 36, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, H.; Makabel, B.; Cui, Q.; Li, J.; Su, C.; Ashby Jr, C.R.; Chen, Z.; Zhang, J. The targeting of non-coding RNAs by curcumin: Facts and hopes for cancer therapy. Oncol. Rep. 2019, 42, 20–34. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015, 35, 645–651. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, Y.; Fu, H.; Zheng, Y.; Bao, D.; Yin, Y.; Chen, Q.; Nie, X.; Hao, Q.; Hou, D. Curcumin Exerts a Protective Effect against Premature Ovarian Failure in Mice. J. Mol. Endocrinol. 2018, 60, 261–271. [Google Scholar] [CrossRef]
- Abhari, S.M.F.; Khanbabaei, R.; Roodbari, N.H.; Parivar, K.; Yaghmaei, P. Curcumin-loaded super-paramagnetic iron oxide nanoparticle affects on apoptotic factors expression and histological changes in a prepubertal mouse model of polycystic ovary syndrome-induced by dehydroepiandrosterone-A molecular and stereological study. Life Sci. 2020, 249, 117515. [Google Scholar] [CrossRef]
- Sirotkin, A.V. The influence of turmeric and curcumin on female reproductive processes. Planta Medica 2022, 88, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Hendarto, H.; Widyanugraha, M.Y.A.; Widjiati, W. Curcumin improves growth factors expression of bovine cumulus-oocyte complexes cultured in peritoneal fluid of women with endometriosis. Int. J. Reprod. Biomed. 2018, 16, ijrm.v16i12.3683. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hsieh, M.-S.; Hsuuw, Y.-D.; Huang, F.-J.; Chan, W.-H. Hazardous effects of curcumin on mouse embryonic development through a mitochondria-dependent apoptotic signaling pathway. Int. J. Mol. Sci. 2010, 11, 2839–2855. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chan, W.-H. Injurious effects of curcumin on maturation of mouse oocytes, fertilization and fetal development via apoptosis. Int. J. Mol. Sci. 2012, 13, 4655–4672. [Google Scholar] [CrossRef]
- Gasparrini, B.; Sayoud, H.; Neglia, G.; de Matos, D.G.; Donnay, I.; Zicarelli, L. Glutathione synthesis during in vitro maturation of buffalo (Bubalus bubalis) oocytes: Effects of cysteamine on embryo development. Theriogenology 2003, 60, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Brad, A.; Bormann, C.; Swain, J.; Durkin, R.; Johnson, A.; Clifford, A.; Krisher, R. Glutathione and adenosine triphosphate content of in vivo and in vitro matured porcine oocytes. Mol. Reprod. Dev. Inc. Gamete Res. 2003, 64, 492–498. [Google Scholar] [CrossRef]
- Ren, J.; Hao, Y.; Liu, Z.; Li, S.; Wang, C.; Wang, B.; Liu, Y.; Liu, G.; Dai, Y. Effect of exogenous glutathione supplementation on the in vitro developmental competence of ovine oocytes. Theriogenology 2021, 173, 144–155. [Google Scholar] [CrossRef]
- Kowalczyk-Zieba, I.; Boruszewska, D.; Suwik, K.; Staszkiewicz-Chodor, J.; Jaworska, J.; Woclawek-Potocka, I. Iloprost affects in vitro maturation and developmental competence of bovine oocytes. Theriogenology 2020, 157, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, A.; Golestanfar, A.; Bashiri, M.; Ahmadi, E.; Shams-Esfandabadi, N. Male pronuclear formation and embryo development following intracytoplasmic injection of ovine pretreated sperm. Avicenna J. Med. Biotechnol. 2018, 10, 41. [Google Scholar]
- Cao, X.; Li, J.; Xue, H.; Wang, S.; Zhao, W.; Du, Z.; Yang, Y.; Yue, Z. Effect of vitrification on meiotic maturation, mitochondrial distribution and glutathione synthesis in immature silver fox cumulus oocyte complexes. Theriogenology 2017, 91, 104–111. [Google Scholar] [CrossRef]
- Furnus, C.C.; De Matos, D.; Picco, S.; García, P.P.; Inda, A.M.; Mattioli, G.; Errecalde, A.L. Metabolic requirements associated with GSH synthesis during in vitro maturation of cattle oocytes. Anim. Reprod. Sci. 2008, 109, 88–99. [Google Scholar] [CrossRef]
| Concentration of Curcumin (µM) | No. of Examined Oocytes | No. (%) of Oocytes with | No. (%) of Oocytes with DNA Fragmented Nuclei | |
|---|---|---|---|---|
| GVBD | M II | |||
| CT (0) | 108 | 103 (95.4 ± 2.3) | 57 (52.8 ± 1.8) c | 12 (12.0 ± 1.9) a |
| 5 | 105 | 101 (96.2 ± 2.1) | 59 (56.2 ± 2.5) b,c | 8 (7.6 ± 2.1) a,b |
| 10 | 107 | 101 (94.4 ± 1.6) | 65 (60.8 ± 2.2) b | 6 (5.6 ± 2.4) b |
| 25 | 109 | 100 (91.7 ± 1.9) | 80 (73.4 ± 1.8) a | 6 (5.5 ± 1.6) b |
| 50 | 108 | 103 (95.4 ± 2.0) | 72 (68.5 ± 2.4) a | 5 (4.6 ± 1.9) b |
| Concentration of Curcumin (µM) | No. Oocytes (n1) | Fertilization Rate (%) | No. Oocytes (n2) | No. (%) of Embryos | |
|---|---|---|---|---|---|
| Cleaved | Blastocyst | ||||
| CT | 102 | 62 (61.8 ± 1.8) b | 186 | 161 (86.3 ± 2.1) | 44 (23.5 ± 2.7) b |
| 25 | 106 | 78 (73.6 ± 2.3) a | 178 | 155 (86.8 ± 2.6) | 64 (35.9 ± 1.9) a |
| 50 | 103 | 78 (75.7 ± 1.5) a | 185 | 156 (84.4 ± 3.4) | 59 (32.0 ± 1.7) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Hu, Z.; Li, Y.; Li, Y.; Ma, W.; Zheng, S.; Zhou, J.; Zhao, Z.; Gan, S.; Chen, Z.; et al. Impact of Curcumin on Frozen Bovine Sperm Quality and In Vitro Bovine Oocyte Maturation. Vet. Sci. 2025, 12, 441. https://doi.org/10.3390/vetsci12050441
Lin H, Hu Z, Li Y, Li Y, Ma W, Zheng S, Zhou J, Zhao Z, Gan S, Chen Z, et al. Impact of Curcumin on Frozen Bovine Sperm Quality and In Vitro Bovine Oocyte Maturation. Veterinary Sciences. 2025; 12(5):441. https://doi.org/10.3390/vetsci12050441
Chicago/Turabian StyleLin, Hao, Zhiye Hu, Yang Li, Yingchun Li, Wenao Ma, Shoujie Zheng, Jianye Zhou, Zhihui Zhao, Shangquan Gan, Zhibao Chen, and et al. 2025. "Impact of Curcumin on Frozen Bovine Sperm Quality and In Vitro Bovine Oocyte Maturation" Veterinary Sciences 12, no. 5: 441. https://doi.org/10.3390/vetsci12050441
APA StyleLin, H., Hu, Z., Li, Y., Li, Y., Ma, W., Zheng, S., Zhou, J., Zhao, Z., Gan, S., Chen, Z., & Zhao, N. (2025). Impact of Curcumin on Frozen Bovine Sperm Quality and In Vitro Bovine Oocyte Maturation. Veterinary Sciences, 12(5), 441. https://doi.org/10.3390/vetsci12050441






