Emergence and Dissemination of the Avian Infectious Bronchitis Virus Lineages in Poultry Farms in South America
Simple Summary
Abstract
1. Introduction
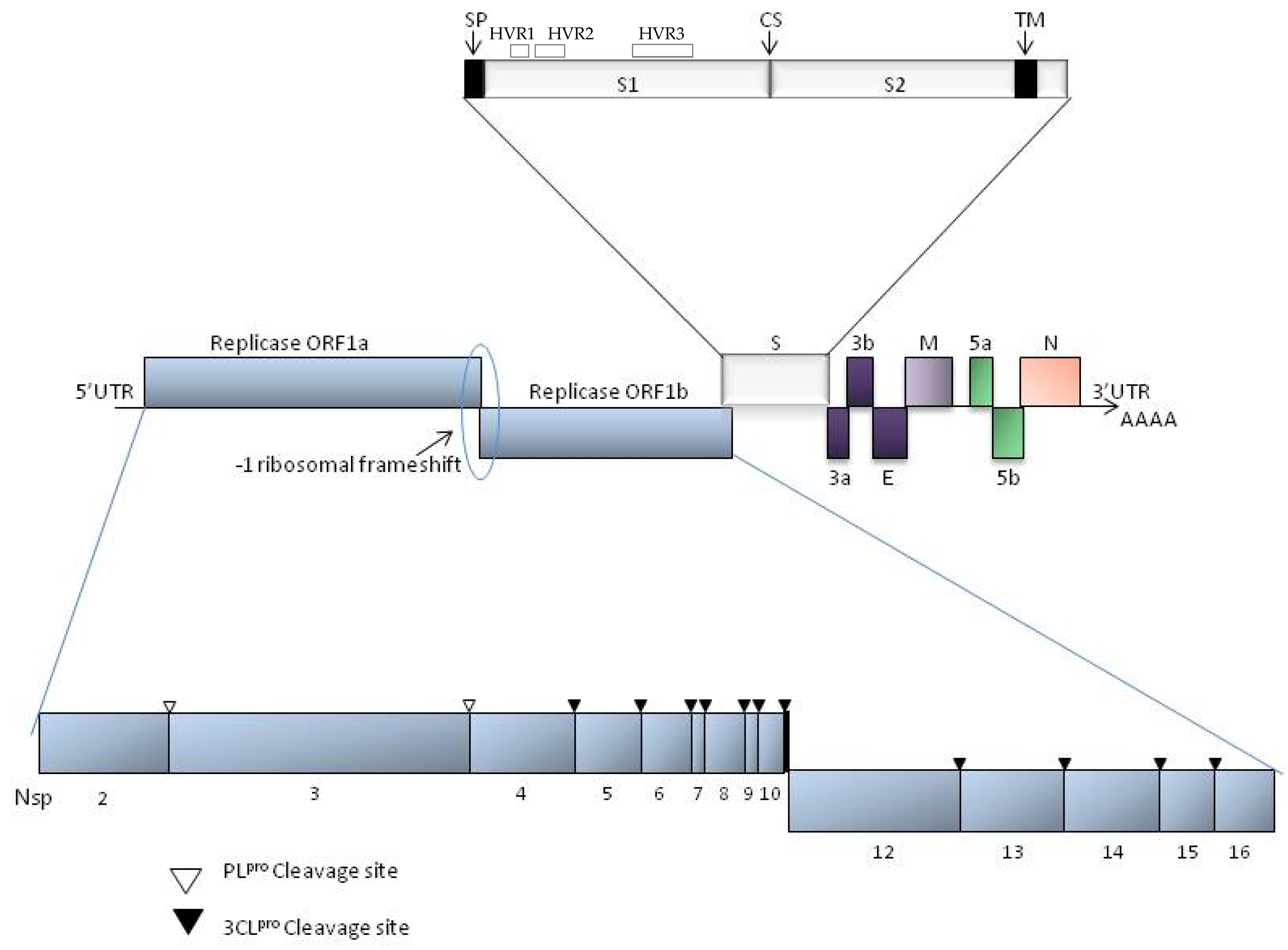
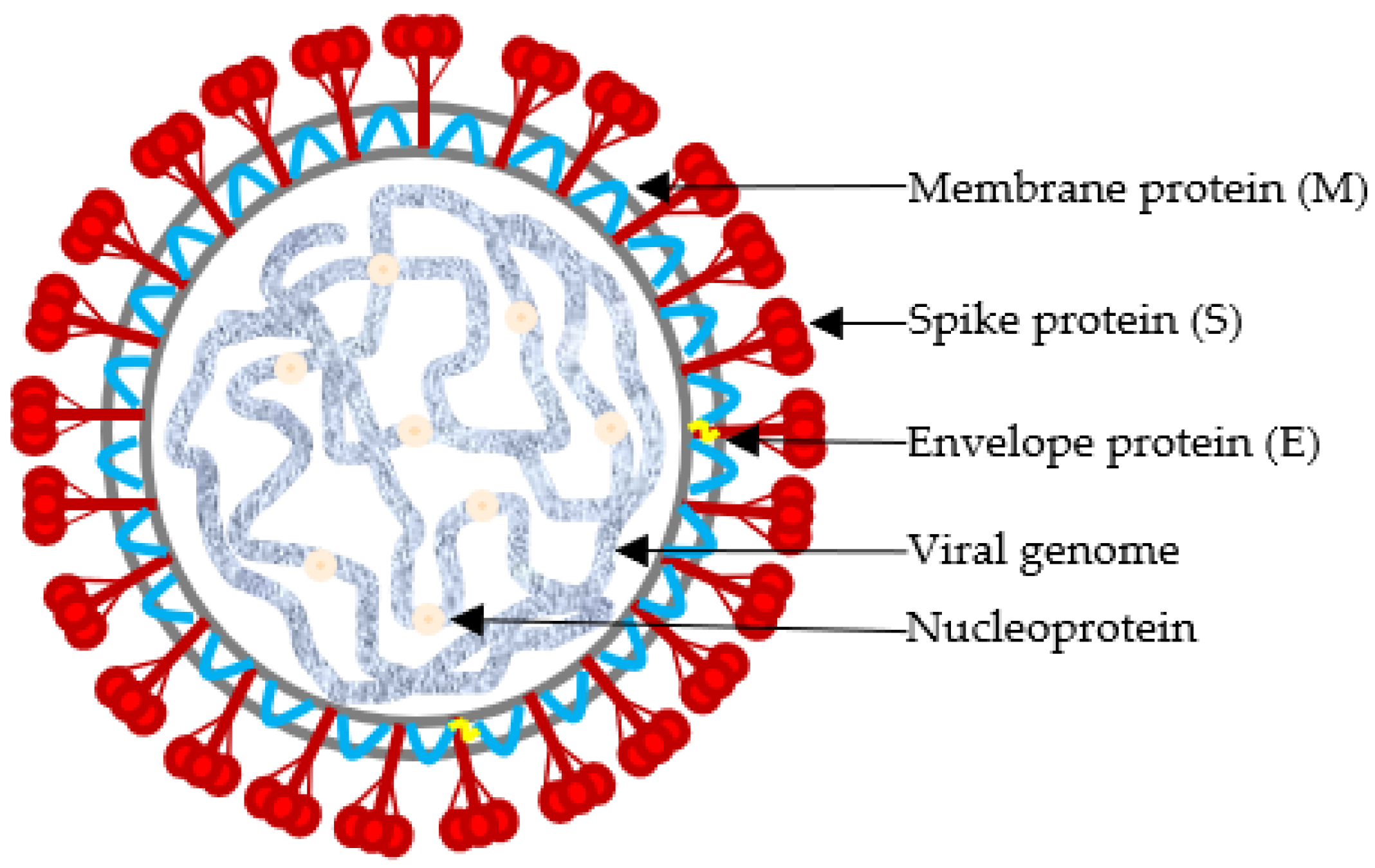
2. Taxonomy and Molecular Structure
3. Genetic and Antigenic Diversity
4. Classification and Identification
5. IBV Emergence and Dissemination in South America
5.1. GI-1
5.2. GI-11
5.3. GI-13
5.4. GI-16
5.5. GI-23
5.6. Current Situation
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cook, J.K.; Jackwood, M.; Jones, R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012, 41, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, J. The early history of infectious bronchitis. Avian Dis. 1998, 42, 648–650. [Google Scholar] [CrossRef]
- Sjaak de Wit, J.J.; Cook, J.K.; van der Heijden, H.M. Infectious bronchitis virus variants: A review of the history, current situation and control measures. Avian Pathol. 2011, 40, 223–235. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Zhang, G. Key Aspects of Coronavirus Avian Infectious Bronchitis Virus. Pathogens 2023, 12, 698. [Google Scholar] [CrossRef]
- Finger, A.; Ashash, U.; Goldenberg, D.; Raviv, Z. Lessons learnt on infectious bronchitis virus lineage GI-23. Avian Pathol. 2025, 54, 27–39. [Google Scholar] [CrossRef]
- Valastro, V.; Holmes, E.C.; Britton, P.; Fusaro, A.; Jackwood, M.W.; Cattoli, G.; Monne, I. S1 gene-based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification. Infect. Genet. Evol. 2016, 39, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Sepotokele, K.M.; O’Kennedy, M.M.; Hayes, M.C.; Wandrag, D.B.R.; Smith, P.; Abolnik, C. Efficacy of a plant-produced infectious bronchitis virus-like particle vaccine in specific pathogen-free chickens. Poult. Sci. 2023, 102, 102953. [Google Scholar] [CrossRef]
- Colvero, L.P.; Villarreal, L.Y.; Torres, C.A.; Brandão, P.E. Assessing the economic burden of avian infectious bronchitis on poultry farms in Brazil. Rev. Sci. Tech. 2015, 34, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.S.A.; Amin, Z.; Rodrigues, K.F.; Saallah, S.; Shaarani, S.M.; Sarker, S.; Siddiquee, S. Infectious Bronchitis Virus (Gammacoronavirus) in Poultry Farming: Vaccination, Immune Response and Measures for Mitigation. Vet. Sci. 2021, 8, 273. [Google Scholar] [CrossRef]
- Legnardi, M.; Tucciarone, C.M.; Franzo, G.; Cecchinato, M. Infectious Bronchitis Virus Evolution, Diagnosis and Control. Vet. Sci. 2020, 7, 79. [Google Scholar] [CrossRef]
- Marandino, A.; Pérez, R. Genetic and Antigenic Diversity of Infectious Bronchitis Virus in South America. Avian Dis. 2021, 65, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Santana-Clavijo, N.F.; Brandão, P.E. Emergence of Avian coronavirus genotype GI-11 in Colombia. Braz. J. Microbiol. 2021, 52, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Fonseca, A.S.K.; Fernando, F.S.; Filho, T.F.; Martins, N.R.D.S.; Lunge, V.R. Emergence and molecular characterization of the avian infectious bronchitis virus GI-23 in commercial broiler farms from South America. Transbound. Emerg. Dis. 2022, 69, 3167–3172. [Google Scholar] [CrossRef]
- Villanueva-Pérez, D.; Tataje-Lavanda, L.; Montalván-Avalos, A.; Paredes-Inofuente, D.; Montoya-Ortiz, S.; Isasi-Rivas, G.; Fernández, M.F.; Fernández-Sánchez, M.; Fernández-Díaz, M. Detection and Molecular Characterization of GI-1 and GI-23 Avian Infectious Bronchitis Virus in Broilers Indicate the Emergence of New Genotypes in Bolivia. Viruses 2024, 16, 1463. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; de Groot, R.J.; Haagmans, B.; Lau, S.K.P.; Neuman, B.W.; Perlman, S.; Sola, I.; van der Hoek, L.; Wong, A.C.P.; Yeh, S.H. ICTV Virus Taxonomy Profile: Coronaviridae 2023. J. Gen. Virol. 2023, 104, 4. [Google Scholar] [CrossRef]
- Yang, D.; Leibowitz, J.L. The structure and functions of coronavirus genomic 3′ and 5′ ends. Virus Res. 2015, 206, 120–133. [Google Scholar] [CrossRef]
- Wickramasinghe, I.N.; van Beurden, S.J.; Weerts, E.A.; Verheije, M.H. The avian coronavirus spike protein. Virus Res. 2014, 194, 37–48. [Google Scholar] [CrossRef]
- Simon-Loriere, E.; Holmes, E.C. Why do RNA viruses recombine? Nat. Rev. Microbiol. 2011, 9, 617–626. [Google Scholar] [CrossRef]
- Promkuntod, N.; van Eijndhoven, R.E.; de Vrieze, G.; Gröne, A.; Verheije, M.H. Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology 2014, 448, 26–32. [Google Scholar] [CrossRef]
- Bali, K.; Bálint, Á.; Farsang, A.; Marton, S.; Nagy, B.; Kaszab, E.; Belák, S.; Palya, V.; Bányai, K. Recombination Events Shape the Genomic Evolution of Infectious Bronchitis Virus in Europe. Viruses 2021, 13, 535. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Hall, D.; Handel, A. Molecular evolution and emergence of avian gammacoronaviruses. Infect. Genet. Evol. 2012, 12, 1305–1311. [Google Scholar] [CrossRef]
- Fraga, A.P.; Gräf, T.; Pereira, C.S.; Ikuta, N.; Fonseca, A.S.K.; Lunge, V.R. Phylodynamic analysis and molecular diversity of the avian infectious bronchitis virus of chickens in Brazil. Infect. Genet. Evol. 2018, 61, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chacón, J.L.; Chacón, R.D.; Sánchez-Llatas, C.J.; Morín, J.G.; Astolfi-Ferreira, C.S.; Piantino Ferreira, A.J. Antigenic and molecular characterization of isolates of the Brazilian genotype BR-I (GI-11) of infectious bronchitis virus supports its recognition as BR-I serotype. Avian Pathol. 2023, 52, 323–338. [Google Scholar] [CrossRef]
- Falchieri, M.; Coward, V.J.; Reid, S.M.; Lewis, T.; Banyard, A.C. Infectious bronchitis virus: An overview of the “chicken coronavirus”. J. Med. Microbiol. 2024, 73, 001828. [Google Scholar] [CrossRef] [PubMed]
- Abozeid, H.H. Global Emergence of Infectious Bronchitis Virus Variants: Evolution, Immunity, and Vaccination Challenges. Transbound. Emerg. Dis. 2023, 1, 1144924. [Google Scholar] [CrossRef]
- Xiong, T.; Xie, H.; Li, L.; Liang, S.; Huang, M.; Yu, C.; Zhuang, T.; Liang, X.; Liu, D.; Chen, R. Prevalence, Genotype Diversity, and Distinct Pathogenicity of 205 Gammacoronavirus Infectious Bronchitis Virus Isolates in China during 2019–2023. Viruses 2024, 16, 930. [Google Scholar] [CrossRef]
- Rafique, S.; Jabeen, Z.; Pervaiz, T.; Rashid, F.; Luo, S.; Xie, L.; Xie, Z. Avian infectious bronchitis virus (AIBV) review by continent. Front. Cell Infect. Microbiol. 2024, 14, 1325346. [Google Scholar] [CrossRef]
- Hipólito, O. Isolamento e identificação do vírus da bronquite infecciosa das galinhas no Brasil. Arq. Esc. Sup Vet. 1957, 10, 131–151. [Google Scholar]
- Silva, E.N. Infectious bronchitis in Brazilian chickens: Current data and observations of field service personnel. Braz. J. Poult. Sci. 2010, 12, 197–203. [Google Scholar] [CrossRef]
- Ramirez-Nieto, G.; Mir, D.; Almansa-Villa, D.; Cordoba-Argotti, G.; Beltran-Leon, M.; Rodriguez-Osorio, N.; Garai, J.; Zabaleta, J.; Gomez, A.P. New Insights into Avian Infectious Bronchitis Virus in Colombia from Whole-Genome Analysis. Viruses 2022, 14, 2562. [Google Scholar] [CrossRef]
- Alvarado, I.R.; Villegas, P.; Mossos, N.; Jackwood, M.W. Molecular characterization of avian infectious bronchitis virus strains isolated in Colombia during 2003. Avian Dis. 2005, 49, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, H.; Gallardo, R.; Rosende, S. Isolation of infectious bronchitis virus from broiler chickens in Chile. Avian Dis. 1976, 20, 601–603. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.J.; Dijkman, R.; Guerrero, P.; Calvo, J.; Gonzalez, A.; Hidalgo, H. Variability in biological behaviour, pathogenicity, protectotype and induction of virus neutralizing antibodies by different vaccination programmes to infectious bronchitis virus genotype Q1 strains from Chile. Avian Pathol. 2017, 46, 666–675. [Google Scholar] [CrossRef]
- Rimondi, A.; Craig, M.I.; Vagnozzi, A.; König, G.; Delamer, M.; Pereda, A. Molecular characterization of avian infectious bronchitis virus strains from outbreaks in Argentina (2001–2008). Avian Pathol. 2009, 38, 149–153. [Google Scholar] [CrossRef]
- Van Roeckel, H.; Bullis, K.L.; Flint, O.S.; Clarke, M.K. Poultry disease control service Massachusetts Agricultural Experiment Station, MA. Annu. Rep. Bull. 1942, 388, 99–103. [Google Scholar]
- Bijlenga, G.; Cook, J.K.; Gelb, J., Jr.; de Wit, J.J. Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: A review. Avian Pathol. 2004, 33, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.; Sáenz, L.; Hidalgo, H. Molecular and Antigenic Characterization of GI-13 and GI-16 Avian Infectious Bronchitis Virus Isolated in Chile from 2009 to 2017 Regarding 4/91 Vaccine Introduction. Animals 2019, 9, 656. [Google Scholar] [CrossRef]
- McKinley, E.T.; Jackwood, M.W.; Hilt, D.A.; Kissinger, J.C.; Robertson, J.S.; Lemke, C.; Paterson, A.H. Attenuated live vaccine usage affects accurate measures of virus diversity and mutation rates in avian coronavirus infectious bronchitis virus. Virus Res. 2011, 158, 225–234. [Google Scholar] [CrossRef]
- Chacón, R.D.; Astolfi-Ferreira, C.S.; Chacón, J.L.; Nuñez, L.F.N.; De la Torre, D.I.; Piantino Ferreira, A.J. A seminested RT-PCR for molecular genotyping of the Brazilian BR-I Infectious Bronchitis Virus Strain (GI-11). Mol. Cell Probes 2019, 47, 101426. [Google Scholar] [CrossRef]
- Marandino, A.; Vagnozzi, A.; Craig, M.I.; Tomás, G.; Techera, C.; Panzera, Y.; Vera, F.; Pérez, R. Genetic and antigenic heterogeneity of infectious bronchitis virus in South America: Implications for control programmes. Avian Pathol. 2019, 48, 270–277. [Google Scholar] [CrossRef]
- Salles, G.B.C.; Pilati, G.V.T.; Savi, B.P.; Dahmer, M.; Muniz, C.; Vogt, J.R.; Lima Neto, A.J.d.; Fongaro, G. Infectious Bronchitis Virus (IBV) in Vaccinated and Non-Vaccinated Broilers in Brazil: Surveillance and Persistence of Vaccine Viruses. Microorganisms 2025, 13, 521. [Google Scholar] [CrossRef] [PubMed]
- Strydom, C.; Abolnik, C. Seven infectious bronchitis virus genotypes including South American-origin G1-11 and Asian-origin GVI-1 circulated in southern African poultry from 2010 to 2020. Virus Res. 2025, 355, 199568. [Google Scholar] [CrossRef]
- Gough, R.E.; Randall, C.J.; Dagless, M.; Alexander, D.J.; Cox, W.J.; Pearson, D. A ‘new’ strain of infectious bronchitis virus infecting domestic fowl in Great Britain. Vet. Rec. 1992, 130, 493–494. [Google Scholar] [CrossRef]
- Parsons, D.; Ellis, M.M.; Cavanagh, D.; Cook, J.K. Characterisation of an infectious bronchitis virus isolated from vaccinated broiler breeder flocks. Vet. Rec. 1992, 131, 408–411. [Google Scholar] [CrossRef]
- Villarreal, L.Y.; Sandri, T.L.; Souza, S.P.; Richtzenhain, L.J.; de Wit, J.J.; Brandao, P.E. Molecular epidemiology of avian infectious bronchitis in Brazil from 2007 to 2008 in breeders, broilers, and layers. Avian Dis. 2010, 54, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Laconi, A.; Listorti, V.; Franzo, G.; Cecchinato, M.; Naylor, C.; Lupini, C.; Catelli, E. Molecular characterization of whole genome sequence of infectious bronchitis virus 624I genotype confirms the close relationship with Q1 genotype. Transbound. Emerg. Dis. 2019, 66, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Capua, I.; Gough, R.E.; Mancini, M.; Casaccia, C.; Weiss, C. A ‘novel’ infectious bronchitis strain infecting broiler chickens in Italy. Zentralbl Vet. B 1994, 41, 83–89. [Google Scholar] [CrossRef]
- Yu, L.; Jiang, Y.; Low, S.; Wang, Z.; Nam, S.J.; Liu, W.; Kwangac, J. Characterization of three infectious bronchitis virus isolates from China associated with proventriculus in vaccinated chickens. Avian Dis. 2001, 45, 416–424. [Google Scholar] [CrossRef]
- Marandino, A.; Vagnozzi, A.; Tomás, G.; Techera, C.; Gerez, R.; Hernández, M.; Williman, J.; Realpe, M.; Greif, G.; Panzera, Y.; et al. Origin of New Lineages by Recombination and Mutation in Avian Infectious Bronchitis Virus from South America. Viruses 2022, 14, 2095. [Google Scholar] [CrossRef]
- Icochea, E.; González, R.; Castro-Sanguinetti, G.; Maturrano, L.; Alzamora, L.; Sesti, L.; Chacón, J.; More-Bayona, J. Genetic Analysis of Infectious Bronchitis Virus S1 Gene Reveals Novel Amino Acid Changes in the GI-16 Lineage in Peru. Microorganisms 2023, 11, 691. [Google Scholar] [CrossRef]
- Trevisol, I.M.; Caron, L.; Mores, M.A.Z.; Voss-Rech, D.; da Silva Zani, G.; Back, A.; Marchesi, J.A.P.; Esteves, P.A. Pathogenicity of GI-23 Avian Infectious Bronchitis Virus Strain Isolated in Brazil. Viruses 2023, 15, 1200. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Kipper, D.; Freitas, D.S.S.; Fonseca, A.S.K.; Lunge, V.R. Evolution and Epidemic Spread of the Avian Infectious Bronchitis Virus (IBV) GI-23 in Brazil. Viruses 2023, 15, 1229. [Google Scholar] [CrossRef] [PubMed]
- Bataille, H.; Molenaar, R.J.; Schaefer, G.; Zuanaze, M.; De Wit, S. The combination of infectious bronchitis virus BR1 and Mass vaccines provides broad protection. Avian Pathol. 2025, 54, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Alhafufi, A.N.; Kasem, S.; Almajhdi, F.N.; Albaqshi, H.A.; Alaql, F.A.; Rihan, E.A.; Abd-Allah, E.M.; Alyousaf, A.A.; Aljasem, Y.K.; Aljehani, N.D.; et al. Full-length genome reveals genetic diversity and extensive recombination patterns of Saudi GI-1 and GI-23 genotypes of infectious bronchitis virus. Virol. J. 2025, 22, 1. [Google Scholar] [CrossRef]
- Shah, A.U.; Peddireddi, L.; Wood, B.; Hemida, M.G. Some novel field isolates belonging to lineage-1 of the genotype GI-avian infectious bronchitis virus (AIBV) show strong evidence of recombination with field/vaccinal strains. Infect. Genet. Evol. 2025, 129, 105723. [Google Scholar] [CrossRef]

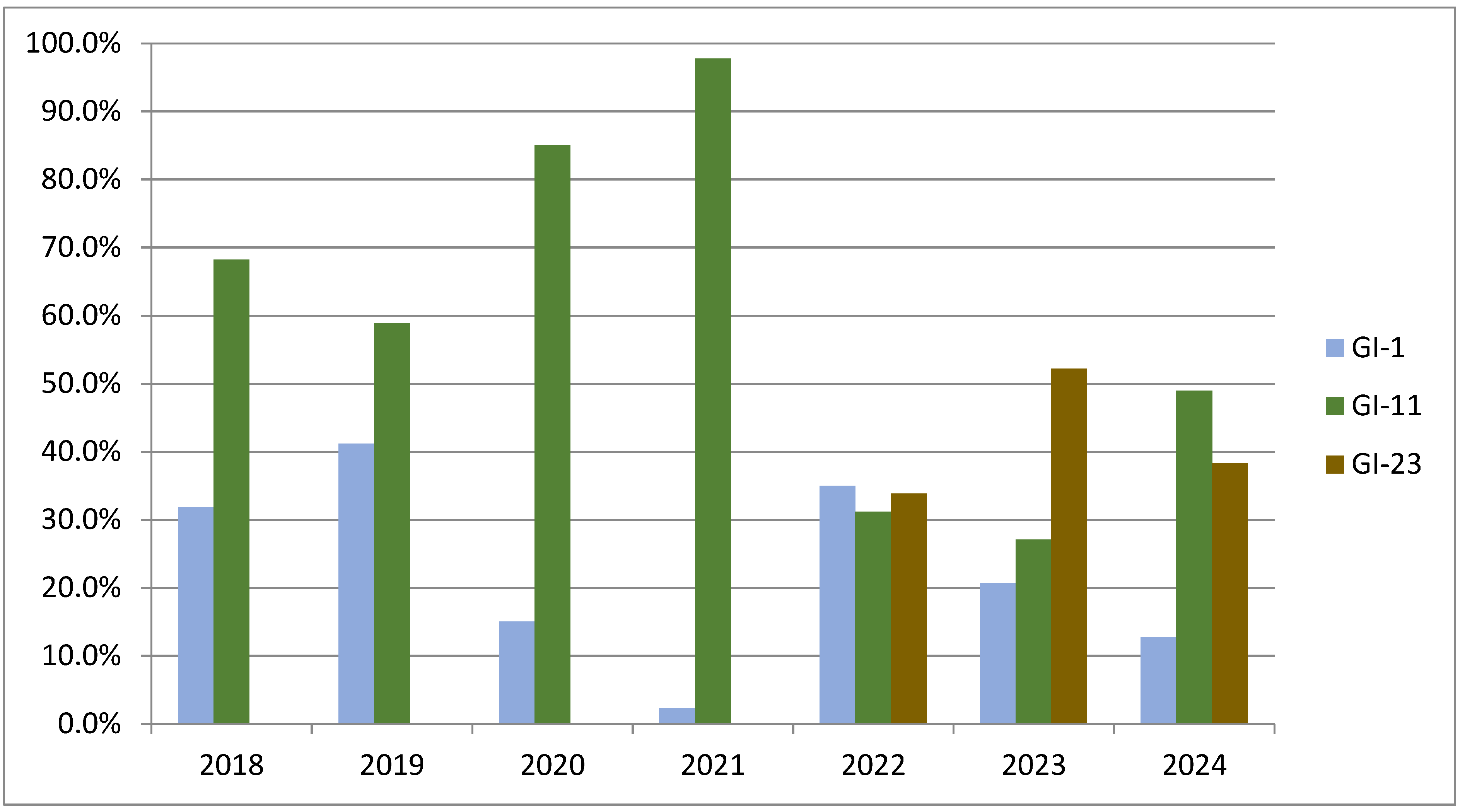
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunge, V.R.; Kipper, D.; Streck, A.F.; Fonseca, A.S.K.; Ikuta, N. Emergence and Dissemination of the Avian Infectious Bronchitis Virus Lineages in Poultry Farms in South America. Vet. Sci. 2025, 12, 435. https://doi.org/10.3390/vetsci12050435
Lunge VR, Kipper D, Streck AF, Fonseca ASK, Ikuta N. Emergence and Dissemination of the Avian Infectious Bronchitis Virus Lineages in Poultry Farms in South America. Veterinary Sciences. 2025; 12(5):435. https://doi.org/10.3390/vetsci12050435
Chicago/Turabian StyleLunge, Vagner Ricardo, Diéssy Kipper, André Felipe Streck, André Salvador Kazantzi Fonseca, and Nilo Ikuta. 2025. "Emergence and Dissemination of the Avian Infectious Bronchitis Virus Lineages in Poultry Farms in South America" Veterinary Sciences 12, no. 5: 435. https://doi.org/10.3390/vetsci12050435
APA StyleLunge, V. R., Kipper, D., Streck, A. F., Fonseca, A. S. K., & Ikuta, N. (2025). Emergence and Dissemination of the Avian Infectious Bronchitis Virus Lineages in Poultry Farms in South America. Veterinary Sciences, 12(5), 435. https://doi.org/10.3390/vetsci12050435





