Effects of Phytogenic Feed Additive on Production Performance, Slaughtering Performance, Meat Quality, and Intestinal Flora of White-Feathered Broilers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Test Materials
2.3. Experimental Design and Feeding Management
2.4. Measurement of the Growth Performance
2.5. Sample Collection
2.6. Measurement of the Carcass Performance
2.7. Determination of the Meat Quality
2.8. Measurement of Cecal Contents
2.9. Statistics and Data Analysis
3. Results
3.1. Impact of Phytogenic Feed Additive on the Growth Performance of White-Feathered Broilers
3.2. Impact of Phytogenic Feed Additive on the Slaughtering Performance of White-Feathered Broilers
3.3. Impact of Phytogenic Feed Additive on the Quality of Meat from White-Feathered Broilers
3.4. Impact of Phytogenic Feed Additive on the Intestinal Flora of White-Feathered Broilers
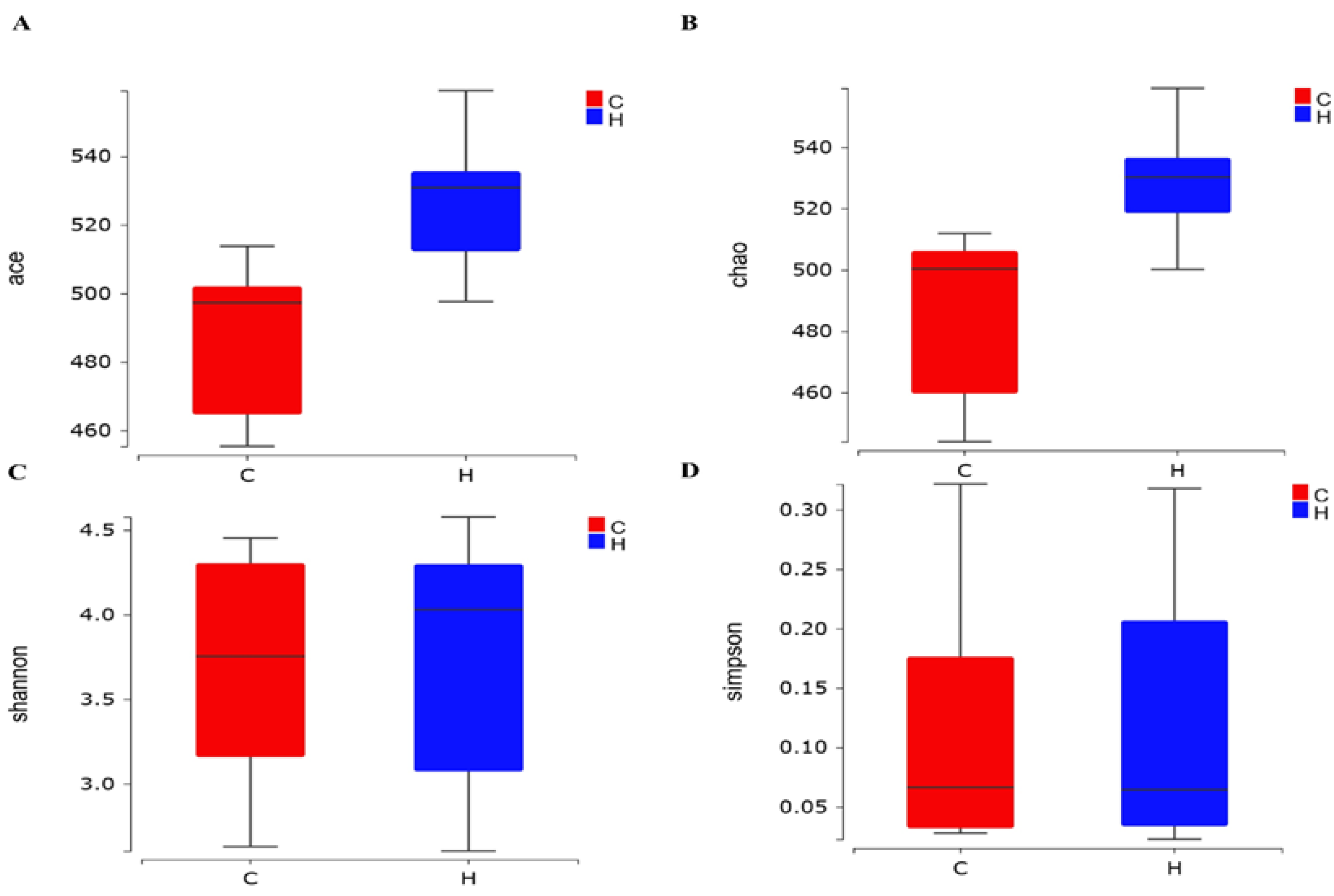
3.4.1. Alpha-Diversity of the Intestinal Flora of White-Feathered Broilers
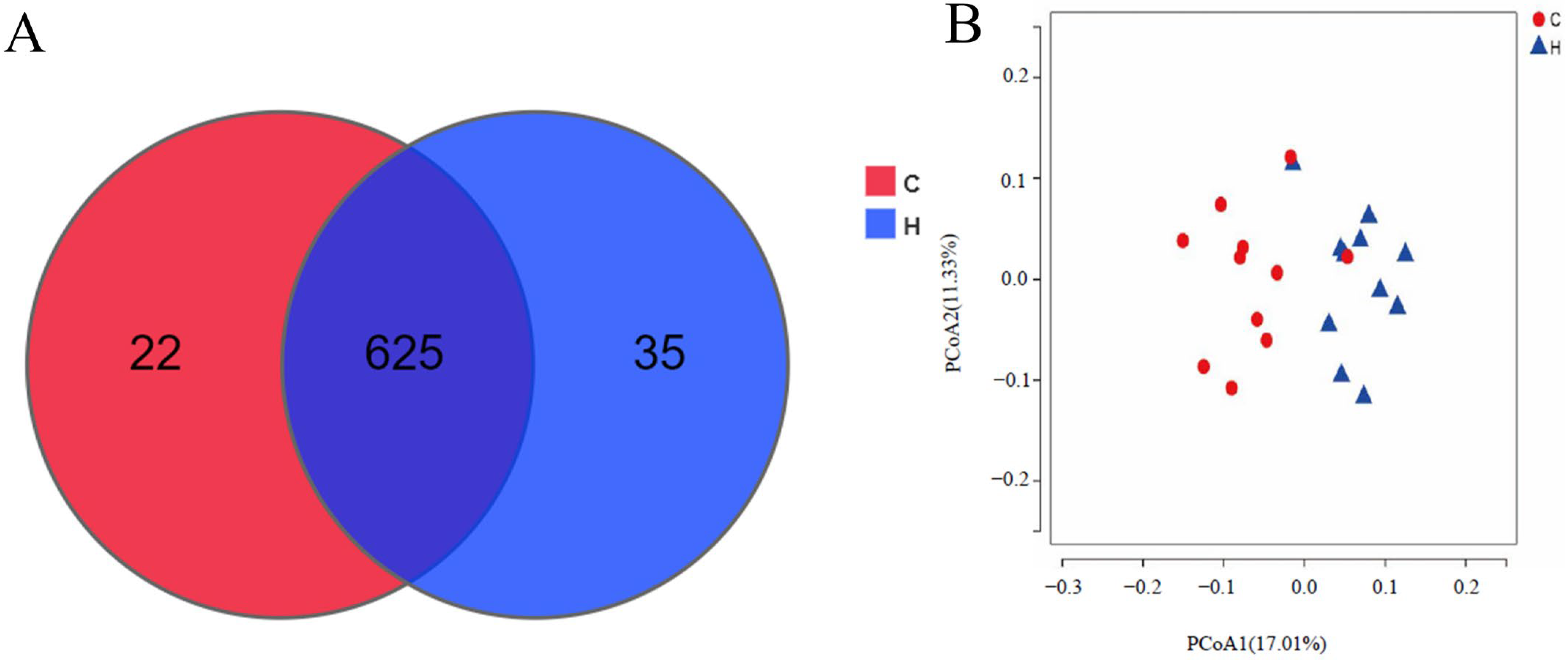
3.4.2. β-Diversity of Intestinal Flora in White-Feathered Broilers
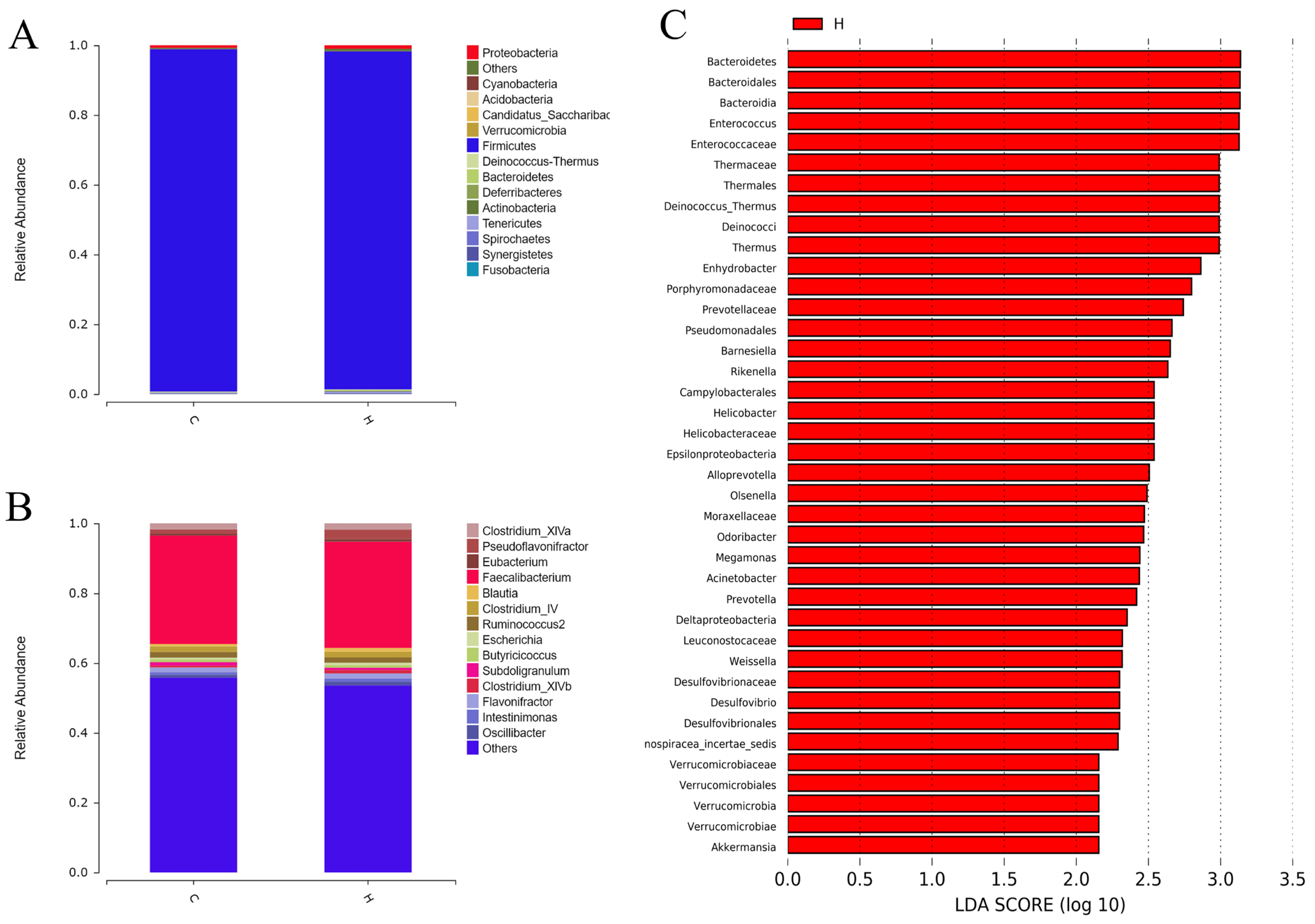
3.5. Composition and Structure of the Intestinal Flora at the Phylum and Genus Levels in White-Feathered Broilers
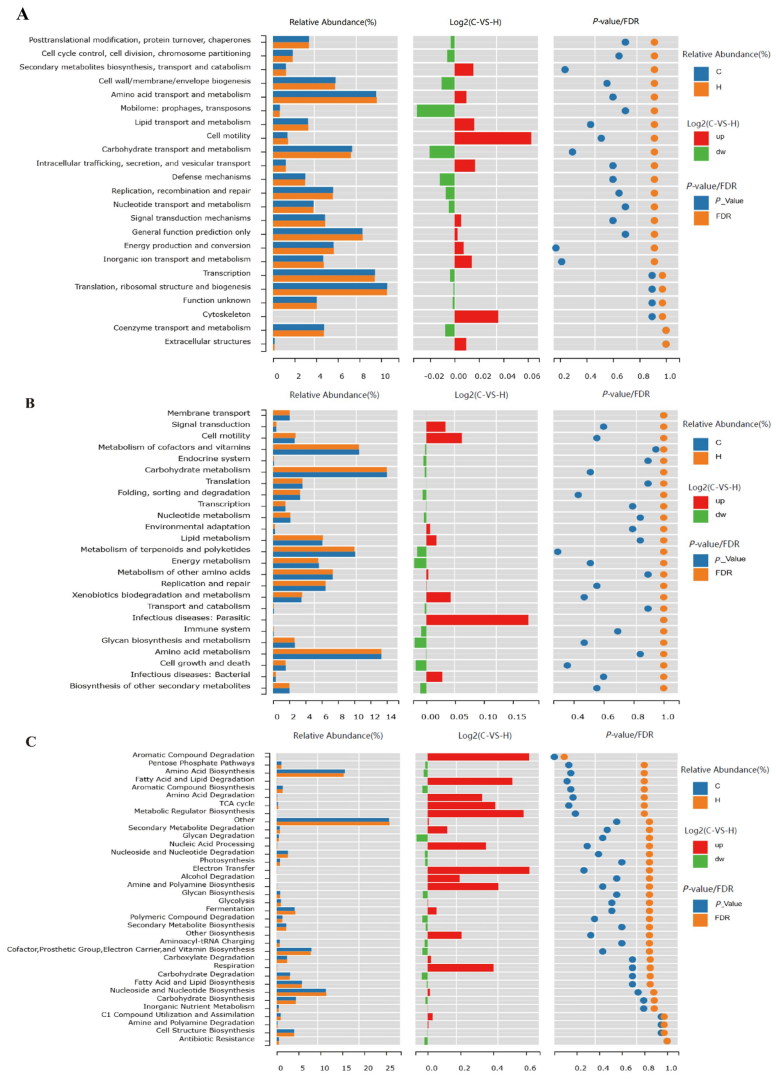
3.6. Predictive Analysis of the COG, KEGG, and Metacyc Pathway Results
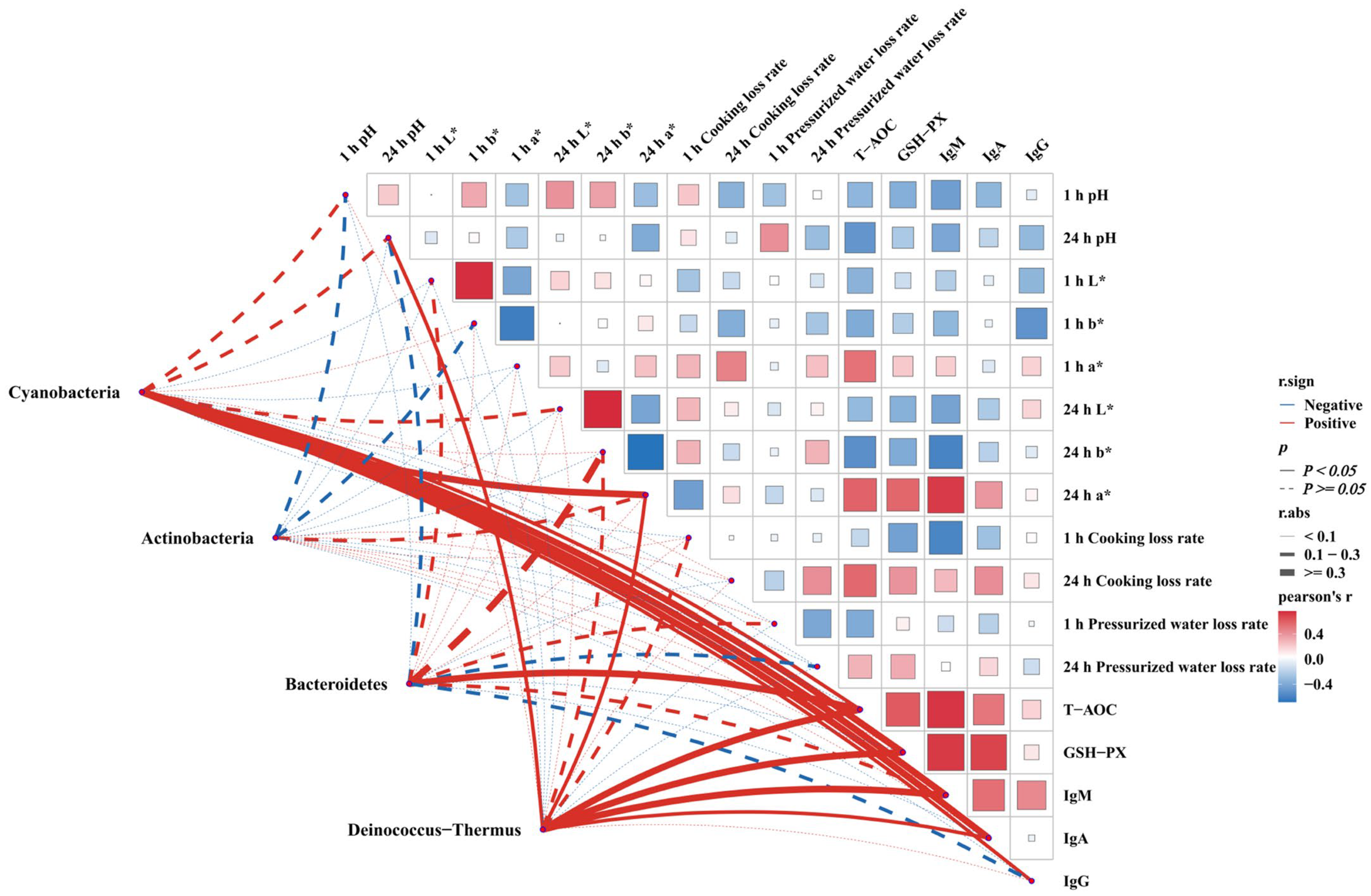
3.7. Analysis of the Correlation Between Clinically Relevant Indices and Gut Contents in White-Feathered Broilers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Zeng, J.; Zhang, L.; Hu, J.; Hao, H.; Zeng, Z.; Li, Y. The fate of antibiotics and antibiotic resistance genes in Large-Scale chicken farm Environments: Preliminary view of the performance of National veterinary Antimicrobial use reduction Action in Guangdong, China. Environ. Int. 2024, 191, 108974. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Halter, B.; Liu, D.; Gilbert, E.R.; Cline, M.A. Dietary Flavonoids as Modulators of Lipid Metabolism in Poultry. Front. Physiol. 2022, 13, 863860. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wei, K.; Liu, L.; Yang, S.; Hu, L.; Zhao, P.; Meng, X.; Shao, M.; Wang, C.; Zhu, L.; et al. Taishan Pinus massoniana pollen polysaccharide inhibits subgroup J avian leucosis virus infection by directly blocking virus infection and improving immunity. Sci. Rep. 2017, 7, 44353. [Google Scholar] [CrossRef] [PubMed]
- Zdunczyk, Z.; Gruzauskas, R.; Juskiewicz, J.; Semaskaite, A.; Jankowski, J.; Godycka-Klos, I.; Jarule, V.; Mieželiene, A.; Alencikiene, G. Growth performance, gastrointestinal tract responses, and meat characteristics of broiler chickens fed a diet containing the natural alkaloid sanguinarine from Macleaya cordata. J. Appl. Poult. Res. 2010, 19, 393–400. [Google Scholar] [CrossRef]
- Ren, J.; Ji, P.; Zhang, X.; Wei, Y.; Zhu, X.; Zhu, Y. Effects of Jianpi Xiaodao Chinese Herbal compound formula for strengthening the spleen and eliminating the conductivity on the growth performance, rumen fermentation parameters and serum indexes of 6-month-old western mongrel cattle. China Anim. Husb. Vet. Med. 2022, 49, 2593–2600. [Google Scholar]
- Liu, L.; Shen, J.; Zhao, C.; Wang, X.; Yao, J.; Gong, Y.; Yang, X. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide. Int. J. Biol. Macromol. 2015, 72, 624–632. [Google Scholar] [CrossRef]
- Ming, K.; He, M.; Su, L.; Du, H.; Wang, D.; Wu, Y.; Liu, J. The inhibitory effect of phosphorylated Codonopsis pilosula polysaccharide on autophagosomes formation contributes to the inhibition of duck hepatitis A virus replication. Poult. Sci. 2020, 99, 2146–2156. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Y.; Guo, F.; Huang, B.; Okyere, S.A.; Wang, H. Comparative analysis of chemical composition, antioxidant and antimicrobial activities of leaves, leaf tea and root from Codonopsis pilosula. Ind. Crops Prod. 2019, 142, 111844. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhang, L.; Yuan, Y.; Yu, D. Codonopsis lanceolata polysaccharide CLPS alleviates high fat/high sucrose diet-induced insulin resistance via anti-oxidative stress. Int. J. Biol. Macromol. 2020, 145, 944–949. [Google Scholar] [CrossRef]
- Xi, N.; Kang, J.; Hao, L.; Li, R.; Bao, Y.; Wanyu, S. Effects of ultrafine powder of the stem and leaf of Astragalus on immunity in chickens. Ital. J. Anim. Sci. 2014, 13, 3022. [Google Scholar] [CrossRef]
- Fu, M.; Wang, J.; Xu, D.; Li, W.; Li, F.; Liu, Z.; Li, Y.; Zhu, C.; Huang, Y.; Huang, Y.; et al. Polysaccharide of Atractylodes macrocephala Koidz alleviates LPS-induced proliferation, differentiation inhibition and excessive apoptosis in chicken embryonic myogenic cells. Vet. Med. Sci. 2024, 103, e1412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Wang, Y.; Hou, W.; Liu, D.; Miao, Z. Research Note: Poria cocos polysaccharide alleviates lipopolysaccharide-induced intestinal inflammation and barrier damage in broiler chickens. Poult. Sci. 2024, 103, 104126. [Google Scholar] [CrossRef] [PubMed]
- Hitache, Z.; Al Dalali, S.; Pei, H.R.; Cao, X.L. Review of the Health Benefits of Cereals and Pseudocereals on Human Gut Microbiota. Food Bioproc. 2023, 16, 2382–2399. [Google Scholar] [CrossRef]
- Koutzoumis, D.N.; Vergara, M.; Pino, J.; Buddendorff, J.; Khoshbouei, H.; Mandel, R.J.; Torres, G.E. Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson’s disease. Exp. Neurol. 2020, 325, 113159. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, D.; Cheng, H.; Wu, J.; Liu, J.; Feng, W.; Peng, C. Repairing gut barrier by traditional Chinese medicine: Roles of gut microbiota. Front. Cell. Infect. Microbiol. 2024, 14, 1389925. [Google Scholar] [CrossRef]
- Ren, S.; Ji, P.; Yan, W.; Liu, W.; Wu, F.; Hua, Y.; Ren, J.; Li, C.; Yang, H. Effects of Jiawei Qiangsan on serum antioxidant and immune functions of white-feathered broilers. Prog. Anim. Med. 2024, 45, 67–71. [Google Scholar]
- Gong, J.; Yin, F.; Hou, Y.; Yin, Y. Review: Chinese herbs as alternatives to antibiotics in feed for swine and poultry production: Potential and challenges in application. Can. J. Anim. Sci. 2014, 94, 223–241. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Meng, S.; Zhang, C.; Guo, L.; Miao, Z. The Effects of Poria cocos Polysaccharides on Growth Performance, Immunity, and Cecal Microflora Composition of Weaned Piglets. Animals 2024, 14, 1121. [Google Scholar] [CrossRef]
- Bai, S.; He, C.; Zhang, K.; Ding, X.; Zeng, Q.; Wang, J.; Peng, H.; Bai, J.; Lu, H.; Xuan, Y.; et al. Effects of dietary inclusion of Radix Bupleuri and Radix Astragali extracts on the performance, intestinal inflammatory cytokines expression, and hepatic antioxidant capacity in broilers exposed to high temperature. Anim. Feed Sci. 2020, 259, 114288. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Ma, C.; Lv, Q.; Yu, H.; Zhang, Q. Effect of ginseng stem leaf extract on the production performance, meat quality, antioxidant status, immune function, and lipid metabolism of broilers. Front. Vet. Sci. 2024, 11, 1463613. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Li, L.; Sun, M.; Li, P.; Yu, Y.; Zhang, Y.; Xu, Z.; Gao, P.; Ma, J.; et al. Effects of dietary supplementation of fermented Artemisia argyi on growth performance, slaughter performance, and meat quality in broilers. Poult. Sci. 2024, 103, 103545. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zou, Z.; Chen, X.; Tan, J.; Liu, L.; Wei, Q.; Xiong, P.; Song, Q.; Chen, J.; Su, W.; et al. Effects of traditional Chinese herbal feed supplement on growth performance, immunity, antioxidant levels, and intestinal health in chickens: A study on Ningdu yellow chickens. Poult. Sci. 2023, 102, 102986. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Gao, J.; Cheng, H.; Guo, G.; Wang, Z. Effects of dietary mulberry leaves on growth, production performance, gut microbiota, and immunological parameters in poultry and livestock: A systematic review and meta-analysis. Anim. Biosci. 2024, 37, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dong, S.; Li, J.; Aizezi, M.; Huang, P.; Abula, S.; Mai, Z.; Liu, D.; Wusiman, A. Effects of Lagenaria siceraria (Molina) Standl polysaccharides on growth performance, immune function, cecum microorganisms and short-chain fatty acids in broilers. Front. Vet. Sci. 2024, 11, 1428623. [Google Scholar] [CrossRef]
- Lu, T.; Abdalla Gibril, B.A.; Xu, J.; Xiong, X. Unraveling the Genetic Foundations of Broiler Meat Quality: Advancements in Research and Their Impact. Genes 2024, 15, 746. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef]
- Jachimowicz, K.; Winiarska-Mieczan, A.; Tomaszewska, E. The Impact of Herbal Additives for Poultry Feed on the Fatty Acid Profile of Meat. Animals 2022, 12, 1054. [Google Scholar] [CrossRef]
- Orlowski, S.; Flees, J.; Greene, E.S.; Ashley, D.; Lee, S.O.; Yang, F.L.; Owens, C.M.; Kidd, M.; Anthony, N.; Dridi, S. Effects of phytogenic additives on meat quality traits in broiler chickens. J. Anim. Sci. 2018, 96, 3757–3767. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, G.; Li, S.; Zhong, X. Effect of a Chinese herbal formula Astragalus immunomodulator on immune function of chickens. Front. Agric. China 2009, 3, 209–215. [Google Scholar] [CrossRef]
- Yue, Y.; Luasiri, P.; Li, J.; Laosam, P.; Sangsawad, P. Research advancements on the diversity and host interaction of gut microbiota in chickens. Front. Vet. Sci. 2024, 11, 1492545. [Google Scholar] [CrossRef]
- Guan, Y.; Zheng, W.; Bai, Y.; Wu, B. Yupingfeng polysaccharide promote the growth of chickens via regulating gut microbiota. Front. Vet. Sci. 2024, 11, 1337698. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Bonos, E.; Skoufos, I.; Tzora, A.; Stylianaki, I.; Lazari, D.; Tsinas, A.; Christaki, E.; Florou-Paneri, P. Effect of herbal feed additives on performance parameters, intestinal microbiota, intestinal morphology and meat lipid oxidation of broiler chickens. Br. Poult. Sci. 2018, 59, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liang, S.; Zhang, Y.; Sun, X.; Li, Y.; Diao, J.; Dong, L.; Ni, H.; Yin, Y.; Ren, J.; et al. Effects of Compound Chinese Herbal Medicine Additive on Growth Performance and Gut Microbiota Diversity of Zi Goose. Animals 2022, 12, 2942. [Google Scholar] [CrossRef]
- McKee, L.S.; La Rosa, S.L.; Westereng, B.; Eijsink, V.G.; Pope, P.B.; Larsbrink, J. Polysaccharide degradation by the Bacteroidetes: Mechanisms and nomenclature. Environ. Microbiol. Rep. 2021, 13, 559–581. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Lewin, G.R.; Carlos, C.; Chevrette, M.G.; Horn, H.A.; McDonald, B.R.; Stankey, R.J.; Fox, B.G.; Currie, C.R. Evolution and Ecology of Actinobacteria and Their Bioenergy Applications. Annu. Rev. Microbiol. 2016, 70, 235–254. [Google Scholar] [CrossRef]
- Tian, B.; Hua, Y. Carotenoid biosynthesis in extremophilic Deinococcus-Thermus bacteria. Trends Microbiol. 2010, 18, 512–520. [Google Scholar] [CrossRef]
- Bhat, V.B.; Madyastha, K.M. C-phycocyanin: A potent peroxyl radical scavenger in vivo and in vitro. Biochem. Biophys. Res. Commun. 2000, 275, 20–25. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Liu, Y.; Yang, Y.; Shi, F.; Cheong, K.L.; Teng, B. Immunostimulatory Effects of Polysaccharides from Spirulina platensis In Vivo and Vitro and Their Activation Mechanism on RAW246.7 Macrophages. Mar. Drugs 2020, 18, 538. [Google Scholar] [CrossRef]
- Pugh, N.; Ross, S.A.; ElSohly, H.N.; ElSohly, M.A.; Pasco, D.S. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001, 67, 737–742. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. Evid. Based Complement. Alternat. Med. 2016, 2016, 7803846. [Google Scholar]
| Item | C Group | L Group | M Group | H Group | p-Value |
|---|---|---|---|---|---|
| Initial weight (g) | 51.53 ± 1.10 | 51.87 ± 0.97 | 51.92 ± 1.20 | 52.22 ± 1.29 | p1 > 0.99 p2 = 0.99 p3 = 0.93 |
| Final weight (g) | 1755.47 ± 54.59 | 2089.99 ± 56.60 * | 2103.06 ± 55.79 * | 2108.40 ± 42.74 * | p1 < 0.001 p2 < 0.001 p3 < 0.001 |
| Average daily weight gain (g/d) | 40.44 ± 1.60 | 48.53 ± 1.34 * | 48.84 ± 1.35 * | 48.96 ± 1.01 * | p1 = 0.001 p2 < 0.001 p3 < 0.001 |
| Average daily feed intake (g/d) | 63.94 ± 0.44 | 66.64 ± 3.65 | 66.65 ± 5.35 | 67.05 ± 5.11 | p1 = 0.94 p2 = 0.94 p3 = 0.92 |
| Material-to-weight ratio | 1.59 ± 0.08 | 1.37 ± 0.08 | 1.36 ± 0.07 | 1.37 ± 0.09 | p1 = 0.20 p2 = 0.17 p3 = 0.19 |
| Item | C Group | H Group | p-Value |
|---|---|---|---|
| Dressing percentage (%) | 91.66 ± 0.89 | 91.76 ± 1.24 | >0.99 |
| Semi-clear chamber rate (%) | 87.66 ± 0.98 | 88.21 ± 1.43 | 0.86 |
| Fully clear chamber rate (%) | 75.38 ± 1.16 | 76.22 ± 1.07 | 0.70 |
| Pectoral muscle rate (%) | 19.82 ± 1.83 | 20.54 ± 0.61 | 0.88 |
| Leg muscle rate (%) | 29.59 ± 2.80 | 29.85 ± 3.04 | >0.99 |
| Belly fat percentage (%) | 1.91 ± 0.22 | 1.77 ± 0.20 | 0.83 |
| Item | C Group | H Group | p-Value |
|---|---|---|---|
| 1 h pH | 6.55 ± 0.11 | 6.43 ± 0.11 * | 0.001 |
| 24 h pH | 5.96 ± 0.20 | 5.83 ± 0.14 | 0.36 |
| 1 h L* (the brightness of meat) | 53.12 ± 0.85 | 52.83 ± 1.11 | 0.89 |
| 1 h b* (the yellowness of meat) | 23.14 ± 1.2 | 22.08 ± 0.90 | 0.10 |
| 1 h a* (the redness of meat) | 4.86 ± 1.85 | 6.38 ± 1.28 * | 0.11 |
| 24 h L* (the brightness of meat) | 55.44 ± 1.68 | 54.22 ± 0.98 | 0.27 |
| 24 h b* (the yellowness of meat) | 23.79 ± 1.63 | 21.59 ± 0.95 * | 0.009 |
| 24 h a* (the redness of meat) | 10.04 ± 3.01 | 15.75 ± 0.94 * | 0.001 |
| 1 h Cooking loss rate (%) | 15.16 ± 2.53 | 12.85 ± 3.23 | 0.56 |
| 24 h Cooking loss rate (%) | 9.66 ± 1.76 | 10.52 ± 2.43 | 0.86 |
| 1 h Pressurized water loss rate (%) | 20.09 ± 2.33 | 19.25 ± 2.75 | 0.96 |
| 24 h Pressurized water loss rate (%) | 33.55 ± 3.45 | 33.34 ± 3.58 | >0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Ren, S.; Yang, H.; Ji, P. Effects of Phytogenic Feed Additive on Production Performance, Slaughtering Performance, Meat Quality, and Intestinal Flora of White-Feathered Broilers. Vet. Sci. 2025, 12, 396. https://doi.org/10.3390/vetsci12050396
Ren J, Ren S, Yang H, Ji P. Effects of Phytogenic Feed Additive on Production Performance, Slaughtering Performance, Meat Quality, and Intestinal Flora of White-Feathered Broilers. Veterinary Sciences. 2025; 12(5):396. https://doi.org/10.3390/vetsci12050396
Chicago/Turabian StyleRen, Jianming, Siyu Ren, Haochi Yang, and Peng Ji. 2025. "Effects of Phytogenic Feed Additive on Production Performance, Slaughtering Performance, Meat Quality, and Intestinal Flora of White-Feathered Broilers" Veterinary Sciences 12, no. 5: 396. https://doi.org/10.3390/vetsci12050396
APA StyleRen, J., Ren, S., Yang, H., & Ji, P. (2025). Effects of Phytogenic Feed Additive on Production Performance, Slaughtering Performance, Meat Quality, and Intestinal Flora of White-Feathered Broilers. Veterinary Sciences, 12(5), 396. https://doi.org/10.3390/vetsci12050396








