Preparation and Evaluation of Novel Epitope-Based ETEC K88-K99 Bivalent Vaccine
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Bacterial Strains and Plasmids
2.2. Bioinformatics-Based Prediction of K99-FanC Epitopes and FaeG-Ep Fusion Construction
2.3. Expression and Identification of the FaeG-Ep Fusion Protein and the FaeG and FanC Proteins
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Mouse Immunization and Infection
- a. Mouse immunization
- b. Inhibition of bacterial adhesion with mouse serum IgG antibodies
- c. Mouse infection
2.6. Statistical Analyses
3. Result
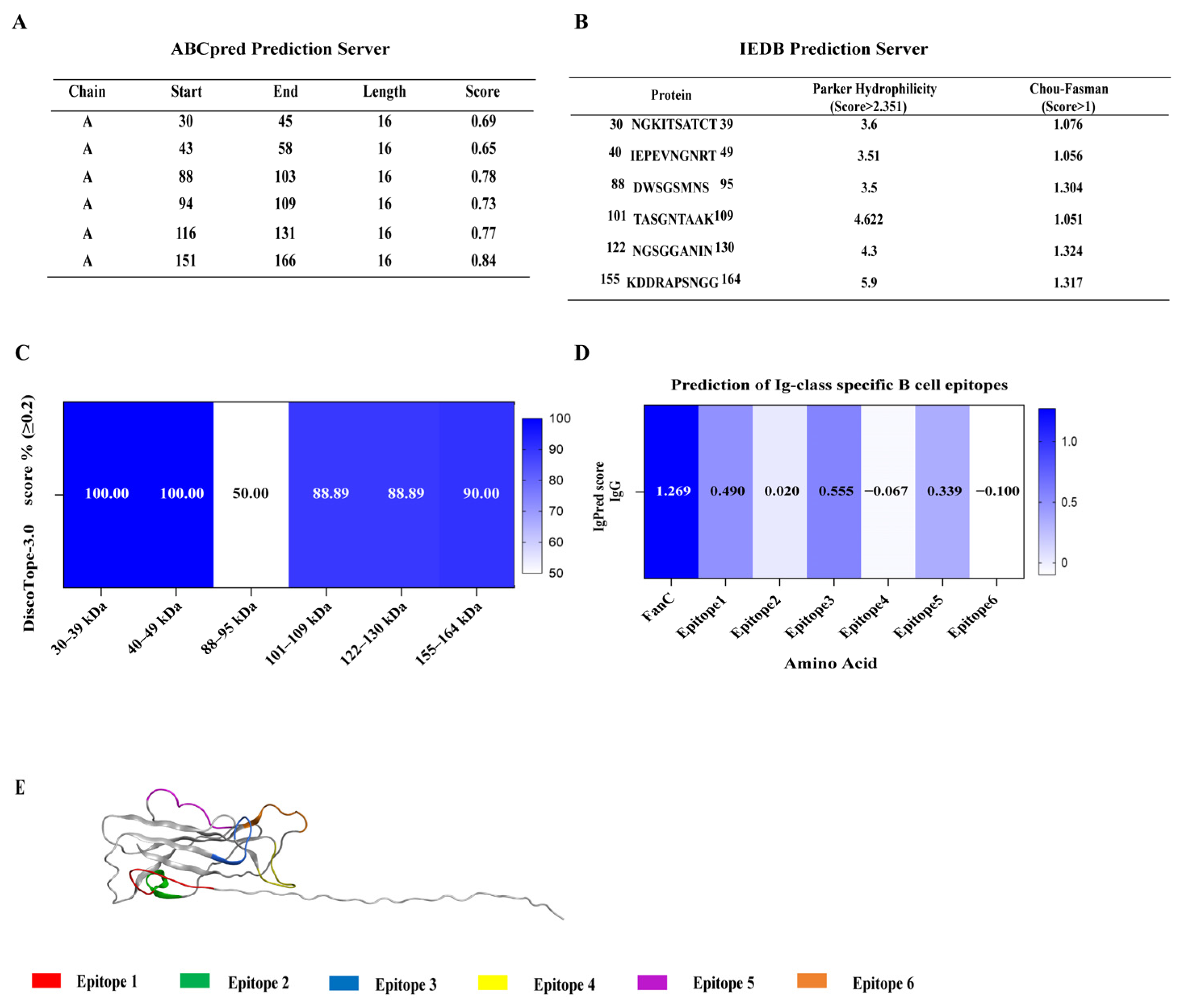
3.1. Selection of K99 Immunodominant B-Cell Epitopes
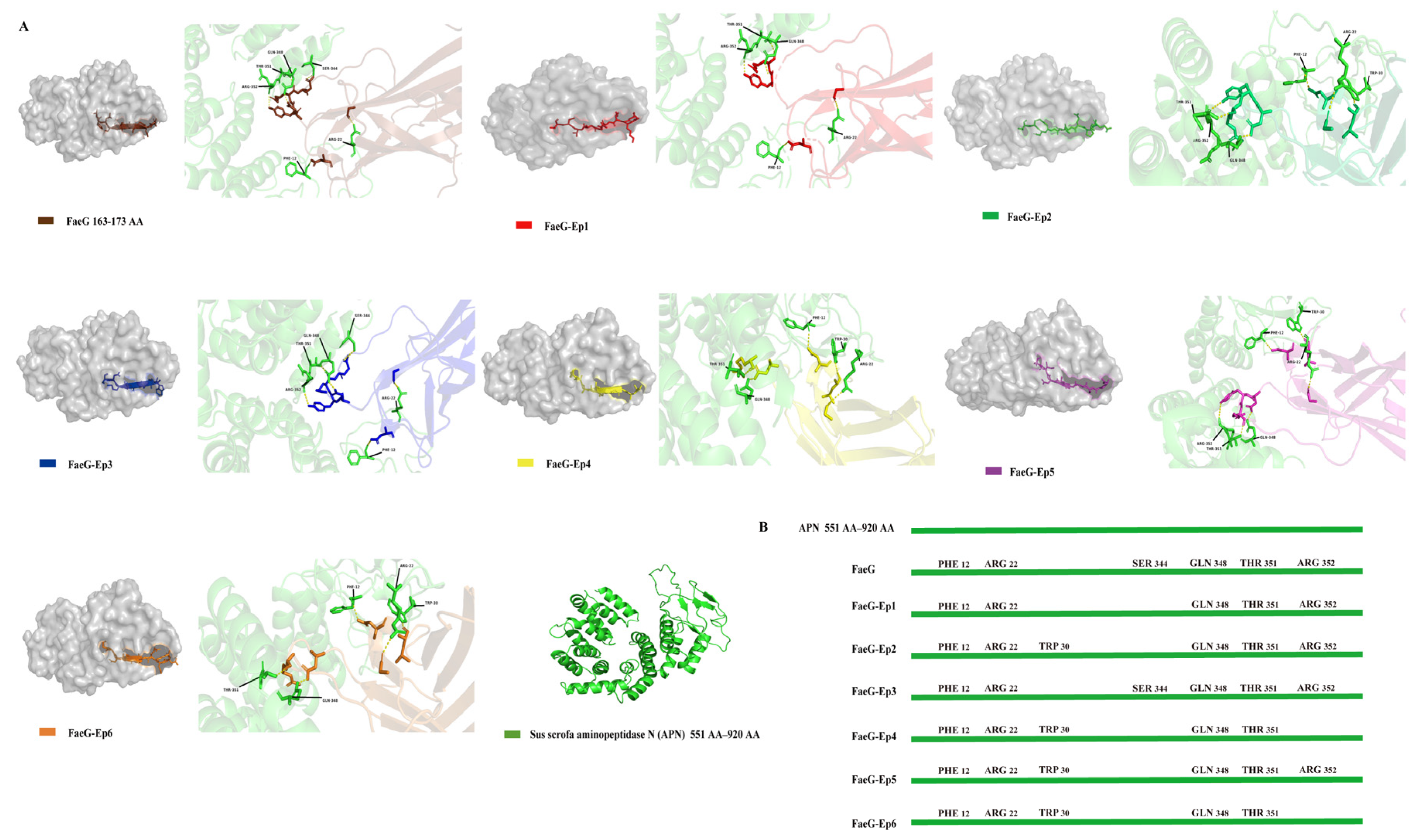
3.2. Design of Peptide-Based Protein and Docking Analysis with Sus Scrofa Aminopeptidase N (APN) Receptors
3.3. Plasmid Construction and Expression of the FaeG-Ep Protein
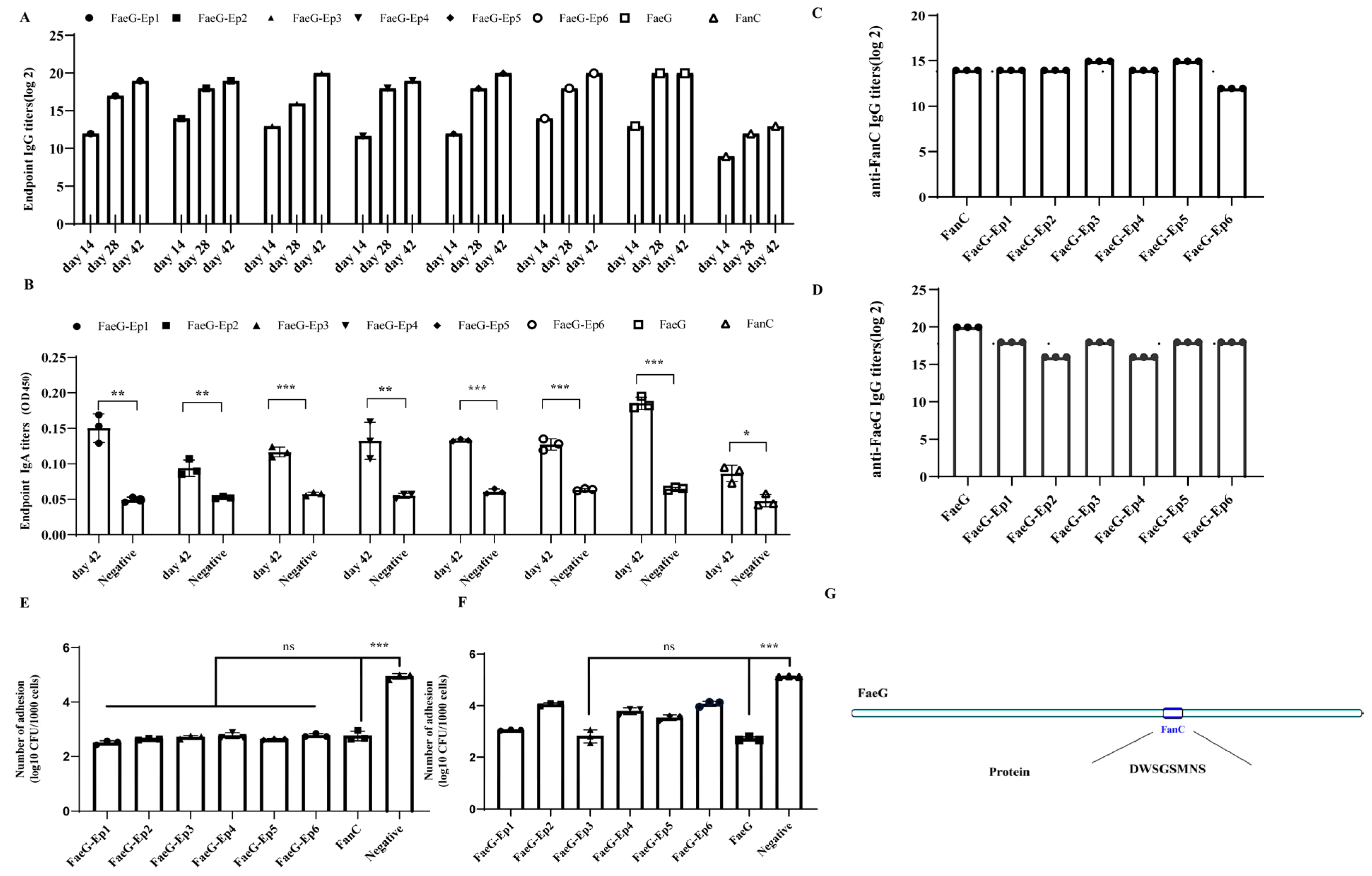
3.4. Verification of the Effective Antigenic Epitope of FanC-Ep on the K88-FaeG-Ep Vaccine In Vitro
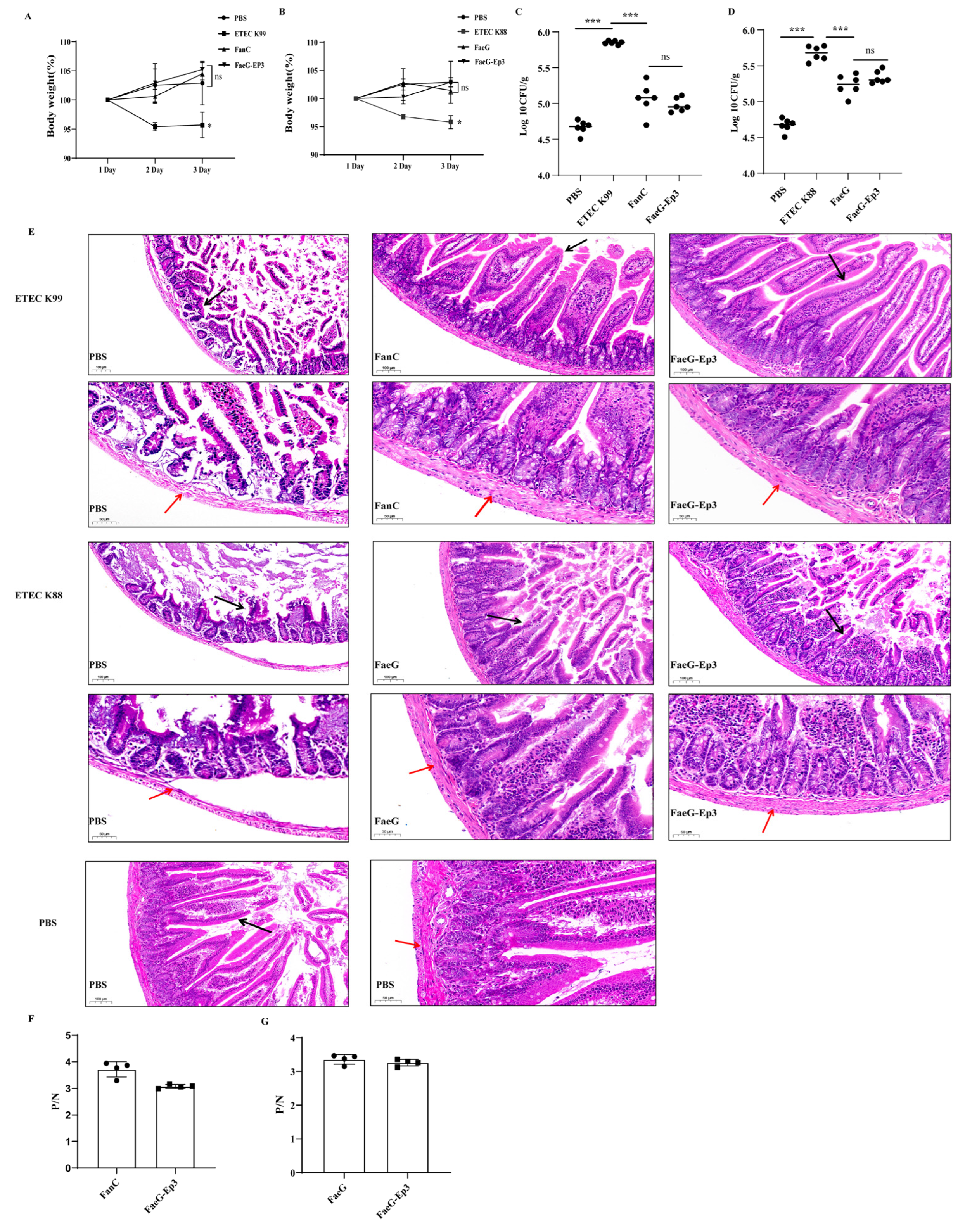
3.5. Verification of the Effective Antigenic Epitope of FanC-Ep in the K88-FaeG-Ep Vaccine In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsueh, F.C.; Lin, C.N.; Chiou, H.Y.; Chia, M.Y.; Chiou, M.T.; Haga, T.; Kao, C.F.; Chang, Y.C.; Chang, C.Y.; Jeng, C.R.; et al. Updated phylogenetic analysis of the spike gene and identification of a novel recombinant porcine epidemic diarrhoea virus strain in Taiwan. Transbound. Emerg. Dis. 2020, 67, 417–430. [Google Scholar] [CrossRef]
- Guo, R.; Fan, B.; Chang, X.; Zhou, J.; Zhao, Y.; Shi, D.; Yu, Z.; He, K.; Li, B. Characterization and evaluation of the pathogenicity of a natural recombinant transmissible gastroenteritis virus in China. Virology 2020, 545, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, E.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef]
- Luppi, A. Swine enteric colibacillosis: Diagnosis, therapy and antimicrobial resistance. Porc. Health Manag. 2017, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Pang, S.; Wu, W.; Jiang, B.; Zhang, W.; Liu, S.; Wang, X.; Pan, Z.; Zhu, G. A multivalent vaccine candidate targeting enterotoxigenic Escherichia coli fimbriae for broadly protecting against porcine post-weaning diarrhea. Vet. Res. 2020, 51, 93. [Google Scholar] [CrossRef]
- Nagy, B.; Whipp, S.C.; Imberechts, H.; Bertschinger, H.U.; Dean-Nystrom, E.A.; Casey, T.A.; Salajka, E. Biological relationship between F18ab and F18ac fimbriae of enterotoxigenic and verotoxigenic Escherichia coli from weaned pigs with oedema disease or diarrhoea. Microb. Pathog. 1997, 22, 1–11. [Google Scholar] [CrossRef]
- Isaacson, R.E.; Richter, P. Escherichia coli 987P pilus: Purification and partial characterization. J. Bacteriol. 1981, 146, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, R.E. K99 surface antigen of Escherichia coli: Purification and partial characterization. Infect. Immun. 1977, 15, 272–279. [Google Scholar] [CrossRef]
- de Graaf, F.K.; Roorda, I. Production, purification, and characterization of the fimbrial adhesive antigen F41 isolated from calf enteropathogenic Escherichia coli strain B41M. Infect. Immun. 1982, 36, 751–758. [Google Scholar] [CrossRef]
- Bakker, D.; Vader, C.E.; Roosendaal, B.; Mooi, F.R.; Oudega, B.; de Graaf, F.K. Structure and function of periplasmic chaperone-like proteins involved in the biosynthesis of K88 and K99 fimbriae in enterotoxigenic Escherichia coli. Mol. Microbiol. 1991, 5, 875–886. [Google Scholar] [CrossRef]
- von Mentzer, A.; Svennerholm, A. Colonization factors of human and animal-specific enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 2024, 32, 448–464. [Google Scholar] [CrossRef]
- Moon, H.W. Colonization factor antigens of enterotoxigenic Escherichia coli in animals. Curr. Top. Microbiol. Immunol. 1990, 151, 147–165. [Google Scholar] [CrossRef]
- Moon, H.W.; Bunn, T.O. Vaccines for preventing enterotoxigenic Escherichia coli infections in farm animals. Vaccine 1993, 11, 200–213. [Google Scholar] [CrossRef]
- Fleckenstein, J.M. Confronting challenges to enterotoxigenic Escherichia coli vaccine development. Front. Trop. Dis. 2021, 2, 709907. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, A.L.; Wierzba, T.F.; Walker, R.I. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 2016, 34, 2880–2886. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.M.; Castro, J.; Araújo, D.; Campos, A.M.; Oliveira, R.; Silva, S.; Outor-Monteiro, D.; Almeida, C. Swine Colibacillosis: Global Epidemiologic and Antimicrobial Scenario. Antibiotics 2023, 12, 682. [Google Scholar] [CrossRef]
- Frydendahl, K. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet. Microbiol. 2002, 85, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Zhou, Y.; Miao, Z. Prevalence and characterization of virulence genes in Escherichia coli isolated from piglets suffering post-weaning diarrhoea in Shandong Province, China. Vet. Med. Sci. 2020, 6, 69–75. [Google Scholar] [CrossRef]
- Blanco, J.; Blanco, M.; Garabal, J.I.; González, E.A. Enterotoxins, colonization factors and serotypes of enterotoxigenic Escherichia coli from humans and animals. Microbiologia 1991, 7, 57–73. [Google Scholar] [PubMed]
- Bakker, D.; Willemsen, P.T.; Simons, L.H.; van Zijderveld, F.G.; de Graaf, F.K. Characterization of the antigenic and adhesive properties of FaeG, the major subunit of K88 fimbriae. Mol. Microbiol. 1992, 6, 247–255. [Google Scholar] [CrossRef]
- Pedersen, P.A.; Andersen, L.N. Deletion and duplication of specific sequences in the K88ab fimbrial subunit protein from porcine enterotoxigenic Escherichia coli. Mol. Gen. Genet. 1991, 229, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Roosendaal, E.; Boots, M.; de Graaf, F.K. Two novel genes, fanA and fanB, involved in the biogenesis of K99 fimbriae. Nucleic Acids Res. 1987, 15, 5973–5984. [Google Scholar] [CrossRef]
- Lu, T.; Moxley, R.A.; Zhang, W. Mapping the Neutralizing Epitopes of Enterotoxigenic Escherichia coli K88 (F4) Fimbrial Adhesin and Major Subunit FaeG. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Wen, L.; Hou, X.; Wang, G.; Yu, L.Y.; Wei, X.M.; Liu, J.K.; Liu, Q.; Wei, C.H. Immunization with recombinant Lactobacillus casei strains producing K99, K88 fimbrial protein protects mice against enterotoxigenic Escherichia coli. Vaccine 2012, 30, 3339–3349. [Google Scholar] [CrossRef]
- Huang, J.; Duan, Q.; Zhang, W. Significance of Enterotoxigenic Escherichia coli (ETEC) Heat-Labile Toxin (LT) Enzymatic Subunit Epitopes in LT Enterotoxicity and Immunogenicity. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Rausch, D.; Ruan, X.; Nandre, R.; Duan, Q.; Hashish, E.; Casey, T.A.; Zhang, W. Antibodies derived from a toxoid MEFA (multiepitope fusion antigen) show neutralizing activities against heat-labile toxin (LT), heat-stable toxins (STa, STb), and Shiga toxin 2e (Stx2e) of porcine enterotoxigenic Escherichia coli (ETEC). Vet. Microbiol. 2017, 202, 79–89. [Google Scholar] [CrossRef]
- Ruan, X.; Robertson, D.C.; Nataro, J.P.; Clements, J.D.; Zhang, W.; STa Toxoid Vaccine Consortium Group. Characterization of heat-stable (STa) toxoids of enterotoxigenic Escherichia coli fused to double mutant heat-labile toxin peptide in inducing neutralizing Anti-STa antibodies. Infect. Immun. 2014, 82, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, C.; Francis, D.H.; Fang, Y.; Knudsen, D.; Nataro, J.P.; Robertson, D.C. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect. Immun. 2010, 78, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Seo, H.; Moxley, R.A.; Zhang, W. Mapping the neutralizing epitopes of F18 fimbrial adhesin subunit FedF of enterotoxigenic Escherichia coli (ETEC). Vet. Microbiol. 2019, 230, 171–177. [Google Scholar] [CrossRef]
- Deng, G.; Li, W.; Wu, X.; Bao, S.; Zeng, J.; Zhao, N.; Luo, M.; Liu, X.; Wang, Y. Immunogenicity and protective efficacy of a recombinant adenoviral based vaccine expressing heat-stable enterotoxin (STa) and K99 adhesion antigen of enterotoxigenic Escherichia coli in mice. Mol. Immunol. 2015, 68, 684–691. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; He, Y.; Chen, Y.; Hu, C.; Chen, J.; Zhang, L.; Chen, X.; Guo, A. A Novel Multiepitope Fusion Antigen as a Vaccine Candidate for the Prevention of Enterotoxigenic E. coli-Induced Calf Diarrhea. Vaccines 2024, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef]
- Lee, C.H.; Koohy, H. In silico identification of vaccine targets for 2019-nCoV. F1000Res 2020, 9, 145. [Google Scholar] [CrossRef]
- Rao, J.; Liu, X.; Zhu, X.; Qi, Y.; Chen, H.; Bei, W. Novel Soluble apxIVA-Truncated Protein and Its Application to Rapid Detection and Distinction of Actinobacillus pleuropneumoniae Wild-Strain-Infected Samples from Those Vaccinated with apxIV-Partially Deleted Vaccine. Vet. Sci. 2025, 12, 278. [Google Scholar] [CrossRef]
- Tang, C.; Xie, B.; Zong, Q.; Sun, Z. Proanthocyanidins and probiotics combination supplementation ameliorated intestinal injury in Enterotoxigenic Escherichia coli infected diarrhea mice. J. Funct. Foods 2019, 62, 103521. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.; Wang, S.; Gao, T.; Liu, W.; Yang, K.; Yuan, F.; Wu, Q.; Li, C.; Guo, R.; et al. Prevalence, Molecular Characterization, and Antimicrobial Resistance Profile of Enterotoxigenic Escherichia coli Isolates from Pig Farms in China. Foods 2025, 14, 1188. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Quan, G.; Yang, Y.; Zhao, J.; Wang, Y.; Zhou, M.; Hardwidge, P.R.; Zhu, J.; Liu, S.; Zhu, G. Binding determinants in the interplay between porcine aminopeptidase N and enterotoxigenic Escherichia coli F4 fimbriae. Vet. Res. 2018, 49, 23. [Google Scholar] [CrossRef] [PubMed]
- Melkebeek, V.; Goddeeris, B.M.; Cox, E. ETEC vaccination in pigs. Vet. Immunol. Immunopathol. 2013, 152, 37–42. [Google Scholar] [CrossRef]
- Fairbrother, J.M.; Nadeau, E.; Belanger, L.; Tremblay, C.L.; Tremblay, D.; Brunelle, M.; Wolf, R.; Hellmann, K.; Hidalgo, Á. Immunogenicity and protective efficacy of a single-dose live non-pathogenic Escherichia coli oral vaccine against F4-positive enterotoxigenic Escherichia coli challenge in pigs. Vaccine 2017, 35, 353–360. [Google Scholar] [CrossRef]
- Nadeau, E.; Fairbrother, J.M.; Zentek, J.; Bélanger, L.; Tremblay, D.; Tremblay, C.L.; Röhe, I.; Vahjen, W.; Brunelle, M.; Hellmann, K.; et al. Efficacy of a single oral dose of a live bivalent E. coli vaccine against post-weaning diarrhea due to F4 and F18-positive enterotoxigenic E. coli. Vet. J. 2017, 226, 32–39. [Google Scholar] [CrossRef]
- Ran, X.; Chen, X.; Wang, S.; Chang, C.; Wen, X.; Zhai, J.; Ni, H. Preparation of porcine enterotoxigenic Escherichia coli (ETEC) ghosts and immunogenic analysis in a mouse model. Microb. Pathog. 2019, 126, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Moxley, R.A.; Zhang, W. Application of a Novel Epitope- and Structure-Based Vaccinology-Assisted Fimbria-Toxin Multiepitope Fusion Antigen of Enterotoxigenic Escherichia coli for Development of Multivalent Vaccines against Porcine Postweaning Diarrhea. Appl. Environ. Microbiol. 2020, 86, e00274-20. [Google Scholar] [CrossRef]
- Deepthi, V.; Sasikumar, A.; Mohanakumar, K.P.; Rajamma, U. Computationally designed multi-epitope vaccine construct targeting the SARS-CoV-2 spike protein elicits robust immune responses in silico. Sci. Rep. 2025, 15, 9562. [Google Scholar] [CrossRef] [PubMed]
- Ascon, M.A.; Ochoa-Reparaz, J.; Walters, N.; Pascual, D.W. Partially assembled K99 fimbriae are required for protection. Infect. Immun. 2005, 73, 7274–7280. [Google Scholar] [CrossRef] [PubMed]
- Jay, C.M.; Bhaskaran, S.; Rathore, K.S.; Waghela, S.D. Enterotoxigenic K99+ Escherichia coli attachment to host cell receptors inhibited by recombinant pili protein. Vet. Microbiol. 2004, 101, 153–160. [Google Scholar] [CrossRef]
- Wei, C.H.; Liu, J.K.; Hou, X.L.; Yu, L.Y.; Lee, J.S.; Kim, C.J. Immunogenicity and protective efficacy of orally or intranasally administered recombinant Lactobacillus casei expressing ETEC K99. Vaccine 2010, 28, 4113–4118. [Google Scholar] [CrossRef]
- Verdonck, F.; De Hauwere, V.; Bouckaert, J.; Goddeeris, B.M.; Cox, E. Fimbriae of enterotoxigenic Escherichia coli function as a mucosal carrier for a coupled heterologous antigen. J. Control Release 2005, 104, 243–258. [Google Scholar] [CrossRef]
- Ogunniyi, A.D.; Kotlarski, I.; Morona, R.; Manning, P.A. Epitope analysis of the FanC subunit protein of the K99 (F5) fimbriae of enterotoxigenic Escherichia coli using a recombinant fusion technique. FEMS Immunol. Med. Microbiol. 2002, 34, 23–31. [Google Scholar] [CrossRef]
- Xia, P.; Wang, Y.; Zhu, C.; Zou, Y.; Yang, Y.; Liu, W.; Hardwidge, P.R.; Zhu, G. Porcine aminopeptidase N binds to F4+ enterotoxigenic Escherichia coli fimbriae. Vet. Res. 2016, 47, 24. [Google Scholar] [CrossRef]
- Diard, M.; Hardt, W. Basic Processes in Salmonella-Host Interactions: Within-Host Evolution and the Transmission of the Virulent Genotype. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Carroll, C.J.; Hocking, D.M.; Azzopardi, K.I.; Praszkier, J.; Bennett-Wood, V.; Almeida, K.; Ingle, D.J.; Baines, S.L.; Tauschek, M.; Robins-Browne, R.M. Re-evaluation of a Neonatal Mouse Model of Infection with Enterotoxigenic Escherichia coli. Front. Microbiol. 2021, 12, 651488. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Warfel, K.F.; Desai, P.; Li, J.; Lee, J.J.; Wong, D.A.; Nguyen, P.M.; Qin, Y.; Sobol, S.E.; Jewett, M.C.; et al. A low-cost recombinant glycoconjugate vaccine confers immunogenicity and protection against enterotoxigenic Escherichia coli infections in mice. Front. Mol. Biosci. 2023, 10, 1085887. [Google Scholar] [CrossRef]
- Yu, M.; Qi, R.; Chen, C.; Yin, J.; Ma, S.; Shi, W.; Wu, Y.; Ge, J.; Jiang, Y.; Tang, L.; et al. Immunogenicity of recombinant Lactobacillus casei-expressing F4 (K88) fimbrial adhesin FaeG in conjunction with a heat-labile enterotoxin A (LTAK63) and heat-labile enterotoxin B (LTB) of enterotoxigenic Escherichia coli as an oral adjuvant in mice. J. Appl. Microbiol. 2017, 122, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Wu, W.; Pang, S.; Pan, Z.; Zhang, W.; Zhu, G. Coimmunization with Two Enterotoxigenic Escherichia coli (ETEC) Fimbrial Multiepitope Fusion Antigens Induces the Production of Neutralizing Antibodies against Five ETEC Fimbriae (F4, F5, F6, F18, and F41). Appl. Environ. Microbiol. 2020, 86, e00217-20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, W. Escherichia coli K88ac fimbriae expressing heat-labile and heat-stable (STa) toxin epitopes elicit antibodies that neutralize cholera toxin and STa toxin and inhibit adherence of K88ac fimbrial E. coli. Clin. Vaccine Immunol. 2010, 17, 1859–1867. [Google Scholar] [CrossRef]
- Pandey, K.K.; Sahoo, B.R.; Pattnaik, A.K. Protein Nanoparticles as Vaccine Platforms for Human and Zoonotic Viruses. Viruses 2024, 16, 936. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Fux, R.; Palic, D. Ferritin Vaccine Platform for Animal and Zoonotic Viruses. Vaccines 2024, 12, 1112. [Google Scholar] [CrossRef]

| Strains | Relevant Properties | Sources |
|---|---|---|
| C83549 | Porcine E. coli field isolate, K88ac | Hubei Academy of Agricultural Sciences |
| C83644 | Porcine E. coli field isolate, K99 | |
| pET/FaeG | FaeG + pET30α (+) in DH5α/BL21 | This study |
| pET/FanC | FanC + pET30α (+) in DH5α/BL21 | This study |
| pET/FaeG-Ep1 | FaeG-Ep1 + pET30α (+) in DH5α/BL21 | This study |
| pET/FaeG-Ep2 | FaeG-Ep2 + pET30α (+) in DH5α/BL21 | This study |
| pET/FaeG-Ep3 | FaeG-Ep3 + pET30α (+) in DH5α/BL21 | This study |
| pET/FaeG-Ep4 | FaeG-Ep4 + pET30α (+) in DH5α/BL21 | This study |
| pET/FaeG-Ep5 | FaeG-Ep5 + pET30α (+) in DH5α/BL21 | This study |
| pET/FaeG-Ep6 | FaeG-Ep6 + pET30α (+) in DH5α/BL21 | This study |
| Antigen Name | Concentration | p-Value | N-Value | p-Value/N-Value |
|---|---|---|---|---|
| K88-FaeG | 11.7 ng/mL | 1.18 | 0.12 | 9.86 |
| K99-FanC | 93.75 ng/mL | 1.01 | 0.06 | 15.78 |
| FaeG-Ep1 | 1 μg/mL | 1.0 | 0.07 | 14.23 |
| FaeG-Ep2 | 46.87 ng/mL | 1.12 | 0.15 | 7.36 |
| FaeG-Ep3 | 4 μg/mL | 0.95 | 0.09 | 10.23 |
| FaeG-Ep4 | 4 μg/mL | 1.0 | 0.09 | 10.23 |
| FaeG-Ep5 | 4 μg/mL | 0.93 | 0.06 | 13.63 |
| FaeG-Ep6 | 187.5 ng/mL | 1.0 | 0.09 | 11.68 |
| Antigen Name | Protein Sequence |
|---|---|
| K88 | FaeG |
| K99 | FanC |
| Epitope 1 | 30NGKITSATCT39 |
| Epitope 2 | 40IEPEVNGNRT49 |
| Epitope 3 | 88DWSGSMNS95 |
| Epitope 4 | 101TASGNTAAK109 |
| Epitope 5 | 122NGSGGANIN130 |
| Epitope 6 | 155KDDRAPSNGG164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yang, Y.; Yue, X.; Liu, Z.; Yuan, F.; Yang, K.; Zhu, J.; Liu, W.; Tian, Y.; Wu, Q.; et al. Preparation and Evaluation of Novel Epitope-Based ETEC K88-K99 Bivalent Vaccine. Vet. Sci. 2025, 12, 381. https://doi.org/10.3390/vetsci12040381
Wang S, Yang Y, Yue X, Liu Z, Yuan F, Yang K, Zhu J, Liu W, Tian Y, Wu Q, et al. Preparation and Evaluation of Novel Epitope-Based ETEC K88-K99 Bivalent Vaccine. Veterinary Sciences. 2025; 12(4):381. https://doi.org/10.3390/vetsci12040381
Chicago/Turabian StyleWang, Shuangshuang, Yuxin Yang, Xinru Yue, Zewen Liu, Fangyan Yuan, Keli Yang, Jiajia Zhu, Wei Liu, Yongxiang Tian, Qiong Wu, and et al. 2025. "Preparation and Evaluation of Novel Epitope-Based ETEC K88-K99 Bivalent Vaccine" Veterinary Sciences 12, no. 4: 381. https://doi.org/10.3390/vetsci12040381
APA StyleWang, S., Yang, Y., Yue, X., Liu, Z., Yuan, F., Yang, K., Zhu, J., Liu, W., Tian, Y., Wu, Q., Gao, T., Li, C., Song, H., Zhou, D., & Bei, W. (2025). Preparation and Evaluation of Novel Epitope-Based ETEC K88-K99 Bivalent Vaccine. Veterinary Sciences, 12(4), 381. https://doi.org/10.3390/vetsci12040381






