Effect of Shearing for Improving the Thermoregulatory Responses of Crossbred Sheep During Heat Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Period and Ethics Approval
2.2. Animals, Climate Chamber, and Climate Data
2.3. Study Design, Heat Exposure, and Experimental Treatments
2.4. Biological Records and Sampling
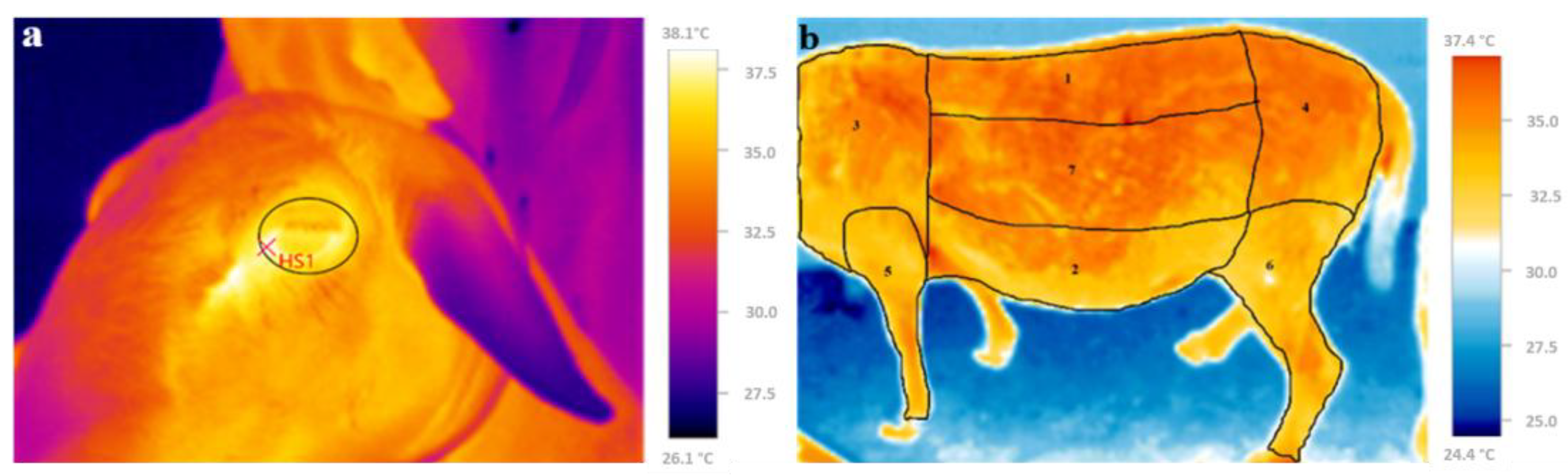
2.4.1. Ocular Surface Temperature and Skin Temperature
2.4.2. Rectal Temperature and Respiration Rate
2.4.3. Sweating Rate and Sample Collection for Cortisol Analysis
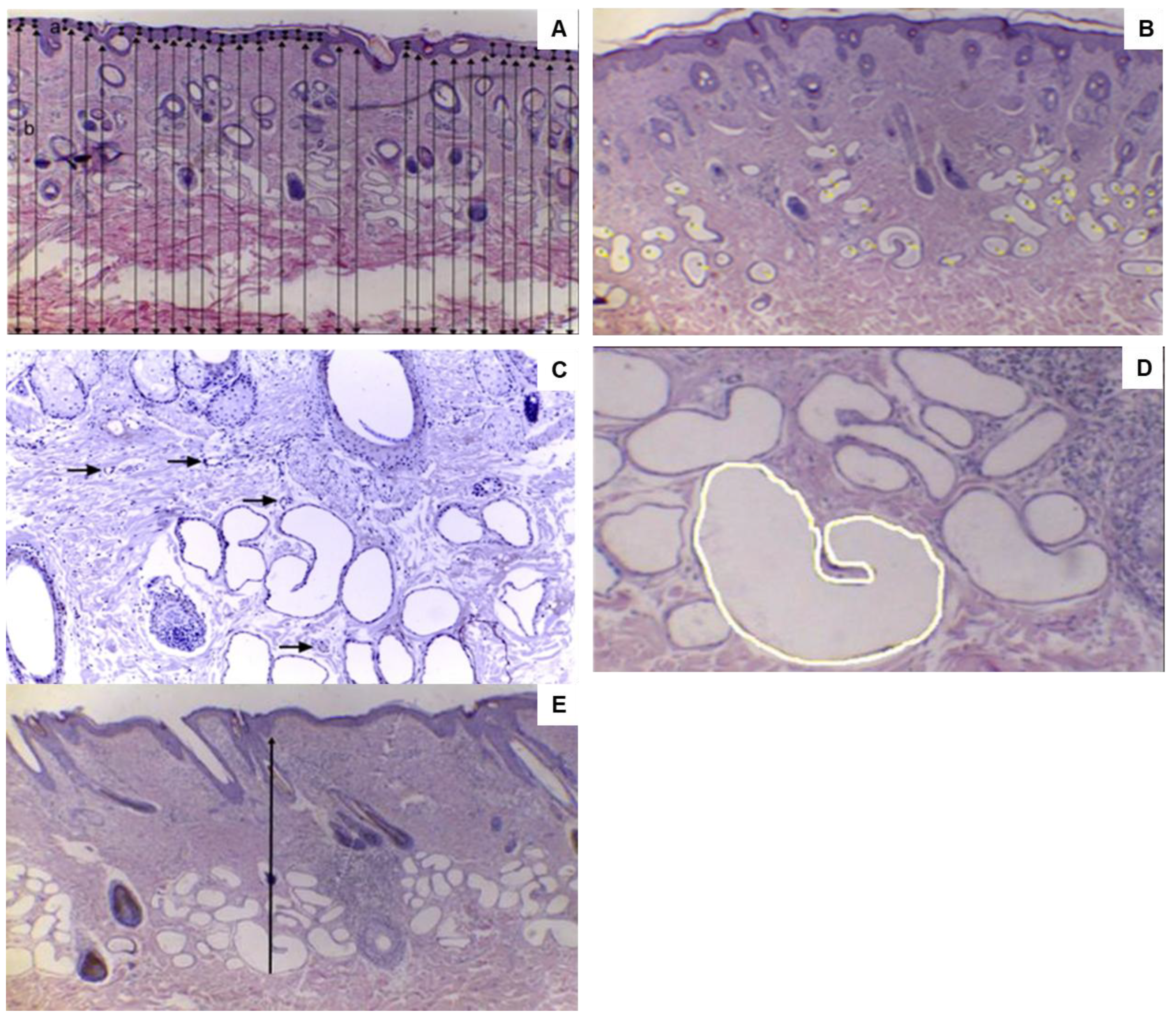
2.4.4. Skin Tissue Biopsies and Histological Analysis
2.5. Statistical Analysis
3. Results
3.1. Climatic Chamber
3.2. Biological Records: Rectal Temperature, Ocular Surface Temperature, Respiration Rate, and Sweating Rate
3.3. Skin Temperature
3.4. Histological Characteristics and Hormone Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OST | Ocular surface temperature |
| ST | Skin temperature |
| RR | Respiration rate |
| RT | Rectal temperature |
| SR | Sweating rate |
| NSG | Number of sweating glands |
| AT | Air temperature |
| RH | Relative humidity |
| THI | Temperature humidity index |
References
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Wisconsin Department of Natural Resources. The Science of Climate Change. Available online: https://dnr.wisconsin.gov/climatechange/science (accessed on 11 December 2023).
- Rashamol, P.V.; Sejian, V.; Bagath, M.; Krishnan, G.; Archana, P.R.; Bhatta, R. Physiological adaptability of livestock to heat Stress: An updated review. J. Anim. Behav. Biometeorol. 2020, 6, 62–71. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of animals to heat stress. Animal 2018, 12, s431–s444. [Google Scholar] [CrossRef]
- McManus, C.; Dallago, B.S.L.; Lehugeur, C.; Ribeiro, L.A.; Hermuche, P.; Guimarães, R.F.; Carvalho Júnior, O.A.; Paiva, S.R. Patterns of heat tolerance in different sheep breeds in Brazil. Small Rumin. Res. 2016, 144, 290–299. [Google Scholar] [CrossRef]
- El-Tarabany, M.S.; El-Tarabany, A.A.; Atta, M.A. Physiological and lactation responses of Egyptian dairy Baladi goats to natural thermal stress under subtropical environmental conditions. Int. J. Biometeorol. 2017, 61, 61–68. [Google Scholar] [CrossRef]
- McManus, C.; Paiva, S.R.; Olegário De Araújo, R. Genetics and breeding of sheep in Brazil. Rev. Bras. Zootec. 2010, 39, 236–246. [Google Scholar] [CrossRef]
- McManus, C.; Pinto, B.F.; Martins, R.F.S.; Louvandini, H.; Paiva, S.R.; Braccini Neto, J.; Paim, T.d.P. Selection objectives and criteria for sheep in Central Brazil. Rev. Bras. Zootec. 2011, 40, 2713–2720. [Google Scholar] [CrossRef]
- Milne, C. The history of the Dorper sheep. Small Rumin. Res. 2000, 36, 99–102. [Google Scholar] [CrossRef]
- Cezar, M.F.; de Souza, B.B.; de Souza, W.H.; Pimenta Filho, E.C.; Tavares, G.d.P.; Medeiros, G.X. Physiologic parameters of the Dorper and Santa Inês sheep and their cross submitted to the climatic conditions of the tropic semi-arid northeasterner. Cienc. Agrotecnol. 2004, 28, 614–620. [Google Scholar] [CrossRef]
- Pulido-Rodríguez, L.F.; Titto, C.G.; de Andrade Bruni, G.; Froge, G.A.; Fuloni, M.F.; Payan-Carrera, R.; Henrique, F.L.; de Mira, A.C.A.P.; Pereira, A.M.F. Effect of solar radiation on thermoregulatory responses of Santa Inês sheep and their crosses with wool and hair Dorper sheep. Small Rum. Res. 2021, 202, 106470. [Google Scholar] [CrossRef]
- Silveira, R.M.F.; Garcia, P.R.; de Castro Júnior, S.L.; Arno, A.; da Silva, I.J.O. Are there differences in the adaptive profile of hair sheep and their crosses with wool breeds? Int. J. Biometeorol. 2024, 68, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, N.M.H.; Furtado, D.A.; de Souza, B.B.; de Sousa, O.B.; da Costa, A.N.L.; Feitosa, J.V.; da Silva, M.R.; Batista, L.F.; Dornelas, K.C. Morphology of coat and skin of small ruminants reared in the Brazilian semi-arid region. J. Therm. Biol. 2023, 112, 103418. [Google Scholar] [CrossRef] [PubMed]
- Marcone, G.; Kaart, T.; Piirsalu, P.; Arney, D.R. Panting scores as a measure of heat stress evaluation in sheep with access and with no access to shade. Appl. Anim. Behav. Sci. 2021, 240, 105350. [Google Scholar] [CrossRef]
- Marai, I.F.M.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological traits as affected by heat stress in sheep—A review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Pantoja, M.H.A.; Mourão, G.B.; Ferreira, M.C.S.; Titto, E.A.L.; Strefezzi, R.F.; Gallo, S.B.; Titto, C.G. Heat tolerance in hair sheep: Individual differences on physiological, endocrine, and behavioral responses. Anim.-Open Space 2024, 3, 100067. [Google Scholar] [CrossRef]
- Piccione, G.; Caola, G.; Refinetti, R. Effect of shearing on the core body temperature of three breeds of Mediterranean sheep. Small Rumin. Res. 2002, 46, 211–215. [Google Scholar] [CrossRef]
- Maia, A.S.C.; Silva, R.G.; Andrade, P.C. Efeitos da temperatura e da movimentação do ar sobre o isolamento térmico do velo de ovinos em câmara climática. R. Bras. Zootec. 2009, 38, 104–108. [Google Scholar] [CrossRef]
- Elhadi, A.; Salama, A.A.K.; Such, X.; Albanell, E.; Toral, P.G.; Hervás, G.; Frutos, P.; Caja, G. Effects of shearing 2 breeds of dairy ewes during lactation under mild winter conditions. J. Dairy Sci. 2019, 102, 1712–1724. [Google Scholar] [CrossRef]
- Corrales-Hlinka, F.; Perez-Clariget, R.; Freitas-de-Melo, A.; Ungerfeld, R. Thermoregulatory, metabolic and stress responses to spring shearing of aged ewes born to undernourished mothers. J. Therm. Biol. 2023, 113, 103503. [Google Scholar] [CrossRef]
- Mendes, L.C.N.; Matsukuma, B.H.; De Oliveira, G.; Peres, L.C.T.; Gerardi, B.; Feitosa, F.L.F.; Venturoli Perri, S.H.; Peiró, J.R. Efeito da tosquia na temperatura corpórea e outros parâmetros clínicos em ovinos. Pesq. Vet. Bras. 2013, 33, 817–825. [Google Scholar] [CrossRef]
- Titto, C.G.; Veríssimo, C.J.; Pereira, A.M.F.; Geraldo, A.d.M.; Katiki, L.M.; Titto, E.A.L. Thermoregulatory response in hair sheep and shorn wool sheep. Small Rumin. Res. 2016, 144, 341–345. [Google Scholar] [CrossRef]
- Mahgoub, O.; Kadim, I.T.; Al-Dhahab, A.; Bello, R.B.; Al-Amri, I.S.; Ambu Ali, A.A.; Khalaf, S. An Assessment of Omani Native Sheep Fiber Production and Quality Characteristics. J. Agric. Mar. Sci. 2010, 49, 193–198. [Google Scholar] [CrossRef]
- McManus, C.M.; Faria, D.A.; Lucci, C.M.; Louvandini, H.; Pereira, S.A.; Paiva, S.R. Heat stress effects on sheep: Are hair sheep more heat resistant? Theriogenology 2020, 155, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.; Schmidt, M.; Ammon, C.; Rose-Meierhöfer, S.; Burfeind, O.; Heuwieser, W.; Berg, W. Monitoring the body temperature of cows and calves using video recordings from an infrared thermography camera. Vet. Res. Commun. 2013, 37, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hooper, H.B.; dos Santos Silva, P.; de Oliveira, S.A.; Merighe, G.K.F.; Negrão, J.A. Acute heat stress induces changes in physiological and cellular responses in Saanen goats. Int. J. Biometeorol. 2018, 62, 2257–2265. [Google Scholar] [CrossRef]
- Pulido-Rodríguez, L.F.; Titto, E.A.L.; Henrique, F.L.; Longo, A.L.S.; Hooper, H.B.; Pereira, T.L.; Pereira, A.M.F.; Titto, C.G. Termografia infravermelha da superfície ocular como indicador de estresse em suínos na fase de creche. Pesq. Vet. Bras. 2017, 37, 453–458. [Google Scholar] [CrossRef][Green Version]
- Kotrba, R.; Knížková, I.; Kunc, P.; Bartoš, L. Comparison between the coat temperature of the eland and dairy cattle by infrared thermography. J. Thermal Biol. 2007, 32, 355–359. [Google Scholar] [CrossRef]
- Schleger, A.; Turner, H. Sweating rates of cattle in the field and their reaction to diurnal and seasonal changes. Aust. J. Agric. Res. 1965, 16, 92–106. [Google Scholar] [CrossRef]
- Silva, W.E.; Leite, J.H.G.M.; de Sousa, J.E.R.; Costa, W.P.; da Silva, W.S.T.; Guilhermino, M.M.; Asensio, L.A.B.; Façanha, D.A.E. Daily rhythmicity of the thermoregulatory responses of locally adapted Brazilian sheep in a semiarid environment. Int. J. Biometeorol. 2017, 61, 1221–1231. [Google Scholar] [CrossRef]
- De, K.; Kumar, D.; Saxena, V.K.; Naqvi, S.M.K. Study of circadian rhythmicity of physiological response and skin temperature of sheep during summer and winter in semi-arid tropical environment. Physiol. Behav. 2017, 169, 16–21. [Google Scholar] [CrossRef]
- Fonsêca, V.D.F.C.; Maia, A.S.C.; Saraiva, E.P.; de Melo Costa, C.C.; da Silva, R.G.; Abdoun, K.A.; Al-Haidary, A.A.; Samara, E.M.; Fuller, A. Bio-thermal responses and heat balance of a hair coat sheep breed raised under an equatorial semi-arid environment. J. Therm. Biol. 2019, 84, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ewart, S.L. Thermoregulation. In Cunningham’s Textbook of Veterinary Physiology, 6th ed.; Klein, B.G., Ed.; Saunders: St. Louis, MO, USA, 2019; pp. 597–607. [Google Scholar]
- Silanikove, N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest. Prod. Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.; Malik, P.K.; Naqvi, S.M.K.; Lal, R. Adapting Sheep Production to Climate Change. In Sheep Production Adapting to Climate Change, 1st ed.; Sejian, V., Bhatta, R., Gaughan, J., Malik, P.K., Naqvi, S.M.K., Lal, R., Eds.; Springer: Singapore, 2017; pp. 1–450. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Schaefer, A.L.; Cook, N.J.; Scott, S.L. Infrared thermography as a non-invasive tool to study animal welfare. Anim. Welf. 2005, 14, 319–325. [Google Scholar] [CrossRef]
- Church, J.S.; Hegadoren, P.R.; Paetkau, M.J.; Miller, C.C.; Regev-Shoshani, G.; Schaefer, A.L.; Schwartzkopf-Genswein, K.S. Influence of environmental factors on infrared eye temperature measurements in cattle. Res. Vet. Sci. 2014, 96, 220–226. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K. Central neural pathways for thermoregulation. Front. Biosci. 2011, 16, 74–104. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Kamijo, Y.; Lee, K.; Mack, G.W.T. Active cutaneous vasodilation in resting humans during mild heat stress. J. Appl. Physiol. 2005, 98, 829–837. [Google Scholar] [CrossRef]
- da Silva, R.G.; Starling, J.M.C. Cutaneous and respiratory evaporation rates of sheep in hot environments. Rev. Bras. Zootec. 2003, 32, 1956–1961. [Google Scholar] [CrossRef]
- McManus, C.; Louvandini, H.; Gugel, R.; Sasaki, L.C.B.; Bianchini, E.; Bernal, F.E.M.; Paiva, S.R.; Paim, T.P. Skin and coat traits in sheep in Brazil and their relation with heat tolerance. Trop. Anim. Health Prod. 2011, 43, 121–126. [Google Scholar] [CrossRef]
- Castanheira, M.; Paiva, S.R.; Louvandini, H.; Landim, A.; Fiorvanti, M.C.S.; Dallago, B.S.; Correa, P.S.; McManus, C. Use of heat tolerance traits in discriminating between groups of sheep in central Brazil. Trop. Anim. Health Prod. 2010, 42, 1821–1828. [Google Scholar] [CrossRef]
- de Andrade Pantoja, M.H.; Esteves, S.N.; Jacinto, M.A.C.; Pezzopane, J.R.M.; de Paz, C.C.P.; da Silva, J.A.R.; Junior, J.D.B.L.; Brandão, F.Z.; Moura, A.B.B.; Romanello, N.; et al. Thermoregulation of male sheep of indigenous or exotic breeds in a tropical environment. J. Therm. Biol. 2017, 69, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Ngadiyono, N.; Maulana, H.; Atmoko, B.A. Effect of shearing on thermo-physiological, behavior, and productivity traits of two Indonesian local sheep breeds. Trop. Anim. Sci. J. 2024, 47, 42–52. [Google Scholar]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef]
- Chesire, W.P. Autonomic Neuroscience: Basic and Clinical Thermoregulatory disorders and illness related to heat and cold stress. Auton. Neurosci. Basic Clin. 2016, 196, 91–104. [Google Scholar] [CrossRef]
- Smith, C.J.; Johnson, J.M. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: Neural control of skin blood flow and sweating in humans. Auton. Neurosci. 2016, 196, 25–36. [Google Scholar] [CrossRef]
- Mobini, B. Studies on the density of various dermal structures in adult rams and ewes. Bulg. J. Vet. Med. 2013, 16, 1–6. [Google Scholar]
- Gatenby, R.M.; Monteith, J.L.; Clark, J.A. Temperature and humidity gradients in a sheeps fleece. 2. The energetic significance of transients. Agric. Meteorol. 1983, 29, 83–101. [Google Scholar] [CrossRef]
- Al-Ramamneh, D.; Gerken, D.M.; Riek, A. Effect of shearing on water turnover and thermobiological variables in German Blackhead mutton sheep. J. Anim. Sci. 2011, 89, 4294–4304. [Google Scholar] [CrossRef]
- Starling, J.M.C.; Silva, R.G.; Negrão, J.A.; Maia, A.S.C.; Bueno, A.R. Seasonal variation of thyroid hormones and cortisol of sheep in tropical environment. R. Bras. Zootec. 2005, 34, 2064–2073. [Google Scholar] [CrossRef]
- Cook, C.J. Basal and stress response cortisol levels and stress avoidance learning in sheep (Ovis ovis). N. Z. Vet. J. 1996, 44, 162–163. [Google Scholar] [CrossRef]
- Alhidary, I.A.; Shini, S.; Al Jassim, R.A.M.; Gaughan, J.B. Physiological responses of Australian Merino wethers exposed to high heat load1. J. Anim. Sci. 2012, 90, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Giudice, E.; Passantino, A.; Zumbo, A.; Di Pietro, S.; Piccione, G. Shearing induces secondary biomarkers responses of thermal stress in sheep. Anim. Sci. Pap. Rep. 2016, 34, 73–80. Available online: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20163164147 (accessed on 20 March 2025).
- Pehlivan, E.; Kaliber, M.; Konca, Y.; Dellal, G. Effect of shearing on some physiological and hormonal parameters in Akkaraman sheep. Asian-Australas. J. Anim. Sci. 2019, 33, 848. [Google Scholar] [CrossRef]
- Taha, E.A. The Influence of Shearing Stress on Thermal Homeostasis and Performance of Barki Ewes in the North Western Desert of Egypt. Int. J. Environ. Agric. Biotechnol. 2000, 5, 412–420. [Google Scholar] [CrossRef]
- Cirne, L.G.A.; Silva Sobrinho, A.D.; Santana, V.T.; Endo, V.; Almeida, F.D.; Franco, M.R.; Silva, F.U.; Oliveira, E.D.; Carvalho, G.D.; Zeola, N.M.B.L. Effect of strategic shearing on feeding behavior in Ile de France sheep in Bermudagrass (Cynodon dactylon cv Vaquero) grazing during breeding season. Semin. Cienc. Agrar. 2014, 35, 1607. [Google Scholar] [CrossRef][Green Version]
- Dikmen, S.; Orman, A.; Ustuner, H. The effect of shearing in a hot environment on some welfare indicators in Awassi lambs. Trop. Anim. Health Prod. 2011, 43, 1327–1335. [Google Scholar] [CrossRef]
- Piccione, G.; Caola, G. Influence of Shearing on the Circadian Rhythm of Body Temperature in the Sheep. J. Vet. Med. Ser. A 2003, 50, 235–240. [Google Scholar] [CrossRef]
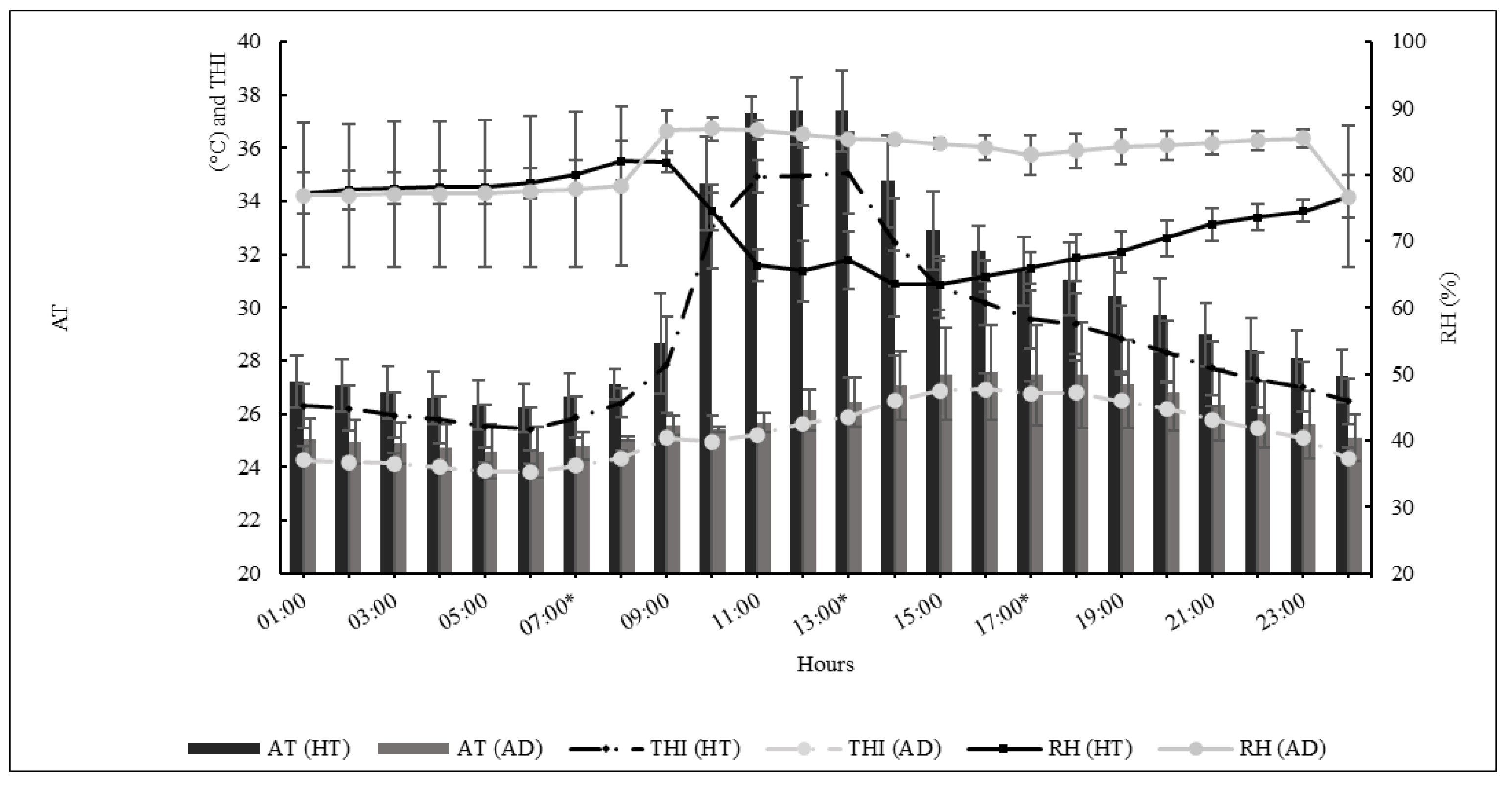
| Meteorology Data | Hour | SE | p Value | ||
|---|---|---|---|---|---|
| 7 h | 13 h | 20 h | |||
| Air Temperature (°C) | 21.93 c | 34.89 a | 25.54 b | 0.21 | <0.0001 |
| Black Globe Temperature (°C) | 23.33 c | 43.45 a | 24.07 b | 0.45 | <0.0001 |
| Relative Humidity (%) | 84.00 a | 61.71 c | 69.92 b | 0.44 | <0.0001 |
| THI | 21.55 c | 32.41 a | 24.50 b | 0.15 | <0.0001 |
| Biological Records | Hour | Control | Post-Shearing |
|---|---|---|---|
| Rectal Temperature (°C) | 7 | 38.43 ± 0.044 bA | 38.49 ± 0.052 cA |
| 10 | 38.82 ± 0.041 aA | 38.60 ± 0.046 cA | |
| 13 | 38.90 ± 0.042 aA | 39.00 ± 0.047 abA | |
| 17 | 38.82 ± 0.044 aA | 38.92 ± 0.054 bA | |
| 20 | 38.89 ± 0.038 aB | 39.10 ± 0.051 aA | |
| Ocular Surface Temperature (°C) | 7 | 37.76 ± 0.078 cB | 38.01 ± 0.059 cA |
| 10 | 38.40 ± 0.062 bB | 38.55 ± 0.065 cA | |
| 13 | 39.02 ± 0.066 aB | 39.24 ± 0.074 aA | |
| 17 | 38.41 ± 0.072 bA | 38.57 ± 0.073 bA | |
| 20 | 38.49 ± 0.065 bB | 38.71 ± 0.085 bA | |
| Respiration Rate (movements.min−1) | 7 | 49 ± 2.2 dA | 48 ± 2.5 dA |
| 10 | 72 ± 2.9 bA | 59 ± 2.9 cB | |
| 13 | 112 ± 3.6 aB | 133 ± 3.7 aA | |
| 17 | 73 ± 3.3 bB | 85 ± 3.6 bA | |
| 20 | 59 ± 2.9 cB | 81 ± 3.7 bA | |
| Sweating Rate (g.m−2.h−1) | 7 | 357.21 ± 22.175 bA | 195.13 ± 12.39 bB |
| 13 | 806.61 ± 96.386 aA | 339.63 ± 53.169 aB | |
| 20 | 381.09 ± 25.284 bA | 239.91 ± 17.847 bB |
| Body Regions | Time | Control | Post-Shearing |
|---|---|---|---|
| Back + Loin | 7 h | 32.1 ± 0.21 eA | 32.3 ± 0.19 dA |
| 10 h | 35.9 ± 0.19 bA | 34.3 ± 0.19 cB | |
| 13 h | 37.8 ± 0.19 aB | 38.9 ± 0.20 aA | |
| 17 h | 34.3 ± 0.19 cB | 34.9 ± 0.19 bA | |
| 20 h | 33.1 ± 0.19 dB | 34.7 ± 0.19 bA | |
| Belly + Flank | 7 h | 34.1 ± 0.17 dA | 33.8 ± 0.17 dA |
| 10 h | 36.4 ± 0.18 bA | 35.0 ± 0.17 cB | |
| 13 h | 37.6 ± 0.18 aB | 38.1 ± 0.17 aA | |
| 17 h | 36.0 ± 0.18 bA | 35.9 ± 0.17 bA | |
| 20 h | 35.2 ± 0.17 cA | 35.7 ± 0.17 bA | |
| Shoulder | 7 h | 33.9 ± 0.19 eA | 33.5 ± 0.18 dA |
| 10 h | 36.6 ± 0.18 bB | 35.1 ± 0.18 cA | |
| 13 h | 37.9 ± 0.19 aB | 39.0 ± 0.18 aA | |
| 17 h | 35.6 ± 0.18 cA | 36.0 ± 0.18 bA | |
| 20 h | 35.1 ± 0.18 dB | 35.7 ± 0.18 bA | |
| Rump | 7 h | 32.2 ± 0.18 eB | 33.1 ± 0.18 dA |
| 10 h | 35.8 ± 0.19 bA | 34.6 ± 0.18 cB | |
| 13 h | 37.5 ± 0.18 aB | 38.9 ± 0.18 aA | |
| 17 h | 34.6 ± 0.19 cB | 35.5 ± 0.18 bA | |
| 20 h | 33.8 ± 0.18 dB | 35.3 ± 0.18 bA | |
| Forearm + knee + cannon | 7 h | 33.6 ± 0.16 eA | 33.8 ± 0.16 dA |
| 10 h | 36.5 ± 0.17 bA | 34.8 ± 0.15 cB | |
| 13 h | 37.6 ± 0.19 aA | 37.8 ± 0.16 aA | |
| 17 h | 35.7 ± 0.17 cA | 35.9 ± 0.16 bA | |
| 20 h | 34.9 ± 0.18 dB | 35.8 ± 0.16 bA | |
| Leg + Shank + Dewclaw | 7 h | 33.1 ± 0.18 eA | 33.3 ± 0.16 dA |
| 10 h | 36.1 ± 0.21 bA | 34.7 ± 0.15 cB | |
| 13 h | 36.9 ± 0.23 aB | 37.7 ± 0.16 aA | |
| 17 h | 35.2 ± 0.18 cB | 35.7 ± 0.15 bA | |
| 20 h | 34.4 ± 0.16 dB | 35.7 ± 0.16 bA | |
| Middle | 7 h | 33.3 ± 0.18 eA | 33.1 ± 0.18 dA |
| 10 h | 36.3 ± 0.18 bA | 34.7 ± 0.18 cB | |
| 13 h | 37.7 ± 0.18 aB | 38.9 ± 0.18 aA | |
| 17 h | 35.1 ± 0.17 cB | 35.6 ± 0.18 bA | |
| 20 h | 34.6 ± 0.18 dB | 35.4 ± 0.18 bA |
| Characteristics | Treatment | p Value | ||
|---|---|---|---|---|
| Baseline | Control | Post-Shearing | ||
| EDT (µm) | 29.96 ± 5.55 | 41.18 ± 5.07 | 36.27 ± 5.07 | 0.36 |
| DT (µm) | 2048.8 ± 43.63 | 1952.7 ± 43.89 | 2077.4 ± 43.89 | 0.08 |
| NSG | 33 ± 4.27 | 40 ± 4.05 | 35 ± 4.05 | 0.28 |
| ASG (µm2) | 16,574 ± 3102 | 19,265 ± 2928 | 15,433 ± 2928 | 0.37 |
| DSG-ED (µm) | 1205.8 ± 79.64 | 1380.9 ± 74.16 | 1407.8 ± 74.16 | 0.09 |
| VSG | 31.89 ± 2.79 | 30.94 ± 2.79 | 23.11 ± 2.79 | 0.08 |
| Treatment | Day | p Value | ||
|---|---|---|---|---|
| 1 | 5 | 9 | ||
| Control | 1.87 ± 0.46 | 1.49 ± 0.48 | 1.04 ± 0.51 | 0.45 |
| Post-shearing | 1.68 ± 0.46 | 1.24 ± 0.49 | 0.92 ± 0.51 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulido-Rodríguez, L.F.; Pereira, A.M.F.; Henrique, F.L.; Strefezzi, R.D.F.; Pantoja, M.H.d.A.; Mota-Rojas, D.; Titto, C.G. Effect of Shearing for Improving the Thermoregulatory Responses of Crossbred Sheep During Heat Stress. Vet. Sci. 2025, 12, 358. https://doi.org/10.3390/vetsci12040358
Pulido-Rodríguez LF, Pereira AMF, Henrique FL, Strefezzi RDF, Pantoja MHdA, Mota-Rojas D, Titto CG. Effect of Shearing for Improving the Thermoregulatory Responses of Crossbred Sheep During Heat Stress. Veterinary Sciences. 2025; 12(4):358. https://doi.org/10.3390/vetsci12040358
Chicago/Turabian StylePulido-Rodríguez, Lina Fernanda, Alfredo Manuel Franco Pereira, Fábio Luís Henrique, Ricardo De Francisco Strefezzi, Messy Hannear de Andrade Pantoja, Daniel Mota-Rojas, and Cristiane Gonçalves Titto. 2025. "Effect of Shearing for Improving the Thermoregulatory Responses of Crossbred Sheep During Heat Stress" Veterinary Sciences 12, no. 4: 358. https://doi.org/10.3390/vetsci12040358
APA StylePulido-Rodríguez, L. F., Pereira, A. M. F., Henrique, F. L., Strefezzi, R. D. F., Pantoja, M. H. d. A., Mota-Rojas, D., & Titto, C. G. (2025). Effect of Shearing for Improving the Thermoregulatory Responses of Crossbred Sheep During Heat Stress. Veterinary Sciences, 12(4), 358. https://doi.org/10.3390/vetsci12040358







