A Window Opens and a Shunt Closes: A New Laparoscopic Approach for the Attenuation of the Gastrophrenic Shunt
Simple Summary
Abstract
1. Introduction
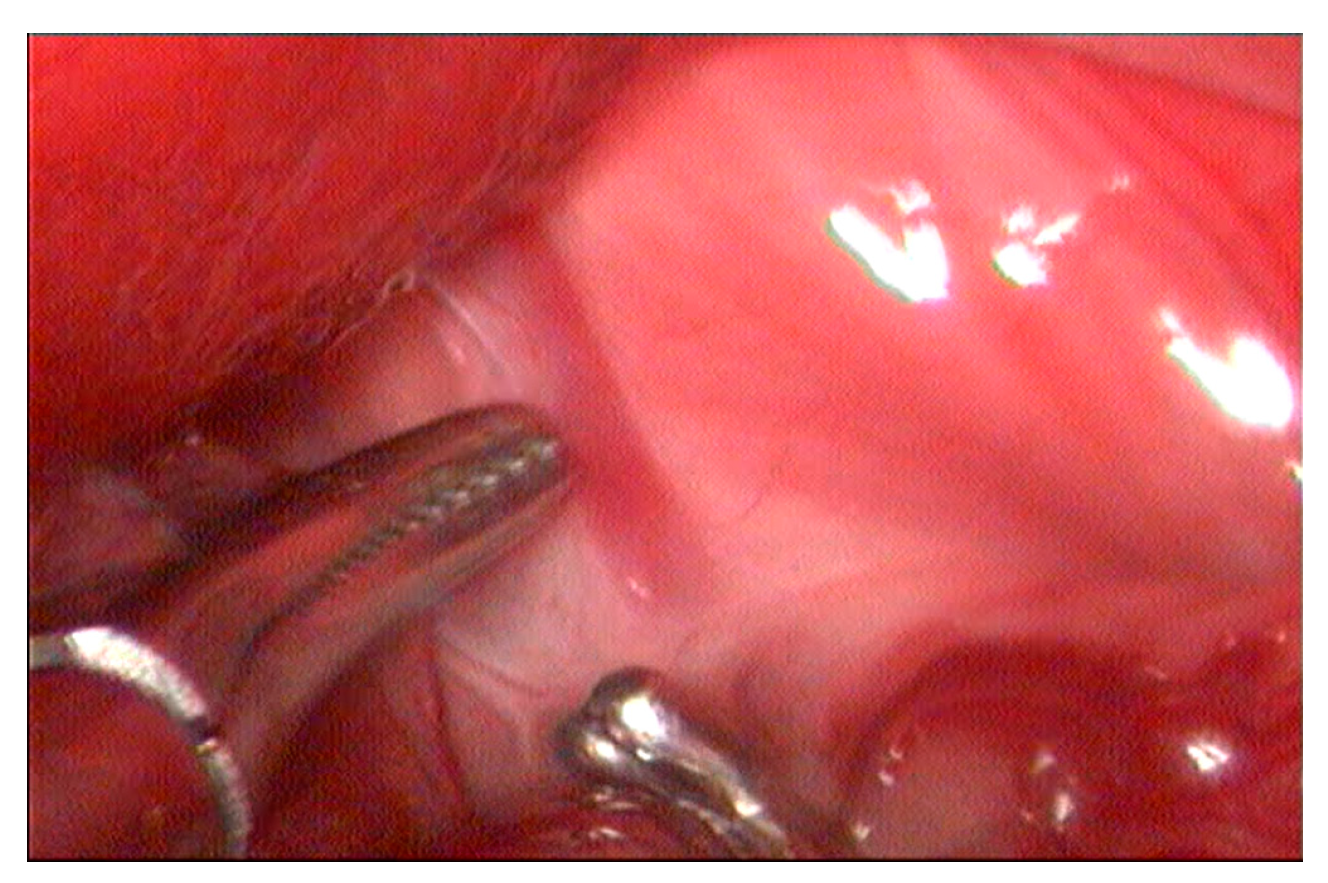
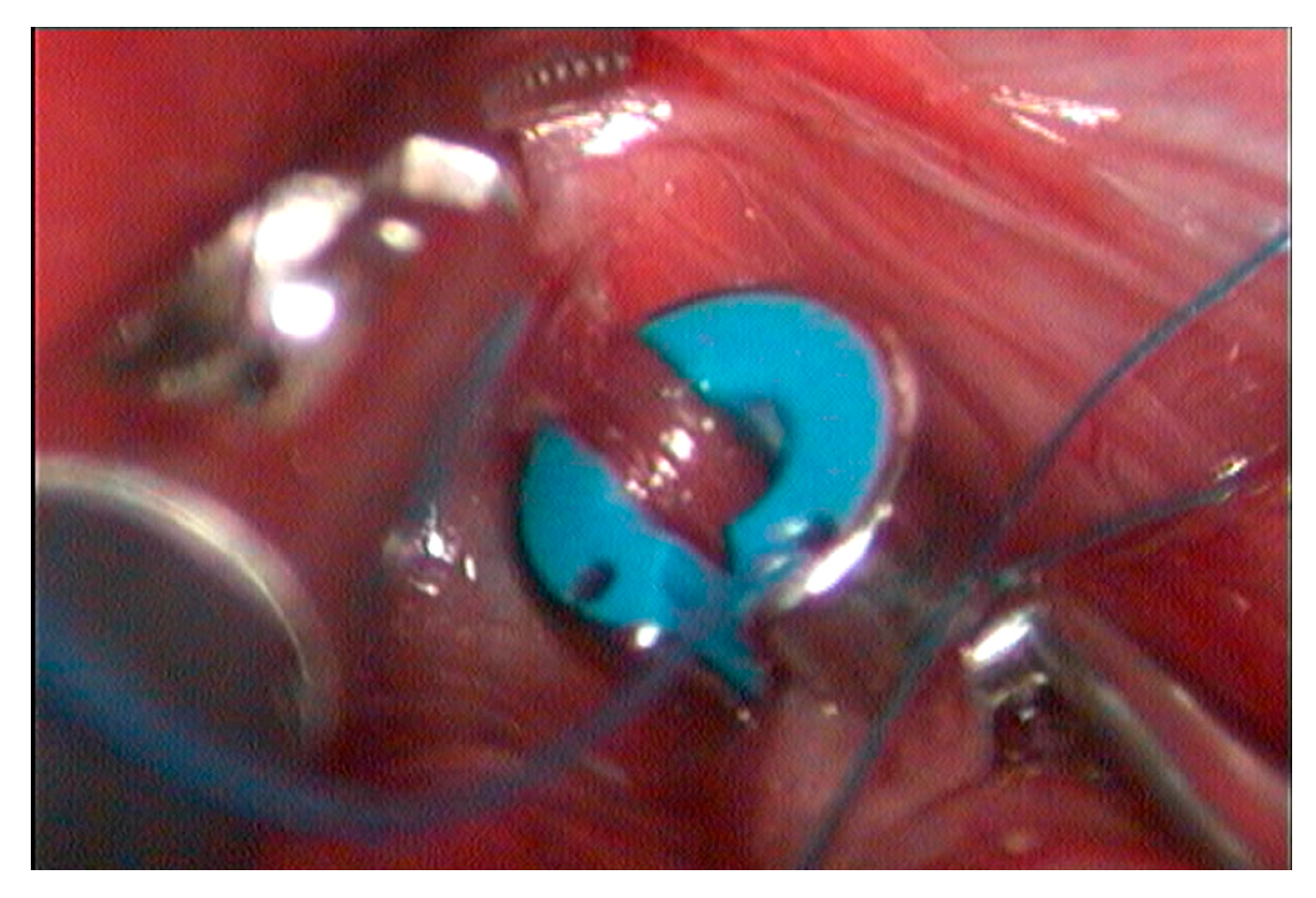
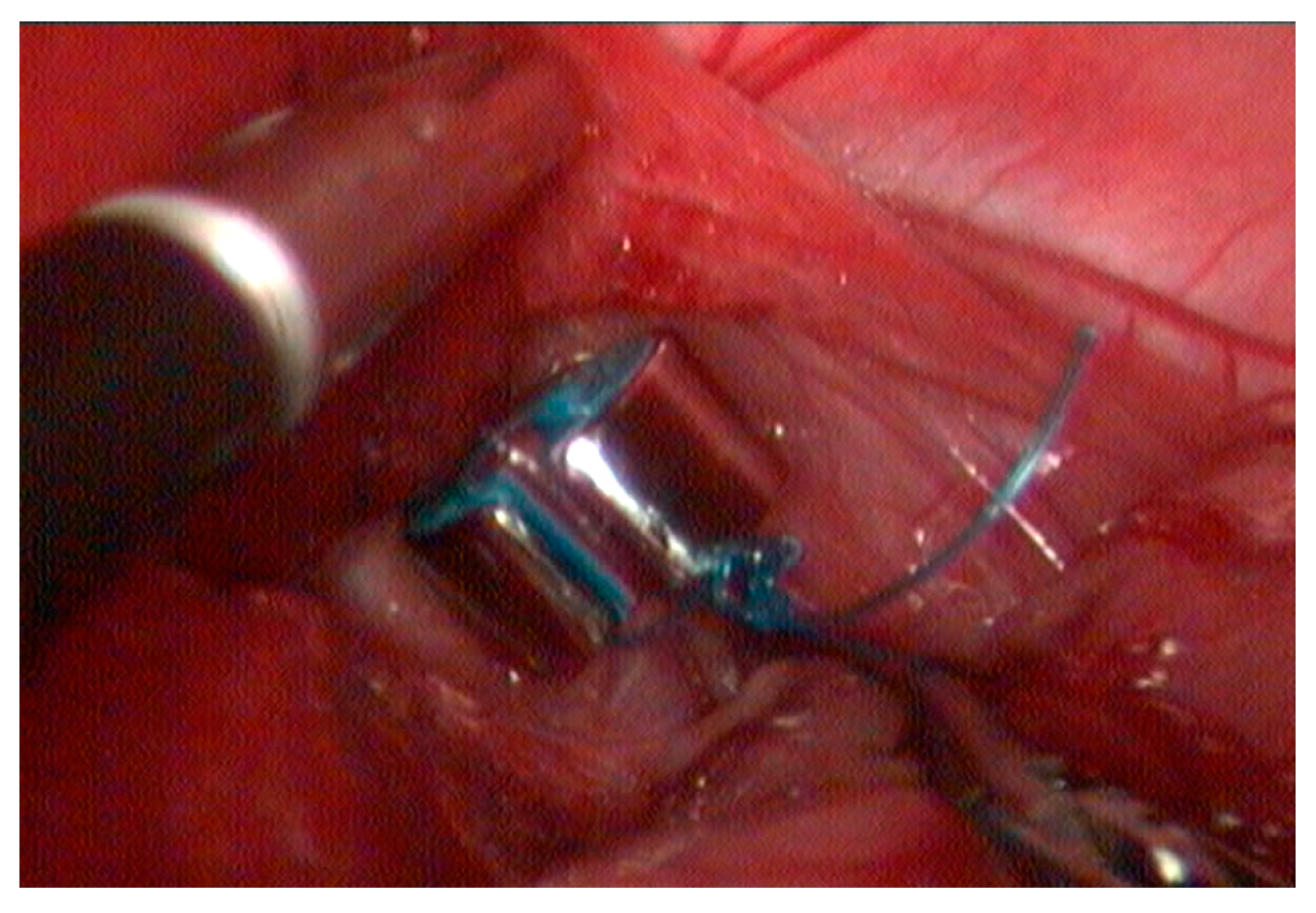
2. Materials and Methods
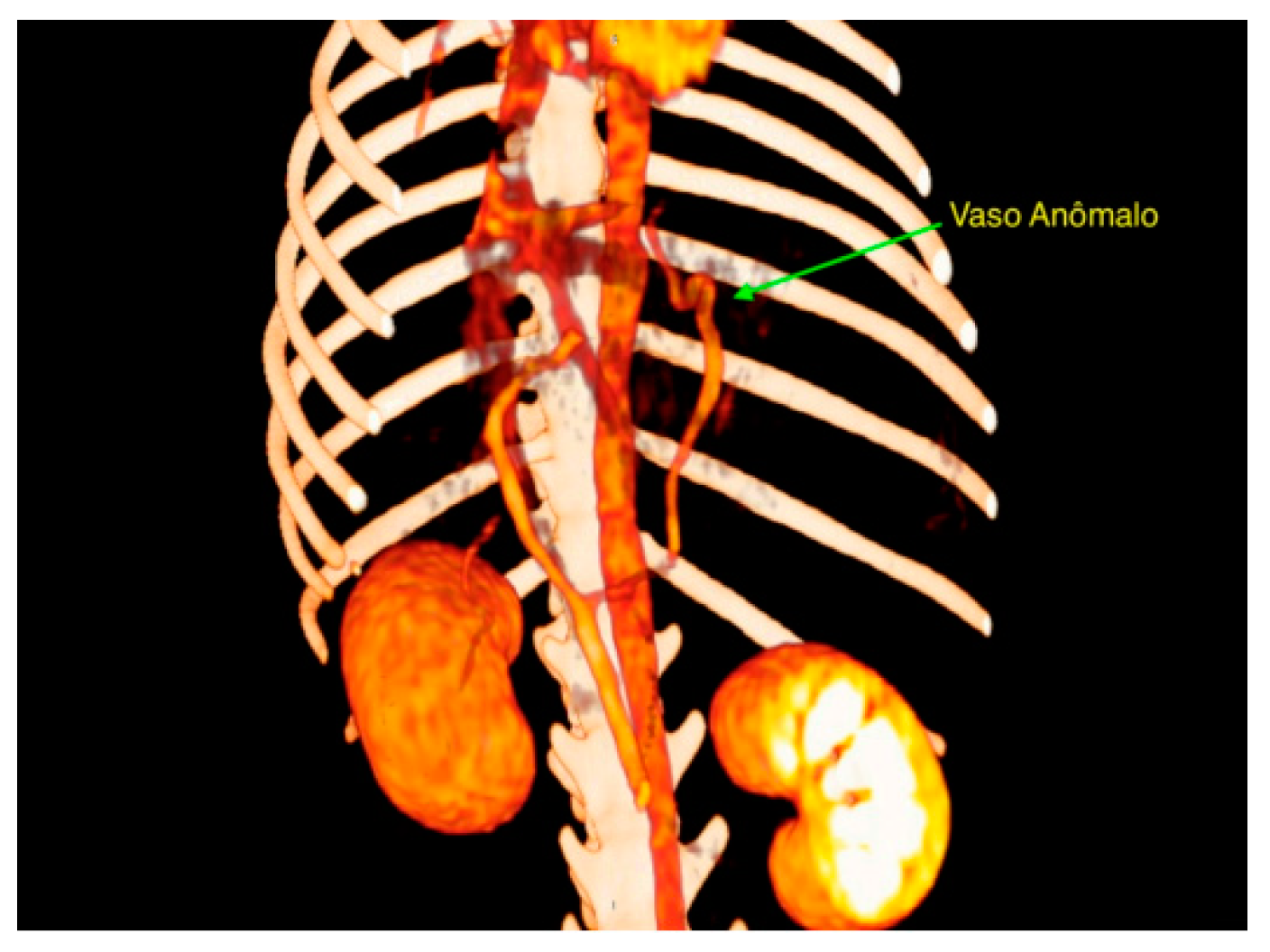
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunt, G.B. Evaluation of In Vivo Behavior of Ameroid Ring Constrictors in Dogs with Congenital Extrahepatic Portosystemic Shunts Using Computed Tomography. Vet. Surg. 2014, 43, 834–842. [Google Scholar] [CrossRef]
- Stedile, R. Glandular surgeries: Liver and spleen. In Videosurgery in Small Animals, 1st ed.; Brun, M.V., Ed.; Roca: Rio de Janeiro, Brazil, 2015; Volume 1, Chapter 18; pp. 251–262. [Google Scholar]
- Gow, A.G. Hepatic Encephalopathy. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Umek, N.; Plut, D.; Krofič Žel, M.; Domanjko Petrič, A. Radiologic Evaluation of Portosystemic Shunts in Humans and Small Animals: Review of the Literature with Clinical Case Reports. Diagnostics 2023, 13, 482. [Google Scholar] [CrossRef] [PubMed]
- Stefănescu, R.A.; Boghian, V.; Solcan, G.; Codreanu, M.D.; Musteata, M. Electroencephalographic Features of Presumed Hepatic Encephalopathy in a Pediatric Dog with a Portosystemic Shunt—ACaseReport. Life 2025, 15, 107. [Google Scholar] [CrossRef]
- Taub, R. Liver regeneration: From myth mechanism. Nat. Rev. Mol. Cell Biol. 2004, 5, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.T.; Martin, R.; Constantinescu, G.M. The anatomy and embryology of portosystemic shunts in dogs and cats. Semin. Vet. Med. Surg. (Small Anim.) 1990, 5, 76–82. [Google Scholar]
- Hunt, G.B. Effect of breed on anatomy of portosystemic shunts resulting from congenital diseases in dogs and cats: A review of 242 cases. Aust. Vet. J. 2004, 82, 746–749. [Google Scholar] [CrossRef]
- Bertolini, G. Anomalies of the Portal Venous System in Dogs and Cats as Seen on Multidetector-Row Computed Tomography: An Overview and Systematization Proposal. Vet. Sci. 2019, 6, 5–10. [Google Scholar] [CrossRef]
- Parry, A.T.; White, R.N. Comparison of computed tomographic angiography and intraoperative mesenteric portovenography for extrahepatic portosystemic shunts. J. Small Anim. Pract. 2017, 58, 49–55. [Google Scholar] [CrossRef]
- Vogt, J.C.; Krahwinkel, D.J., Jr.; Bright, R.M.; Daniel, G.B.; Toal, R.L.; Rohrbach, B. Gradual occlusion of extrahepatic portosystemic shunts in dogs and cats using the ameroid constrictor. Vet. Surg. 1996, 25, 495–502. [Google Scholar] [CrossRef]
- Leshem, S.S.; Lotsikas, P.J.; Reimer, S.B. In vitro expansion patterns of ameroid ring constrictors. Am. J. Vet. Res. 2008, 69, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Jericó, M.M.; Kogika, M.M.; Andrade Neto, J.P. Treatise on Internal Medicine for Dogs and Cats, 1st ed.; Roca: Rio de Janeiro, Brazil, 2015; p. 2464. [Google Scholar]
- Wallace, M.L. Updates in Hepatobiliary Surgery: New Data on Portosystemic Shunts and Cholecystectomy in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2023, 52, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Poggi, E.; Rubio, D.G.; Duarte, F.J.P.; Del Sol, J.G.; Borghetti, L.; Izzo, F.; Cinti, F. Laparoscopic portosystemic shunt attenuation in 20 dogs (2018–2021). Vet. surg. 2022, 51, 138–149. [Google Scholar] [CrossRef]
- Brun, M.V. Use of ameroid constrictor plus intracorporeal suture in the laparoscopic treatment of extrahepatic shunts in dogs. Veterinary Endoscopy Society Annual Conference Scientific Presentation Abstracts. Vet. Surg. 2023, 52, 1–15. [Google Scholar]
- White, R.N.; Shales, C.; Parry, A.T. New perspectives on the development of extrahepatic portosystemic shunts. J. Small Anim. Pract. 2017, 58, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.C.; Nelson, L.L. Anatomy of extrahepatic portosystemic shunts in dogs as determined by computed tomography angiography. Vet. Radiol. Ultrasound 2011, 52, 498–506. [Google Scholar] [CrossRef]
- White, R.N.; Parry, A.T. Morphology of congenital portosystemic shunts emanating from the left gastric vein in dogs and cats. J. Small Anim. Pract. 2013, 54, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, L.; Van Steenbeek, F.G.; Favier, R.P.; Kummeling, A.; Leegwater, P.A.J.; Rothuizen, J. Distribution of extrahepatic congenital portosystemic shunt morphology 629 in predisposed dog breeds. BMC Vet. Res. 2012, 8, 112. [Google Scholar] [CrossRef]
- Konstantinidis, A.O.; Patsikas, M.N.; Papazoglou, L.G.; Adamama-Moraitou, K.K. Congenital Portosystemic Shunts in Dogs and Cats: Classification, Pathophysiology, Clinical Presentation and Diagnosis. Vet. Sci. 2023, 10, 160. [Google Scholar] [CrossRef]
- Williams, A.; Gow, A.; Kilpatrick, S.; Tivers, M.; Lipscomb, V.; Smith, K.; Day, M.O.; Jeffery, N.; Mellanby, R.J. Astrocyte Lesions in Cerebral Cortex and Cerebellum of Dogs with Congenital Ortosystemic Shunting. Vet. Sci. 2020, 21, 44. [Google Scholar] [CrossRef]
- Greenhalgh, S.N.; Dunning, M.D.; McKinley, T.J.; Goodfellow, M.R.; Kelman, K.R.; Freitag, T.; O’Neill, E.J.; Hall, E.J.; Watson, P.J.; Jeffery, N.D. Comparison of survival after surgical or medical treatment in dogs with congenital portosystemic shunt. J. Am. Vet. Med. Assoc. 2010, 236, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, S.N.; Reeve, J.A.; Johnstone, T.; Goodfellow, M.R.; Dunning, M.D.; O’Neill, E.J.; Hall, E.J.; Watson, P.J.; Jeffery, N.J. Long-term survival with congenital portosystemic shunt after surgery or medical treatment. J. Am. Vet. Med. Assoc. 2014, 245, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Brum, A.M.; Champion, T.; Zanatta, R.; Costa, M.T.; Canola, J.C. Use of probiotics and lactulose in the control of hyperammonemia caused by congenital portosystemic vascular shunt in a dog. Ciênc. Rural 2007, 37, 572–574. [Google Scholar] [CrossRef]
- Traverson, M.; Lussier, B.; Huneault, L.; Gatineau, M. Comparative outcomes between ameroid ring constrictor and cellophane banding for treatment of single congenital extrahepatic portosystemic shunts in 49 dogs (1998–2012). Vet. Surg. 2017, 47, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Brun, M.V.; Pasquale, R. Laparoscopic treatment of extrahepatic portosystemic shunt with ameroid constrictor. In Laparoscopy and Thoracoscopy in the Dog and Cat, 1st ed.; Andrea, M.P., Roberto, P., Eds.; Edra Publishing: Palm Beach Gardens, FL, USA, 2023; Volume 1, pp. 412–417. [Google Scholar]
- Besancon, M.F.; Kyles, A.E.; Griffey, S.M.; Gregory, C.R. Evaluation of the characteristics of venous occlusion after ameroid constrictor placement in dogs. Vet. Surg. 2004, 33, 597–605. [Google Scholar] [CrossRef]
- Szatmari, V.; Van Sluijs, F.J.; Rothuizen, J.; Voorhout, G. Ultrasonographic assessment of hemodynamic changes in the portal vein during surgical attenuation of congenital extrahepatic portosystemic shunts in dogs. J. Am. Vet. Med. Assoc. 2004, 224, 395–402. [Google Scholar] [CrossRef]
- Serrano, G.; Charalambous, M.; Devriendt, N.; de Rooster, H.; Mortier, F.; Paepe, D. Treatment of congenital extrahepatic portosystemic shunts in dogs: A systematic review and meta-analysis. J. Vet. Intern. Med. 2019, 33, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Hyun, J. Migration of ameroid constrictor without dislodgement of the ameroid key after extrahepatic portosystemic shunt surgery in a dog. J. Prev. Vet. Med. 2024, 48, 58–63. [Google Scholar] [CrossRef]
- García-Bengoch, J.; Caínzos, M.; Fernández, A.L.; Santos, F.; Gonzalez, F. Intraperitoneal migration of epicardial pacemakers. Tex. Heart Inst. J. 2007, 34, 376–378. [Google Scholar]
- Kaplan, M.; Ozel, S.K.; Dönmez, O.; Kazez, A. Treatment approaches for abdominal migration of peritoneal catheter of ventriculoperitoneal shunt. Turk. Neurosurg. 2007, 17, 158–162. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socolhoski, B.V.G.; Paraguassú, A.O.; Pozzobon, F.M.; Caye, P.; Gasparotto, J.C.; Schiefler, O.H.d.M.; Reinstein, R.d.S.; Müller, D.C.d.M.; Brun, M.V. A Window Opens and a Shunt Closes: A New Laparoscopic Approach for the Attenuation of the Gastrophrenic Shunt. Vet. Sci. 2025, 12, 351. https://doi.org/10.3390/vetsci12040351
Socolhoski BVG, Paraguassú AO, Pozzobon FM, Caye P, Gasparotto JC, Schiefler OHdM, Reinstein RdS, Müller DCdM, Brun MV. A Window Opens and a Shunt Closes: A New Laparoscopic Approach for the Attenuation of the Gastrophrenic Shunt. Veterinary Sciences. 2025; 12(4):351. https://doi.org/10.3390/vetsci12040351
Chicago/Turabian StyleSocolhoski, Brenda Viviane Götz, Amanda Oliveira Paraguassú, Franciéli Mallmann Pozzobon, Pâmela Caye, Jean Carlos Gasparotto, Otávio Henrique de Melo Schiefler, Rainer da Silva Reinstein, Daniel Curvello de Mendonça Müller, and Maurício Veloso Brun. 2025. "A Window Opens and a Shunt Closes: A New Laparoscopic Approach for the Attenuation of the Gastrophrenic Shunt" Veterinary Sciences 12, no. 4: 351. https://doi.org/10.3390/vetsci12040351
APA StyleSocolhoski, B. V. G., Paraguassú, A. O., Pozzobon, F. M., Caye, P., Gasparotto, J. C., Schiefler, O. H. d. M., Reinstein, R. d. S., Müller, D. C. d. M., & Brun, M. V. (2025). A Window Opens and a Shunt Closes: A New Laparoscopic Approach for the Attenuation of the Gastrophrenic Shunt. Veterinary Sciences, 12(4), 351. https://doi.org/10.3390/vetsci12040351






