Simple Summary
Liver injury, a prevalent disorder in animals with a multifactorial etiology, is critically influenced by ferroptosis—an iron-dependent cell death mechanism. Although Euphorbia humifusa Willd. ex Schltdl. (EHW) exhibits pharmacological properties, its role in modulating ferroptosis-associated liver injury remains underexplored. This study evaluated EHW’s therapeutic effects using a CCl4-induced murine hepatotoxicity model. EHW administration significantly attenuated liver dysfunction, mitigated ferroptosis, and restored the regulatory pathways linked to iron metabolism and lipid peroxidation. These findings position EHW as a promising therapeutic agent for veterinary liver diseases, primarily through ferroptosis pathway regulation.
Abstract
Liver injury poses major health risks in livestock, necessitating effective therapeutic interventions. This study elucidates the hepatoprotective mechanisms of Euphorbia humifusa Willd. ex Schltdl. (EHW) by integrating network pharmacology, molecular docking, and experimental validation. Using a CCl4-induced liver injury model mimicking veterinary clinical scenarios, EHW markedly alleviated hepatic damage, demonstrated by reduced liver index, serum ALT and AST levels, histopathological lesions, iron accumulation, inflammatory cytokines, and ferroptosis-associated gene expression. Network pharmacology identified EHW’s core bioactive components (quercetin, kaempferol, and β-sitosterol) and critical targets (IL-6, STAT3, HIF-1α, PTGS2, NFE2L2, and KEAP1) which were linked to ferroptosis and oxidative stress. Molecular docking revealed robust binding affinities between these compounds and ferroptosis-related proteins. In vivo validation confirmed that EHW inhibited KEAP1, activated NFE2L2-mediated antioxidant defenses (upregulating SOD1 and NQO1), restored iron homeostasis (lowering TFR1, elevating FTH1), and attenuated phospholipid peroxidation by suppressing ACSL4 and ALOX12. These results indicate that EHW mitigates ferroptosis-driven liver injury via KEAP1-NFE2L2 signaling to restore iron homeostasis and reduce oxidative stress, offering a mechanistic foundation for its clinical application in veterinary hepatoprotection.
1. Introduction
The liver is one of the most vital organs in livestock, playing a crucial role in key physiological functions such as synthesis, metabolism, and immunity [1]. Once liver damage occurs, it severely disrupts these essential physiological processes [2]. In veterinary practice, liver injury is commonly caused by factors such as poor feed quality [3], pathogenic microbial infections [4], the ingestion of toxic substances [5], or improper management practices [6]. When liver damage occurs, it can lead to significant harm to the animal’s health. If timely interventions are not implemented, liver injury may rapidly progress, resulting in liver failure and potentially leading to death [7]. Therefore, there is an urgent need to identify effective therapeutic agents to address the common issue of liver damage in veterinary clinical settings.
As a key organ for iron storage and metabolism in the body, maintaining normal iron homeostasis is crucial for the liver to preserve its proper function [8]. Ferroptosis is a recently emerging form of iron-dependent programmed cell death, characterized by the peroxidation of membrane lipids [9]. Various pathways, including redox balance, lipid metabolism, and iron metabolism, can influence the progression of ferroptosis in cells [10]. Research has demonstrated that ferroptosis exerts a dual function in liver diseases. Specifically, in the context of hepatocellular carcinoma, the induction of ferroptosis has been established as a significant strategy to retard tumor progression [11]. However, in the case of non-alcoholic fatty liver disease, alcoholic liver injury, and liver fibrosis, inhibiting ferroptosis has demonstrated beneficial effects in alleviating the condition [12,13,14]. Furthermore, in liver injury studies, Yang et al. [15] found that Maresin 1 could inhibit ferroptosis by activating the NFE2L2/HMOX1/GPX4 pathway, thereby reducing liver damage. Similarly, Wei et al. [16] found that Gan Dan Kang, a traditional Chinese medicine (TCM) compound, could alleviate CCL4-induced hepatocellular ferroptosis in mice through the activation of the KEAP1/NFE2L2 pathway. In veterinary clinical practice, inhibiting ferroptosis in liver tissue has also been widely recognized as an important approach to mitigating hepatic injury in livestock. For instance, Zhao et al. [17] found that Phlorotannins could alleviate heat-stress-induced liver damage in broiler chickens by reducing hepatic ferroptosis. Similarly, Huang et al. [18] demonstrated that the total flavonoids of Rhizoma Drynaria could reverse AFB1-induced liver injury in broilers by inhibiting ferroptosis.
Euphorbia humifusa Willd. ex Schltdl. (EHW) is an annual herbaceous plant widely distributed in China. As a TCM, it is also known as Dijincao and has been documented in the Compendium of Materia Medica (Ben-Cao-Gang-Mu in Chinese) since ancient times [19]. According to TCM theory, EHW is considered to have a pungent and neutral nature, with properties that include clearing heat, detoxifying and cooling the blood, and stopping bleeding, as well as promoting diuresis and alleviating jaundice. Modern medical studies have shown that EHW possesses anti-inflammatory [20], antiviral [21], hypoglycemic [22], and anti-tumor effects [23], and its therapeutic efficacy in treating intestinal and hepatic inflammation is widely acknowledged [24]. However, the potential of EHW to alleviate liver damage through the ferroptosis pathway has not yet been reported. Therefore, this study aims to construct a liver injury model in mice using CCL4, a classic hepatotoxic substance, and to employ network pharmacology to predict potential targets, followed by experimental validation. The goal is to explore whether EHW can mitigate liver damage through the regulation of ferroptosis and to investigate the underlying mechanisms. The findings from this study may contribute to a deeper understanding of the mechanisms of EHW’s action and provide a theoretical basis for its clinical application.
2. Materials and Methods
2.1. Reagents and Materials
EHW was purchased from Kangyiyin Biotechnology Co., Ltd. (Bozhou, China). CCL4 was obtained from Guangdong Puhui Chemical Technology Co., Ltd. (Shaoguan, China). The reagent kits for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Hematoxylin and eosin staining kits were from BKMAM Biology Technology Co., Ltd. (Changde, China), and the Prussian blue staining kit and 2 × Universal Blue SYBR Green qPCR Master Mix were from Wuhan Servicebio Technology Co., Ltd. (Wuhan, China). RNAiso Plus was purchased from TaKaRa Bio Lnc. (Tokyo, Japan), and the All-in-One 5 × RT MasterMix reverse transcription kit was obtained from Applied Biological Materials Inc. (Vancouver, BC, Canada).
2.2. Preparation of the Drug
Distilled water was added to a total of 50 g of EHW at a ratio of 15:1. After soaking for 50 min, the mixture was brought to a boil over a high heat and then simmered for 45 min. The liquid was filtered through gauze, and the filtrate was collected. The remaining drug residue was then re-extracted with an equal amount of distilled water, brought to a boil over high heat, and simmered for 45 min. This process was repeated three times. Then, the combined liquid was concentrated using a rotary evaporator to a final concentration of 0.4 g/mL. Finally, a portion of this concentrated liquid was diluted with distilled water to achieve a concentration of 0.2 g/mL. Both concentrations were aliquoted and stored in a refrigerator at 4 °C for later use.
2.3. Animal Grouping and Treatment
A total of 24 SPF-grade male KM mice (purchased from Southwest Medical University) were randomly assigned into four groups: the control group (CON), model group (CCL4), high-dose EHW group (EHW-H), and low-dose EHW group (EHW-L), with 6 mice in each group. After 3 d of acclimatization, all mice were treated with daily oral gavage for 7 consecutive days at a dose of 0.1 mL/10 g of EHW decoction. The high-dose group received EHW at 4 g/kg, and the low-dose group received 2 g/kg. The CON and CCL4 groups were given an equivalent volume of sterile water. One hour after the final dose, the mice were injected intraperitoneally with CCL4 solution (0.1% CCL4 in olive oil) at a dose of 0.1 mL/10 g. Analogously, the CON group received an equivalent volume of olive oil. Eighteen hours later, the mice were gavaged once more and then anesthetized for blood collection from the ocular sinus. Finally, the mice were euthanized via cervical dislocation, and their liver tissues were harvested. Part of the liver was fixed in 4% paraformaldehyde, and another portion was rapidly frozen in liquid nitrogen and stored at −80 °C for long-term preservation. The experimental procedure and treatment flow was created with the BioGDP website (https://biogdp.com/ (accessed on 17 October 2024)) [25], as shown in Figure 1. All animal experiments were conducted in accordance with the guidelines approved by the Ethics Committee of Southwest University for Animal Welfare (Ethics No. LAC2024-1-0346).
Figure 1.
Animal experimental flowchart.
2.4. Measurement of Liver Organ Index
The liver was carefully removed from the mice, and the surface moisture was gently blotted with bibulous paper. The organs were then precisely weighed using an electronic balance, and the organ index was calculated using the following formula:
Liver Index = Organ Weight (g)/Body Weight (g)
2.5. Serum AST and ALT Activity Assays in Mice
Serum samples from mice were used to measure the activities of AST and ALT using the respective test kits, following the instructions provided by the manufacturers. These assays were performed to evaluate the liver function status of the mice.
2.6. Hematoxylin and Eosin (HE) Staining
Fresh liver tissue samples were fixed in 4% paraformaldehyde and processed through gradient ethanol dehydration. The samples were then embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE). After mounting, the pathological changes in the liver tissue of the mice from each group were observed under a microscope (CX31RTSF, OLYMPUS).
2.7. Prussian Blue Staining
The process of tissue embedding and sectioning was performed as described in Section 2.6. For Prussian blue staining, the protocol provided by the manufacturer was strictly followed. After mounting, the liver tissue sections were examined for iron deposition in the livers of the mice across different groups.
2.8. Collection of EHW Chemical Constituents
The primary active components of EHW were retrieved from the TCMSP database (https://www.tcmsp-e.com/tcmsp.php (accessed on 3 November 2024)) by searching for “Diercao”. The active ingredients were selected based on the criteria of oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18.
2.9. Screening of Active Ingredient Targets
SMILES identifiers for all compounds of EHW were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/ (accessed on 3 November 2024)). Potential targets were identified by using the Swiss Target Prediction database (http://www.swisstargetprediction.ch/ (accessed on 3 November 2024)), with targets with a probability greater than 0 considered. Additionally, the Super-PRED database (https://prediction.charite.de/ (accessed on 3 November 2024)) was used to further identify potential targets of EHW active components. The active ingredient targets were then combined with those from the TCMSP database and duplicates were removed, resulting in a comprehensive list of drug targets.
2.10. Screening of Liver Injury and Ferroptosis Targets, and Identification of Potential EHW Targets for Liver Injury and Ferroptosis Treatment
Liver-injury-related targets were collected using the GeneCards database (https://www.genecards.org/ (accessed on 18 October 2024)) with the keyword “liver injury,” selecting those with a relevance score > 8.0. Additional liver-injury-related targets were retrieved from the DisGeNET database (https://www.disgenet.org/ (accessed on 2 March 2024)). The two target sets were combined and duplicates removed, forming the disease target set. Ferroptosis-related targets were compiled from the FerrDb database (http://www.zhounan.org/ferrdb (accessed on 27 September 2024)), and duplicates were similarly removed to generate the ferroptosis target set. The three sets of drug active ingredient targets, disease targets, and ferroptosis targets were then intersected using Venn diagrams created by https://www.bioinformatics.com.cn (accessed on 25 December 2024), an online platform to identify potential therapeutic targets for EHW in liver injury through ferroptosis modulation.
2.11. Construction of the “Drug–Disease–Active Ingredient–Intersecting Target” Network
The intersecting targets obtained in Section 2.10 were used to construct network and type files, which were then imported into Cytoscape 3.9.1 software to create a “drug–disease–active ingredient–intersecting target” network diagram.
2.12. Protein–Protein Interaction (PPI) Network Construction for Intersecting Targets
The intersecting targets identified in Section 2.10 were imported into the String platform (https://string-db.org/ (accessed on 3 November 2024)) to retrieve protein–protein interaction (PPI) data while removing isolated targets. The resulting PPI data were then imported into the Cytoscape 3.9.1 software for topological analysis and visualization.
2.13. GO Enrichment and KEGG Pathway Enrichment Analysis
The intersecting targets obtained in Section 2.10 were imported into the DAVID database (https://david.ncifcrf.gov/ (accessed on 3 November 2024)) for Gene Ontology (GO) functional and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. This provided insights into the potential biological processes (BPs), cellular components (CCs), molecular functions (MFs), and signaling pathways involved in the treatment of liver injury through ferroptosis by EHW. Enriched GO functions and KEGG pathways were visualized using bubble charts generated by https://www.bioinformatics.com.cn (accessed on 3 November 2024), an online platform for data analysis and visualization.
2.14. Molecular Docking
The SDF format files for the active ingredients of EHW were obtained from the PubChem database. Core protein PDB numbers were determined using Uniprot (https://www.uniprot.org/ (accessed on 5 December 2024)), and the PDB format files for these proteins were downloaded from the PDB database (https://www.rcsb.org/ (accessed on 5 December 2024)). Molecular docking and visualization were performed using the MOE 2022.02 software.
2.15. RNA Extraction and Quantitative PCR (qPCR) Analysis
Total RNA was extracted strictly according to the manufacturer’s instructions for the Trizol reagent kit. RNA concentration and quality were assessed using a NanoDrop 200 ultra-micro spectrophotometer. cDNA was synthesized following the guidelines provided by the reverse transcription kit. GAPDH was used as the internal reference gene, and RT-qPCR reactions were performed using a fluorescence quantitative PCR machine (FQD-96X, BIOER). The relative expression of target genes was analyzed using the 2−ΔΔCt method [26]. Primer sequences for the relevant genes were synthesized by Shanghai Sangon Biotech Co., Ltd. (Shanghai, China), with the sequence details provided in Table 1.

Table 1.
Sequences of primers for RT-qPCR.
2.16. Statistical Analysis
Data were analyzed using IBM SPSS Statistics 26, and the results were expressed as the mean ± standard error of the mean (SEM), and statistical significance was determined using one-way ANOVA followed by Duncan’s multiple range test. GraphPad Prism 9 was utilized for data visualization. In all statistical analyses, a p-value of less than 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. Liver Organ Index and Liver Function Analysis
The results of the liver organ index for each group of mice are shown in Figure 2A. Compared to the CON group, the liver organ index in the CCL4 group was significantly elevated (p < 0.05). However, in comparison to the CCL4 group, the liver organ index in both the EHW-H and EHW-L groups significantly decreased (p < 0.05). The liver function indicators for the EHW treatment groups are presented in Figure 2B,C. Compared to the CON group, serum AST and ALT activities were markedly increased in the CCL4 group (p < 0.01). Treatment with EHW reversed this trend, as the serum ALT activity in the EHW-H group significantly decreased compared to the CCL4 group (p < 0.05), and there was a downward trend in the serum ALT activity in the EHW-L group. Additionally, serum AST activity in both the EHW-H and EHW-L groups was significantly reduced (p < 0.01), with the EHW-H group showing a more pronounced effect than the EHW-L group.
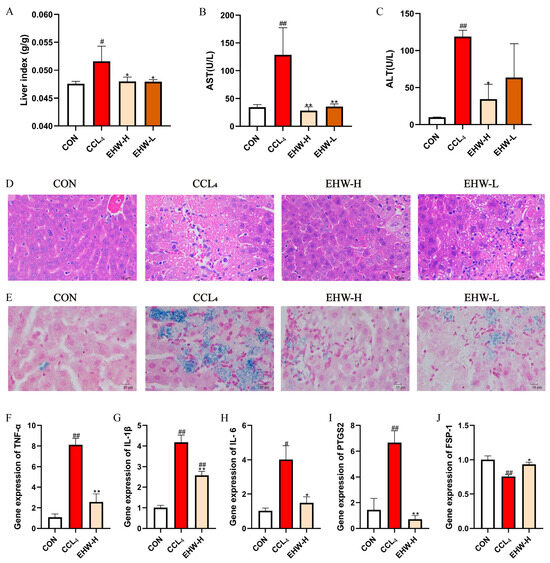
Figure 2.
(A) Changes in the organ index of mouse livers. (B) Changes in serum AST levels. (C) Changes in serum ALT levels. (D) HE staining results of mouse liver tissue. (E) Prussian blue staining results of mouse liver tissue. (F) Relative mRNA expression of TNF-α in mouse liver. (G) Relative mRNA expression of IL-1β in mouse liver. (H) Relative mRNA expression of IL-6 in mouse liver. (I) Relative mRNA expression of PTGS2 in mouse liver. (J) Relative mRNA expression of FSP-1 in mouse liver. Note: * indicates a statistically significant difference compared to the CCL4 group, # indicates a statistically significant difference compared to the CON group, * p < 0.05, ** p < 0.01; # p < 0.05, ## p < 0.01.
3.2. Pathological Histological Examination of HE Staining
The HE staining results of the liver tissues from the mice in each experimental group are shown in Figure 2D. In the CON group, the liver tissue exhibited a normal structure, with intact hepatocytes and no significant pathological damage or inflammatory cell infiltration. In the CCL4 group, hepatic cords were disorganized, and prominent fatty degeneration of the liver was observed, along with extensive hepatocyte necrosis and inflammatory cell infiltration. In contrast, the liver architecture in both the EHW-H and EHW-L groups was partially restored, fatty degeneration was alleviated, hepatocyte necrosis was notably improved, and the number of inflammatory cells infiltrating the liver was significantly reduced. The EHW-H group showed a better therapeutic effect compared to the EHW-L group.
3.3. Prussian Blue Staining Examination
The Prussian blue staining results for the liver tissues from each experimental group are shown in Figure 2E. No significant blue iron ferricyanide deposits were observed in the liver tissues of the CON group. In the CCL4 group, noticeable blue deposits were present in the areas of liver necrosis, indicating iron overload. In the EHW-H and EHW-L groups, the liver necrosis areas were significantly reduced, and the blue deposition at the necrotic sites was markedly lower than in the CCL4 group. Furthermore, the EHW-H group demonstrated a more pronounced reduction in iron deposition compared to the EHW-L group.
3.4. Expression of Liver Inflammatory Cytokines and Ferroptosis-Related Genes
The relative expression levels of liver inflammatory cytokines and ferroptosis-related genes in mice are shown in Figure 2F–J. Compared to the CON group, the relative mRNA expression levels of TNF-α, IL-1β, and PTGS2 were significantly elevated in the CCL4 group (p < 0.01), and IL-6 mRNA expression was also significantly increased (p < 0.05). In contrast, the mRNA levels of TNF-α, IL-1β, and PTGS2 were significantly reduced in the EHW-H group compared to the CCL4 group (p < 0.01), and IL-6 expression also decreased significantly (p < 0.05). Additionally, the mRNA expression of FSP-1 was significantly increased in the EHW-H group (p < 0.05), while it was significantly decreased in the CCL4 group (p < 0.01).
3.5. Major Active Components of EHW
Based on the criteria of OB ≥ 30% and DL ≥ 0.18 in the TCMSP database, six major active components of EHW were identified. Detailed information on these compounds is presented in Table 2.

Table 2.
Information on the major active components of EHW.
3.6. Screening of Drug Active Component Targets
Using the TCMSP, Swiss Target Prediction, and SuperPred databases, the targets of the screened active components of EHW were collected and analyzed. After removing duplicates, a total of 578 potential targets for the active components of EHW were obtained.
3.7. Potential Targets of EHW for Improving Ferroptosis-Related Liver Injury
By searching the GeneCards database with the keyword “liver injury” and applying the filtering criterion of having a relevance score > 8, the target information was merged with data from the DisGeNET database. After removing duplicate entries, 2360 disease-related targets for liver injury were identified. In the FerrDb database, 564 ferroptosis-related disease targets were obtained. By intersecting the 578 targets of EHW with the 564 ferroptosis-related targets and the 2360 liver injury-related targets, 52 potential targets for EHW in improving ferroptosis-related liver injury were identified. The Venn diagram illustrating these results is shown in Figure 3A.
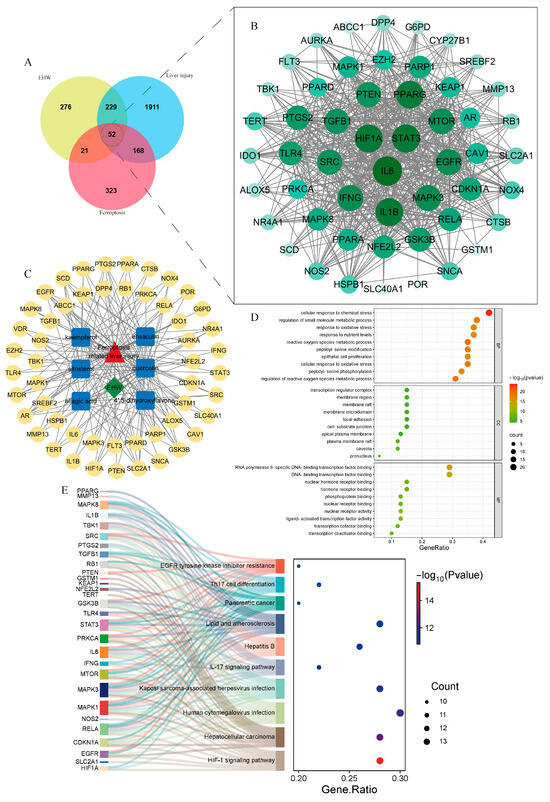
Figure 3.
(A) Venn diagram of EHW component targets, liver injury targets, and ferroptosis targets. (B) PPI network diagram of the intersecting targets. (C) “Drug–active component–intersecting target” diagram. (D) GO enrichment analysis of EHW’s effects on ferroptosis-related liver injury. (E) KEGG enrichment analysis of EHW’s effects on ferroptosis-related liver injury.
3.8. Construction of PPI Network for the Intersecting Targets
The potential targets of EHW for improving ferroptosis-related liver injury were input into the STRING database to construct a PPI network, and the data were visualized using Cytoscape 3.9.1 software (Figure 3B). By calculating the degree values, the top core targets were found to be IL-6, STAT3, HIF1A, PTGS2, NFE2L2, and KEAP1, among others.
3.9. “Drug–Disease–Active Component–Intersecting Target” Network Construction
As shown in Figure 3C, the “drug–disease–active component–intersecting target” relationship network was constructed. The network contained 60 nodes and 184 edges. Based on the degree values, the core active components of EHW for improving ferroptosis-related liver injury were identified. The top-ranked active components included quercetin, kaempferol, and sitosterol.
3.10. GO Enrichment Analysis and KEGG Pathway Enrichment Analysis
The intersecting targets were subjected to GO functional enrichment analysis and KEGG pathway enrichment analysis (p < 0.05). A total of 2214 BPs, 75 CCs, 132 MFs, and 156 signaling pathways were identified. As shown in Figure 3D,E, the potential targets of EHW for alleviating ferroptosis-related liver injury mainly involved BPs such as response to oxidative stress, the regulation of reactive oxygen species, metabolic processes, and ROS metabolic processes. These targets were predominantly located in cellular components like the transcription regulator complex, membrane regions, and membrane rafts. Molecular functions such as RNA polymerase II-specific DNA-binding transcription factor binding, transcription coactivator binding, and DNA-binding transcription factor binding were the most prominent. The KEGG pathway enrichment results indicated that the enriched pathways were primarily associated with Hepatitis B, the HIF-1 signaling pathway, and the IL-17 signaling pathway.
3.11. Molecular Docking Results
Figure 4 presents the molecular docking results between the main active components of EHW and the potential key target proteins associated with ferroptosis-related liver injury. The key target proteins identified through topological analysis, including NFE2L2, KEAP1, LPCAT3, ALOX12, and ACSL4, were pre-processed and subjected to molecular docking alongside the core active components. The results showed that the top three active components, based on their degree value, had binding energies with ferroptosis-related proteins NFE2L2, KEAP1, LPCAT3, ALOX12, and ACSL4 which were lower than −4.5 kcal/mol. Based on the binding energies, the stronger binding component–target combinations were selected for visualization (Figure 5). The results demonstrated that quercetin interacts with the target ACSL4 at the Tyr468 and ASP573 sites, sitosterol interacts with ALOX12 at the Asn287 site, sitosterol interacts with KEAP1 at the Arg326 site, quercetin interacts with LPCAT3 at the Cys383 site, and quercetin interacts with NFE2L2 at the Val606 and Val463 sites.
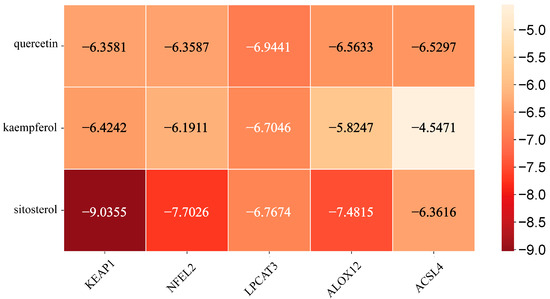
Figure 4.
Heatmap of the binding affinity between the key targets related to ferroptosis and liver injury and the active components of EHW.
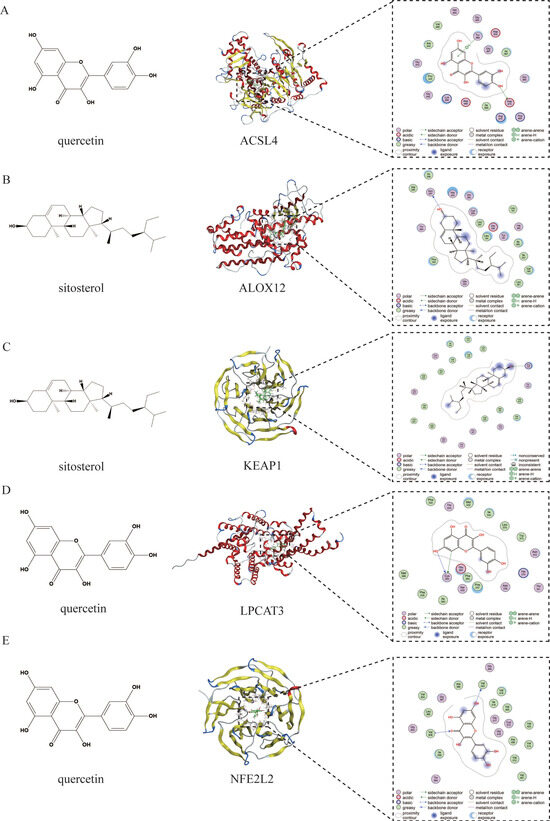
Figure 5.
(A) Molecular docking visualization of quercetin and ACSL4. (B) Molecular docking visualization of sitosterol and ALOX12. (C) Molecular docking visualization of sitosterol and KEAP1. (D) Molecular docking visualization of quercetin and LPCAT3. (E) Molecular docking visualization of quercetin and NFE2L2.
3.12. Effect of EHW on the KEAP1-NFE2L2 Signaling Pathway
As shown in Figure 6, compared to the CON group, the mRNA expression level of KEAP1 in the CCL4 group was significantly elevated (p < 0.05), while the mRNA expression of SOD-1 was significantly reduced (p < 0.01). Additionally, the mRNA expression levels of P62, NFE2L2, and NQO1 were significantly decreased (p < 0.05), and the mRNA expression level of HMOX1 showed a downward trend. However, in comparison to the CCL4 group, the EHW-H group exhibited a significant reduction in KEAP1 mRNA expression (p < 0.05). Moreover, the mRNA expression of P62 and SOD-1 was significantly increased (p < 0.01), and the mRNA levels of NFE2L2, NQO1, and HMOX1 were all significantly elevated (p < 0.05).
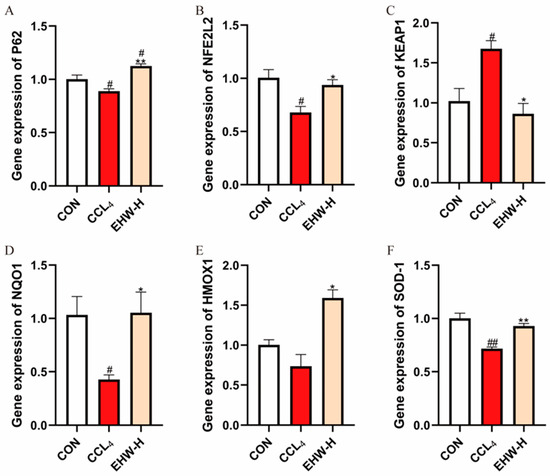
Figure 6.
The effect of EHW on the KEAP1-NFE2L2 signaling pathway. (A) Relative P62 mRNA expression. (B) Relative NFE2L2 mRNA expression. (C) Relative KEAP1 mRNA expression. (D) Relative NQO1 mRNA expression. (E) Relative HMOX1 mRNA expression. (F) Relative SOD-1 mRNA expression. Note: * indicates a statistically significant difference compared to the CCL4 group, # indicates a statistically significant difference compared to the CON group, * p < 0.05, ** p < 0.01; # p < 0.05, ## p < 0.01.
3.13. Effect of EHW on the Expression of Iron Homeostasis-Related Genes
As shown in Figure 7, compared to the CON group, the mRNA expression of TFR1 was significantly elevated in the CCL4 group (p < 0.01), while the mRNA expression of FTH1 was significantly reduced (p < 0.01). Additionally, the mRNA expression level of FPN was significantly decreased (p < 0.05). In contrast, when compared to the CCL4 group, the EHW-H group showed a significant reduction in TFR1 mRNA expression (p < 0.05), while FTH1’s mRNA expression was significantly increased (p < 0.01), and the mRNA expression of FPN exhibited an upward trend.
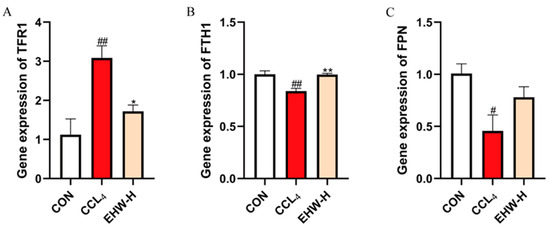
Figure 7.
The effect of EHW on iron homeostasis-related genes.(A) Relative TFR1 mRNA expression. (B) Relative FTH1 mRNA expression. (C) Relative FPN mRNA expression. Note: * indicates a statistically significant difference compared to the CCL4 group, # indicates a statistically significant difference compared to the CON group, * p < 0.05, ** p < 0.01; # p < 0.05, ## p < 0.01.
3.14. Effect of EHW on the Expression of Genes in the PLOOH Metabolism Pathway
As shown in Figure 8, compared to the CON group, the mRNA expression levels of ACSL4 and ALOX12 were significantly increased in the CCL4 group (p < 0.01), and the mRNA expression of LPCAT3 was also significantly elevated (p < 0.05). On the other hand, the mRNA expression of GPX4 was significantly decreased (p < 0.01). In contrast, when compared to the CCL4 group, the EHW-H group exhibited a significant reduction in the mRNA expression of ACSL4 and ALOX12 (p < 0.01), as well as a decrease in the mRNA expression of LPCAT3 (p < 0.05). Moreover, the mRNA expression of GPX4 was significantly increased in the EHW-H group (p < 0.01).
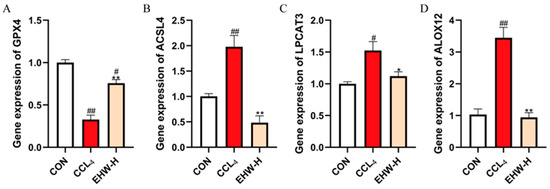
Figure 8.
The effect of EHW on gene expression in the PLOOH metabolism pathway. (A) Relative GPX4 mRNA expression. (B) Relative ACSL4 mRNA expression. (C) Relative LPCAT3 mRNA expression. (D) Relative ALOX12 mRNA expression. Note: * indicates a statistically significant difference compared to the CCL4 group, # indicates a statistically significant difference compared to the CON group, * p < 0.05, ** p < 0.01; # p < 0.05, ## p < 0.01.
4. Discussion
Liver injury is a common condition in veterinary clinical practice and poses a significant threat to livestock production [27]. Inhibiting excessive hepatocyte death is an effective approach to protect the liver and treat liver diseases [28]. Increasing evidence suggests that ferroptosis plays a crucial role in the occurrence and progression of liver-injury-related diseases [29,30]. Fortunately, with the rapid development of traditional Chinese (veterinary) medicine research, its potential use in liver injury treatment demonstrating safety, reliability, and multi-target effects has garnered increasing attention [31]. In this study, we established a mouse model of liver injury using CCL4, a classic hepatotoxic substance. The results showed that, compared to the CON group, the CCL4 group had a significantly increased liver index, and serum AST and ALT activities were markedly elevated. Histological examination via HE staining revealed disorganized hepatic cords, hepatocyte fatty degeneration, and considerable hepatocyte necrosis with inflammatory cell infiltration in the CCL4 group. However, EHW successfully reversed these trends. Compared to the CCL4 group, both the EHW-H and EHW-L groups exhibited a significant reduction in the liver index, and serum AST activity was markedly decreased. Serum ALT activity in the EHW-L group showed a decreasing trend. Consistently, in the EHW-H group, serum ALT activity significantly decreased. HE staining also revealed that liver architecture was restored in both the EHW-H and EHW-L groups, hepatocyte fatty degeneration was alleviated, hepatocyte necrosis was notably improved, and the area of inflammatory cell infiltration was substantially reduced, with the EHW-H group showing a better therapeutic effect than the EHW-L group. The excessive production and release of pro-inflammatory cytokines is one of the major characteristics of liver injury, and TNF-α generation is considered an important early marker of CCL4-induced liver injury [32]. In this study, compared to the CON group, the relative mRNA expression levels of TNF-α and IL-1β in the CCL4 group were significantly increased, and IL-6 mRNA expression was also significantly elevated. In contrast, compared to the CCL4 group, the EHW-H group showed a significant decrease in the relative mRNA expression levels of TNF-α and IL-1β, and IL-6 mRNA expression was significantly reduced. These results suggest that EHW has a protective effect against CCL4-induced liver injury, and this protective effect appears to be dose-dependent.
Ferroptosis is an iron-dependent form of programmed cell death, and abnormal iron accumulation is a key feature in the onset of ferroptosis [33]. PTGS2 is considered an important marker of ferroptosis [34], and FSP-1 is a recently identified inhibitor that plays a crucial role in suppressing ferroptosis [35]. To further investigate whether the hepatoprotective effect of EHW is related to the alleviation of ferroptosis in hepatocytes, we first performed Prussian blue staining. The results revealed that in the CCL4 group, there was a significant increase in blue precipitates in the areas of liver necrosis, indicating the presence of iron overload. In contrast, in the EHW treatment groups, the areas of liver necrosis were significantly reduced, and the extent of blue precipitate staining in the liver tissue was markedly less than in the CCL4 group. Furthermore, we measured the relative mRNA expression levels of PTGS2 and FSP-1 in the liver. Compared to the CON group, the relative mRNA expression of PTGS2 was significantly elevated in the CCL4 group, while the expression of FSP-1 was significantly reduced. In contrast, in the EHW-H group, the relative mRNA expression of PTGS2 was significantly decreased, while the expression of FSP-1 was notably increased compared to the CCL4 group. These results suggest that CCL4-induced liver injury is closely associated with the excessive occurrence of ferroptosis, and that EHW may alleviate ferroptosis, thereby exerting its hepatoprotective effects.
Network pharmacology, an interdisciplinary research approach that integrates knowledge from fields such as systems biology and pharmacology, enables the analysis of the systemic effects of multi-component drugs on the human body [36]. It has significant advantages in exploring the therapeutic targets of TCM and investigating disease mechanisms [37,38]. In this study, by constructing an intersecting target PPI network, we identified the core targets with higher degree values, including PTGS2, NFE2L2, KEAP1, and others. These targets exhibited frequent interactions with the major active compounds of EHW, such as quercetin, kaempferol, and sitosterol, suggesting that EHW may exert its hepatoprotective effects by targeting these core molecules. GO enrichment analysis, which linked the genes identified in this study to functional categories in the Gene Ontology database, revealed statistically significant differences in specific biological processes, cellular components, and molecular functions [39]. KEGG analysis aids in understanding the regulatory processes of various metabolic pathways and cellular processes in biological systems, thus shedding light on key biological mechanisms and interactions [40]. In this study, GO enrichment analysis indicated that the potential targets of EHW in alleviating ferroptosis-induced liver injury were mainly involved in BPs such as the response to oxidative stress, regulation of reactive oxygen species (ROS) metabolic processes, and ROS metabolic processes. These targets were predominantly localized in cellular components, including the transcription regulator complex, membrane regions, and membrane rafts. In terms of molecular functions, they were primarily associated with RNA polymerase II-specific DNA-binding transcription factor binding, transcription coactivator binding, and DNA-binding transcription factor binding. KEGG pathway enrichment analysis revealed that the enriched pathways were primarily related to the Hepatitis B, HIF-1 signaling, and IL-17 signaling pathways. Molecular docking is a computational biology method typically used to study the interactions between drugs and their targets. In this study, molecular docking of the three top-ranking active compounds with key ferroptosis targets (KEAP1, NFE2L2, LPCAT3, ALOX12, and ACSL4) demonstrated that the binding energies of the target small molecule complexes were all significantly lower than −4.5 kcal/mol, suggesting a strong affinity between the active components and the targets. The visualization results further indicated that the active components and core targets were closely connected in a specific spatial structure, implying that the major active compounds of EHW have a strong biological affinity with the ferroptosis-related core targets. These findings suggest that EHW may exert its hepatoprotective effects by acting on the KEAP1-NFE2L2 signaling pathway and reducing the accumulation of PLOOH, thereby mitigating hepatocyte ferroptosis.
Ferroptosis is a form of cell death closely associated with oxidative stress [41]. The KEAP1-NFE2L2 pathway is a crucial regulator of cellular oxidative stress [42]. Upon activation, NFE2L2 exerts potent antioxidant effects and plays a key role in the regulation of ferroptosis [43]. KEAP1 negatively regulates NFE2L2 [44], while P62 can interact with KEAP1 at the NFE2L2 binding site, competing with KEAP1 and thereby activating NFE2L2 [45]. Once activated, NFE2L2 induces an increase in P62, creating a positive feedback loop [46]. More importantly, when NFE2L2 is activated, the downstream antioxidant genes such as HMOX1, NQO2, and SOD-1 are activated, directly exerting antioxidant cell protection and alleviating ferroptosis [47]. In this study, compared to the CON group, the CCL4 group showed a significant increase in KEAP1 mRNA expression, a marked decrease in SOD-1 mRNA expression, and significant reductions in the mRNA expression levels of P62, NFE2L2, and NQO1, with HMOX1 mRNA expression also showing a decreasing trend. In contrast, compared to the CCL4 group, the EHW-H group showed a significant decrease in KEAP1 mRNA expression, a dramatic increase in P62 and SOD-1 mRNA expression, and significant increases in the mRNA expression levels of NFE2L2, NQO1, and HMOX1. These results suggest that EHW can activate NFE2L2, upregulate the expression of antioxidant genes, reduce cellular oxidative stress and ferroptosis, and thereby exert a protective effect against liver injury.
Studies have shown that the dysregulation of iron metabolism is a critical trigger for ferroptosis [48]. The excessive accumulation of iron in the labile iron pool (LIP) is a key factor in the initiation of ferroptosis [49]. The iron content in the LIP is tightly regulated by various molecules. Under normal physiological conditions, iron ions typically interact with transferrin, after which they are internalized into the cell via TFR1 [50]. Once inside the cell, iron is either stored in the iron storage protein complex or, alternatively, exported by FPN [51,52]. Notably, FTH1 serves as a critical component of the iron storage protein complex [53]. When TFR1 is overexpressed [54], FTH1 is insufficient [55] or FPN is suppressed [56], and iron accumulates abnormally in the LIP, leading to the Fenton reaction, which triggers lipid peroxidation and ultimately induces ferroptosis [57]. Interestingly, recent studies have discovered that the activation of the KEAP1-NFE2L2 pathway not only regulates the body’s antioxidant response but also plays a critical role in iron metabolism. Numerous studies have confirmed that key regulators of iron metabolism, such as FTH1 and FPN, are controlled by NFE2L2 [58]. In this study, compared to the CON group, the CCL4 group showed a significant increase in TFR1 mRNA expression, a marked decrease in FTH1 mRNA expression, and a significant reduction in FPN mRNA expression. In contrast, the EHW-H group exhibited a significant decrease in TFR1 mRNA expression, a dramatic increase in FTH1 mRNA expression, and an upward trend in FPN mRNA expression compared to the CCL4 group. These results suggest that EHW may regulate iron metabolism by activating the KEAP1-NFE2L2 pathway, restoring cellular iron homeostasis, and inhibiting excessive ferroptosis, thereby alleviating liver injury.
The accumulation of phospholipid hydroperoxides (PLOOHs) is the most direct cause of ferroptosis [59]. Timely clearance of PLOOHs or the inhibition of the synthesis of their precursors, phospholipid-containing polyunsaturated fatty acid chains (PUFA-PL), can exert a positive effect on ferroptosis [60]. GPX4 is the most well-known reductase of PLOOHs, as it reduces PLOOHs to their corresponding alcohols [34]. Notably, the expression of this gene is also regulated by NFE2L2 [61]. Another critical pathway to prevent ferroptosis is the inhibition of PUFA-PL synthesis. ACSL4 is a fatty acid synthetase that specifically catalyzes the activation of long-chain polyunsaturated fatty acids (PUFAs) [62]. Under the catalysis of ACSL4, these long-chain PUFAs are conjugated with coenzyme A (CoA) to form PUFA-CoA intermediates [63]. Subsequently, LPCAT3 esterifies these intermediates to generate PUFA-PL [64], which is ultimately converted into PLOOHs through the action of lipoxygenases such as ALOX12 [65]. In this study, compared to the CON group, the CCL4 group showed a dramatic increase in the mRNA expression of ACSL4 and ALOX12, a significant increase in LPCAT3 mRNA expression, and a marked decrease in GPX4 mRNA expression. In contrast, the EHW-H group exhibited a significant reduction in the mRNA expression of ACSL4 and ALOX12, a notable decrease in LPCAT3 mRNA expression, and a dramatic increase in GPX4 mRNA expression compared to the CCL4 group. These results suggest that EHW may reduce the generation of PLOOHs and accelerate their reduction, thereby decreasing the accumulation of PLOOHs, inhibiting ferroptosis, and alleviating liver injury.
5. Conclusions
In summary, EHW may exert its hepatoprotective effects by regulating the KEAP1-NFE2L2 signaling pathway to alleviate oxidative stress, restore cellular iron homeostasis, reduce the accumulation of PLOOHs, and ultimately mitigate ferroptosis in the liver. On the other hand, despite promising results, the direct applicability of EHW in veterinary practice requires further pharmacokinetic, toxicity, and large-scale clinical trials to confirm its safety and effectiveness across different livestock breeds.
Author Contributions
Conceptualization, H.D.; methodology, K.Y.; software, J.Y. and Y.P.; validation, H.D., J.W. and W.S.; formal analysis, S.X., C.C. and J.L.; resources, S.X., C.C. and H.D.; data curation, K.Y.; writing—original draft preparation, K.Y. and J.Y.; writing—review and editing, H.D.; visualization, K.Y.; supervision, H.D.; project administration, H.D.; funding acquisition, H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32402934), the Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0470), and the College Students’ Innovative Entrepreneurial Training Plan Program of Chongqing (S202410635371).
Institutional Review Board Statement
All animal experiments were conducted in accordance with the guidelines approved by the Ethics Committee of Southwest University for Animal Welfare (Ethics No. LAC2024-1-0346; date: 31 October 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We are grateful to all other staff in the Department of Traditional Chinese Veterinary Medicine of Southwest University for their assistance in the experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EHW | Euphorbia humifusa Willd. ex Schltdl. |
| TCM | Traditional Chinese Medicine |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| HE | Hematoxylin–eosin staining |
| OB | Oral bioavailability |
| DL | Drug-likeness |
| PPI | Protein–protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| BP | Biological process |
| CC | Cellular component |
| MF | Molecular function |
| SEM | Standard error of the mean |
| LIP | Labile iron pool |
| PLOOHs | Phospholipid hydroperoxides |
| PUFA-PL | Phospholipid-containing polyunsaturated fatty acid chains |
| PUFAs | Polyunsaturated fatty acids |
| CoA | Coenzyme A |
References
- Peleman, C.; Hellemans, S.; Veeckmans, G.; Arras, W.; Zheng, H.; Koeken, I.; Van San, E.; Hassannia, B.; Walravens, M.; Kayirangwa, E.; et al. Ferroptosis is a targetable detrimental factor in metabolic dysfunction-associated steatotic liver disease. Cell Death Differ. 2024, 31, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, W.; Mao, X.; Ge, L.; Du, H.; Li, J.; Hou, L.; Liu, D.; Yin, Y.; Liu, Y.; et al. Melatonin mitigates aflatoxin B1-induced liver injury via modulation of gut microbiota/intestinal FXR/liver TLR4 signaling axis in mice. J. Pineal. Res. 2022, 73, e12812. [Google Scholar] [CrossRef]
- Twedt, D.C.; Cullen, J.; Mccord, K.; Janeczko, S.; Dudak, J.; Simpson, K. Evaluation of fluorescence in situ hybridization for the detection of bacteria in feline inflammatory liver disease. J. Feline. Med. Surg. 2014, 16, 109–117. [Google Scholar] [CrossRef]
- Weingarten, M.A.; Sande, A.A. Acute liver failure in dogs and cats. J. Vet. Emerg. Crit. Care 2015, 25, 455–473. [Google Scholar] [CrossRef]
- Dedeaux, A.M.; Flesner, B.K.; Reinhart, J.M.; Langohr, I.M.; Husnik, R.; Geraci, S.N.; Taboada, J.; Rademacher, N.; Thombs, L.A.; Bryan, J.N.; et al. Biochemical, functional, and histopathologic characterization of lomustine-induced liver injury in dogs. Am. J. Vet. Res. 2020, 81, 810–820. [Google Scholar] [CrossRef]
- Wu, Y.; He, X.; Chen, H.; Lin, Y.; Zheng, C.; Zheng, B. Extraction and characterization of hepatoprotective polysaccharides from Anoectochilus roxburghii against CCL4-induced liver injury via regulating lipid metabolism and the gut microbiota. Int. J. Biol. Macromol. 2024, 277 Pt 3, 134305. [Google Scholar] [CrossRef]
- Xu, W.; Hu, Z.; Zhang, J.; Tang, Y.; Xing, H.; Xu, P.; Ma, Y.; Niu, Q. Cross-talk between autophagy and ferroptosis contributes to the liver injury induced by fluoride via the mtROS-dependent pathway. Ecotoxicol. Environ. Saf. 2023, 250, 114490. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Olzmann, J.A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022, 289, 7038–7050. [Google Scholar] [CrossRef]
- Yang, R.; Gao, W.; Wang, Z.; Jian, H.; Peng, L.; Yu, X.; Xue, P.; Peng, W.; Li, K.; Zeng, P. Polyphyllin I induced ferroptosis to suppress the progression of hepatocellular carcinoma through activation of the mitochondrial dysfunction via Nrf2/HO-1/GPX4 axis. Phytomedicine 2024, 122, 155135. [Google Scholar] [CrossRef]
- Yin, X.; Mi, Y.; Wang, X.; Li, Y.; Zhu, X.; Bukhari, I.; Wang, Q.; Zheng, P.; Xue, X.; Tang, Y. Exploration and Validation of Ferroptosis-Associated Genes in ADAR1 Deletion-Induced NAFLD through RNA-seq Analysis. Int. Immunopharmacol. 2024, 134, 112177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiao, M.; Li, Y.; Chitrakar, B.; Sheng, Q.; Zhao, W. Ursolic Acid Ameliorates Alcoholic Liver Injury through Attenuating Oxidative Stress-Mediated Ferroptosis and Modulating Gut Microbiota. J. Agric. Food Chem. 2024, 72, 21181–21192. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Feng, B.; Yu, J.; Yan, L.; Che, L.; Zhuo, Y.; Luo, Y.; Yu, B.; Wu, D.; Chen, D. Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis. Redox Biol. 2021, 46, 102131. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Zhang, C.; Huang, Y.; Yu, J.; Shi, L.; Zhang, P.; Yin, Y.; Li, R.; Tao, K. Maresin1 Protect Against Ferroptosis-Induced Liver Injury Through ROS Inhibition and Nrf2/HO-1/GPX4 Activation. Front. Pharmacol. 2022, 13, 865689. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Y.; Wang, H.R.; Fan, Y.M.; Gu, J.H.; Zhang, X.Y.; Gong, X.H.; Hao, Z.H. Acute liver injury induced by carbon tetrachloride reversal by Gandankang aqueous extracts through nuclear factor erythroid 2-related factor 2 signaling pathway. Ecotoxicol. Environ. Saf. 2023, 251, 114527. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Yuan, Y.M.; Zhao, Z.H.; Yao, Q.H.; Ye, X.Q.; Wang, Y.Y.; Liu, H.M.; Jha, R.; Balasubramanian, B.; Liu, W.C. Phlorotannin Alleviates Liver Injury by Regulating Redox Balance, Apoptosis, and Ferroptosis of Broilers under Heat Stress. Antioxidants 2024, 13, 1048. [Google Scholar] [CrossRef]
- Huang, S.; Lin, L.; Wang, S.; Ding, W.; Zhang, C.; Shaukat, A.; Xu, B.; Yue, K.; Zhang, C.; Liu, F. Total Flavonoids of Rhizoma Drynariae Mitigates Aflatoxin B1-Induced Liver Toxicity in Chickens via Microbiota-Gut-Liver Axis Interaction Mechanisms. Antioxidants 2023, 12, 819. [Google Scholar] [CrossRef]
- Wu, X.; Ma, G.L.; Chen, H.W.; Zhao, Z.Y.; Zhu, Z.P.; Xiong, J.; Yang, G.X.; Hu, J.F. Antibacterial and antibiofilm efficacy of the preferred fractions and compounds from Euphorbia humifusa (herba euphorbiae humifusae) against Staphylococcus aureus. J. Ethnopharmacol. 2023, 306, 116177. [Google Scholar] [CrossRef]
- Luyen, B.T.; Tai, B.H.; Thao, N.P.; Thao, N.P.; Eun, K.J.; Cha, J.Y.; Xin, M.J.; Lee, Y.M.; Kim, Y. Anti-inflammatory components of Euphorbia humifusa Willd. Bioorg. Med. Chem. Lett. 2014, 24, 1895–1900. [Google Scholar] [CrossRef]
- Chang, S.Y.; Park, J.H.; Kim, Y.H.; Kang, J.S.; Min, J.Y. A natural component from Euphorbia humifusa Willd displays novel, broad-spectrum anti-influenza activity by blocking nuclear export of viral ribonucleoprotein. Biochem. Biophys. Res. Commun. 2016, 471, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Rakotondrabe, T.F.; Fan, M.; Guo, M. Exploring potential antidiabetic and anti-inflammatory flavonoids from Euphorbia humifusa with an integrated strategy. Front. Pharmacol. 2022, 13, 980945. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Kim, C.G.; Jung, Y.J.; Jung, Y.; Jung, H.; Im, J.; Lim, Y.; Lee, Y.H. Euphorbia humifusa Willd exerts inhibition of breast cancer cell invasion and metastasis through inhibition of TNFα-induced MMP-9 expression. BMC Complement. Altern. Med. 2016, 16, 413. [Google Scholar] [CrossRef]
- The State Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2005; p. 84. [Google Scholar]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025, 53, D1670–D1676. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wang, L.; Yang, P.; Liu, Z.; Rajput, S.A.; Hassan, M.; Qi, D. Epigallocatechin Gallate and Glutathione Attenuate Aflatoxin B-Induced Acute Liver Injury in Ducklings via Mitochondria-Mediated Apoptosis and the Nrf2 Signalling Pathway. Toxins 2022, 14, 876. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Yang, N.; Huang, Y.; Hu, T.; Rao, C. Endoplasmic reticulum stress-mediated cell death in liver injury. Cell Death Dis. 2022, 13, 1051. [Google Scholar] [CrossRef]
- Ge, Y.; Yang, S.; Zhang, T.; Gong, S.; Wan, X.; Zhu, Y.; Fang, Y.; Hu, C.; Yang, F.; Yin, L.; et al. Ferroptosis participated in inhaled polystyrene nanoplastics-induced liver injury and fibrosis. Sci. Total Environ. 2024, 916, 170342. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Jiang, R.; Xue, R.; Yin, X.; Wu, M.; Meng, Q. Ferroptosis in liver disease: New insights into disease mechanisms. Cell Death Discov. 2021, 7, 276. [Google Scholar] [CrossRef]
- Sun, Y.K.; Zhang, Y.F.; Xie, L.; Rong, F.; Zhu, X.Y.; Xie, J.; Zhou, H.; Xu, T. Progress in the treatment of drug-induced liver injury with natural products. Pharmacol. Res. 2022, 183, 106361. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, J.; Lee, K.H.; Lee, D.U.; Kwak, J.H.; Kim, Y.S.; Lee, S.M. Ferulic acid protects against carbon tetrachloride-induced liver injury in mice. Toxicology. 2011, 282, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Rittase, W.B.; Slaven, J.E.; Suzuki, Y.J.; Muir, J.M.; Lee, S.H.; Rusnak, M.; Brehm, G.V.; Bradfield, D.T.; Symes, A.J.; Day, R.M. Iron Deposition and Ferroptosis in the Spleen in a Murine Model of Acute Radiation Syndrome. Int. J. Mol. Sci. 2022, 23, 11029. [Google Scholar] [CrossRef]
- Yang, W.S.; Sriramaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; Da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, Y.; Li, J.; Zhou, F.; Xiao, K.; Liu, Y.; Zhang, M.; Wang, S.; Yang, S. Mechanism of Sijunzi Decoction in the treatment of colorectal cancer based on network pharmacology and experimental validation. J. Ethnopharmacol. 2023, 302 (Pt A), 115876. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Li, M.Z.; Li, W.J.; Ouyang, J.F.; Gou, X.J.; Huang, Y. Mechanism of action of Daqinjiao decoction in treating cerebral small vessel disease explored using network pharmacology and molecular docking technology. Phytomedicine. 2023, 108, 154538. [Google Scholar] [CrossRef]
- Cai, B.; Qi, M.; Zhang, X.; Zhang, D. Integrating Network Pharmacology with in vitro Experiments to Validate the Efficacy of Celastrol Against Hepatocellular Carcinoma Through Ferroptosis. Drug Des. Devel. Ther. 2024, 18, 3121–3141. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, X.; Khan, G.J.; Cheng, J.; He, J.; Zhai, K.; Mao, Y. Exploring the molecular mechanism of Artemisia rupestris L. for the treatment of hepatocellular carcinoma via PI3K/AKT pathway. J. Ethnopharmacol. 2024, 322, 117572. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef]
- Adinolfi, S.; Patinen, T.; Deen, A.J.; Pitkänen, S.; Härkönen, J.; Kansanen, E.; Küblbeck, J.; Levonen, A.L. The KEAP1-NRF2 pathway: Targets for therapy and role in cancer. Redox Biol. 2023, 63, 102726. [Google Scholar] [CrossRef] [PubMed]
- Kerins, M.J.; Ooi, A. The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid. Redox Signal. 2018, 29, 1756–1773. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, J.S.; Lee, Y.S.; Han, J.; Lee, D.K.; Kwon, S.W.; Han, D.H.; Lee, Y.H.; Bae, S.H. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy 2020, 16, 1949–1973. [Google Scholar] [CrossRef]
- Song, W.; Zhang, L.; Cui, X.; Wang, R.; Ma, J.; Xu, Y.; Jin, Y.; Wang, D.; Lu, Z. Nobiletin alleviates cisplatin-induced ototoxicity via activating autophagy and inhibiting NRF2/GPX4-mediated ferroptosis. Sci. Rep. 2024, 14, 7889. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Lei, L.; Yuan, J.; Dai, Z.; Xiang, S.; Tu, Q.; Cui, X.; Zhai, S.; Chen, X.; He, Z.; Fang, B.; et al. Targeting the Labile Iron Pool with Engineered DFO Nanosheets to Inhibit Ferroptosis for Parkinson’s Disease Therapy. Adv. Mater. 2024, 36, e2409329. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef]
- Cui, J.; Chen, Y.; Yang, Q.; Zhao, P.; Yang, M.; Wang, X.; Mang, G.; Yan, X.; Wang, D.; Tong, Z.; et al. Protosappanin A Protects DOX-Induced Myocardial Injury and Cardiac Dysfunction by Targeting ACSL4/FTH1 Axis-Dependent Ferroptosis. Adv. Sci. 2024, 11, e2310227. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Lu, D.; Yin, M.; Shan, M.; Gao, Y. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum. Reprod. 2021, 36, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, X.; Tan, Q.; Zhou, H.; Xu, J.; Gu, Q. Inhibiting Ferroptosis through Disrupting the NCOA4-FTH1 Interaction: A New Mechanism of Action. ACS Cent. Sci. 2021, 7, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liang, W.; Huo, D.; Wang, H.; Wang, Y.; Cong, C.; Zhang, C.; Yan, S.; Gao, M.; Su, X.; et al. SPY1 inhibits neuronal ferroptosis in amyotrophic lateral sclerosis by reducing lipid peroxidation through regulation of GCH1 and TFR1. Cell Death Differ. 2023, 30, 369–382. [Google Scholar] [CrossRef]
- Kong, N.; Chen, X.; Feng, J.; Duan, T.; Liu, S.; Sun, X.; Chen, P.; Pan, T.; Yan, L.; Jin, T.; et al. Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1. Acta Pharm. Sin. B 2021, 11, 4045–4054. [Google Scholar] [CrossRef]
- Bao, W.D.; Zhou, X.T.; Zhou, L.T.; Wang, F.; Yin, X.; Lu, Y.; Zhu, L.Q.; Liu, D. Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell. 2020, 19, e13235. [Google Scholar] [CrossRef]
- Gan, B. Mitochondrial regulation of ferroptosis. J. Cell Biol. 2021, 220, e202105043. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Castro-portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Zeng, Y.; Wang, Y.; Pei, T.; Xie, Z.; Xiong, Q.; Wei, H.; Li, W.; Li, J.; et al. Salidroside alleviates cognitive impairment by inhibiting ferroptosis via activation of the Nrf2/GPX4 axis in SAMP8 mice. Phytomedicine 2023, 114, 154762. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Soupene, E.; Kuypers, F.A. Mammalian long-chain acyl-CoA synthetases. Exp. Biol. Med. 2008, 233, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Winter, G.E.; Musavi, L.S.; Lee, E.D.; Snijder, B.; Rebsamen, M.; Superti-Furga, G.; Stockwell, B.R. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem. Biol. 2015, 10, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Banthiya, S.; Van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta. 2015, 1851, 308–330. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).