Effects of Dietary Supplementation Using Phytobiotics with Different Functional Properties on Expression of Immunity Genes, Intestinal Histology, Growth, and Meat Productivity of Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
- -
- 31–33 °C—in the first week;
- -
- 24–30 °C—in the second and third weeks;
- -
- 20–23 °C—in the fourth and fifth (final) week of the experiment.
- -
- On the last day of incubation when the chicks hatched—constant light;
- -
- Days 1–7—23 h of light and 1 h of darkness;
- -
- Days 8–34—four cycles of 5 h of light and 1 h of darkness;
- -
- Day 35—23 h of light and 1 h of darkness.
2.2. Broiler Chicken Nutrition and Plant Extracts
2.3. Growth and Meat Productivity of Broiler Chickens
2.4. Sample Collection
2.5. RNA Isolation and Real-Time PCR
2.6. Histological Studies of Blind Intestinal Pouches
2.7. Statistical Analysis
3. Results
3.1. Individual Consumption of Phytochemicals by Chickens
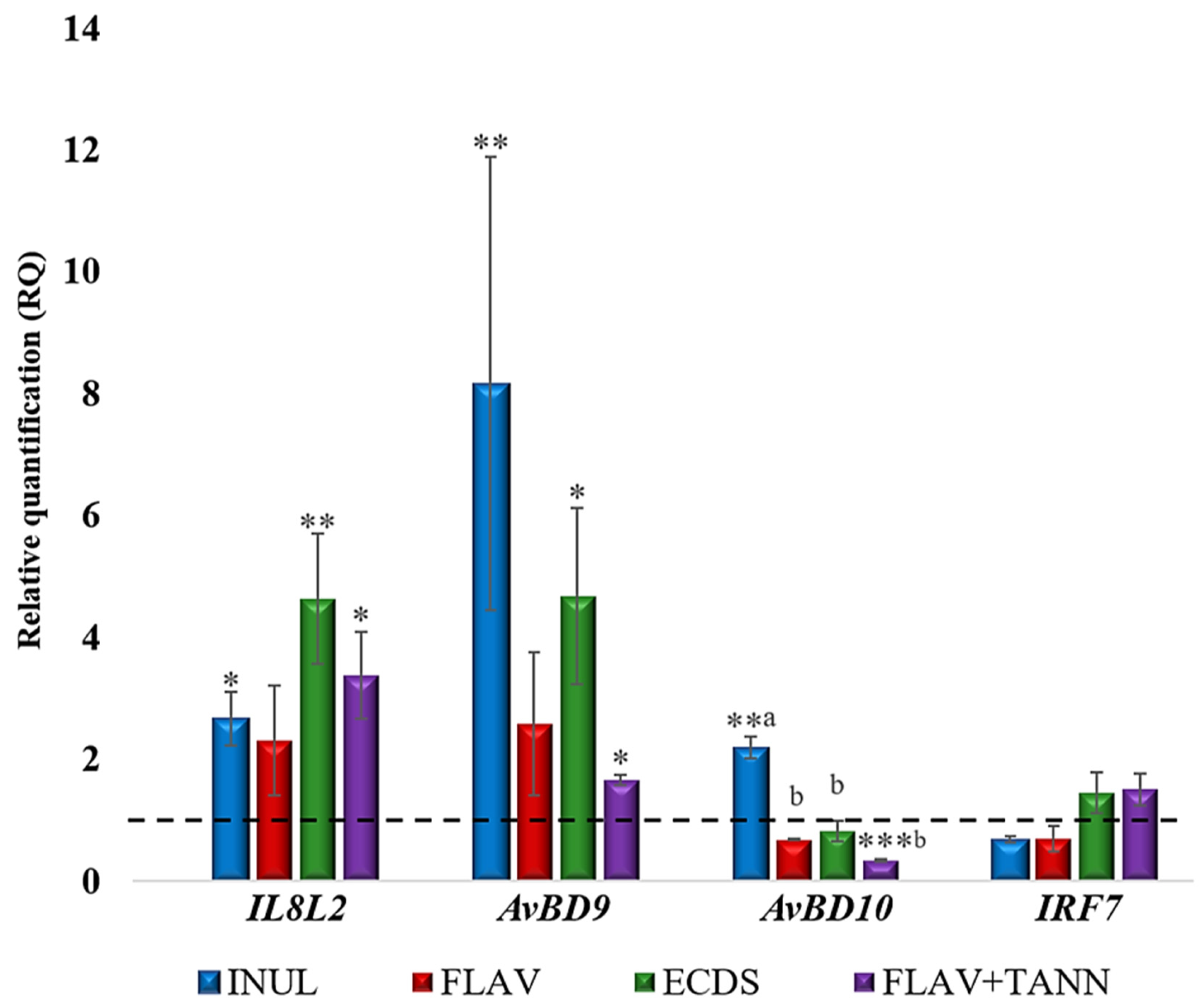
3.2. Expression of Immunity Genes in the Cecum of Broiler Chickens
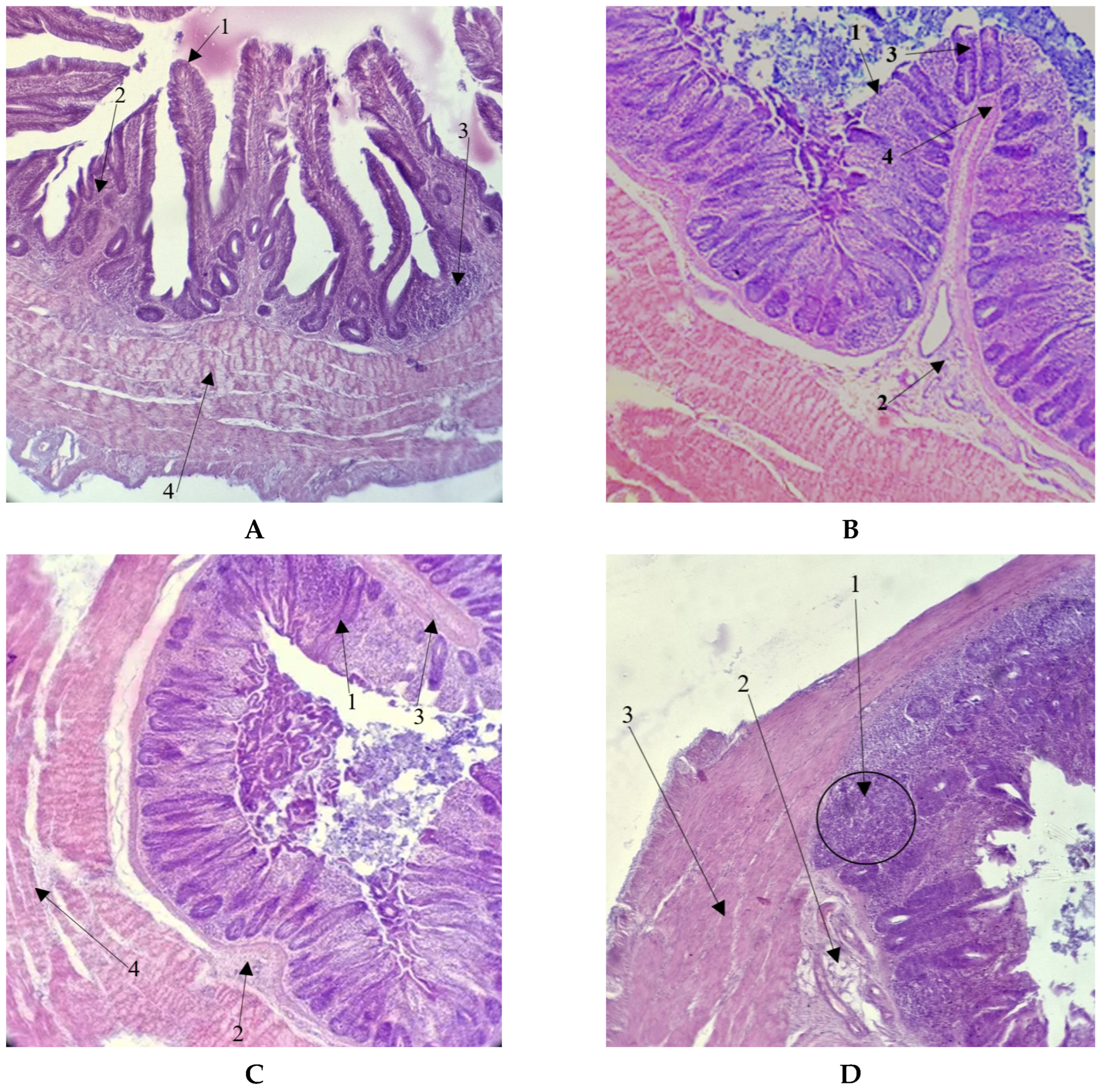
3.3. Morphological Parameters of Ceca
3.4. Growth and Meat Productivity of Broiler Chickens
4. Discussion
4.1. Expression of Immunity Genes in the Ceca of Broiler Chickens
4.2. Morphological Parameters of Ceca
4.3. Growth and Meat Productivity of Broiler Chickens
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, J.R.G.; Mehat, J.; Ragione, R.; Behboudi, S. Preventing bacterial disease in poultry in the post-antibiotic era: A case for innate immunity modulation as an alternative to antibiotic use. Front. Immunol. 2023, 14, 1205869. [Google Scholar] [CrossRef]
- Jiang, A.; Liu, Z.; Lv, X.; Zhou, C.; Ran, T.; Tan, Z. Prospects and Challenges of Bacteriophage Substitution for Antibiotics in Livestock and Poultry Production. Biology 2024, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Khan, G.; Khan, F.; Javed, M.; Ali, H.; Ahmad, M. Use of antibiotics and the farmers’ awareness level of antibiotics resistance of broiler farms. Biol. Clin. Sci. Res. J. 2024, 1, 856. [Google Scholar] [CrossRef]
- Balenović, M.; Janječić, Z.; Savić, V.; Kasap, A.; Popović, M.; Šimpraga, B.; Sokolović, M.; Bedeković, D.; Kiš, G.; Zglavnik, T.; et al. Immunostimulatory and Antibacterial Effects of Cannabis sativa L. Leaves on Broilers. Animals 2024, 14, 1159. [Google Scholar] [CrossRef]
- Deminicis, R.G.; Meneghetti, C.; de Oliveira, E.B.; Garcia Junior, A.A.P.; Farias Filho, R.V.; Deminicis, B.B. Systematic review of the use of phytobiotics in broiler nutrition. Rev. Ciências Agrovet. 2021, 20, 98–106. [Google Scholar]
- Gilani, S.M.H.; Rashid, Z.; Galani, S.; Ilyas, S.; Sahar, S.; Zahoor-Ul-Hassan; Al-Ghanim, K.; Zehra, S.; Azhar, A.; Al-Misned, F.; et al. Growth performance, intestinal histomorphology, gut microflora and ghrelin gene expression analysis of broiler by supplementing natural growth promoters: A nutrigenomics approach. Saudi J. Biol. Sci. 2021, 28, 3438–3447. [Google Scholar] [CrossRef]
- Pandey, S.; Kim, E.S.; Cho, J.H.; Song, M.; Doo, H.; Kim, S.; Keum, G.B.; Kwak, J.; Ryu, S.; Choi, Y.; et al. Cutting-edge knowledge on the roles of phytobiotics and their proposed modes of action in swine. Front. Vet. Sci. 2023, 10, 1265689. [Google Scholar] [CrossRef]
- Balenović, M.; Savić, V.; Janječić, Z.; Popović, M.; Šimpraga, B.; Carović-Stanko, K.; Bedeković, D.; Zelenika, T.A. Immunomodulatory and antimicrobial effects of selected herbs on laying hens. Vet. Arch. 2018, 88, 673–686. [Google Scholar] [CrossRef]
- Moharreria, M.; Vakilia, R.; Oskoueianb, E.; Rajabzadehc, G. Phytobiotic role of essential oil-loaded microcapsules in improving the health parameters in Clostridium perfringens-infected broiler chickens. Ital. J. Anim. Sci. 2021, 20, 2075–2085. [Google Scholar] [CrossRef]
- Ndomou, S.C.H.; Mube, H.K. The Use of Plants as Phytobiotics: A New Challenge. In Phytochemicals in Agriculture and Food; Soto-Hernández, M., Aguirre-Hernández, E., Palma-Tenango, M., Surguchov, A., Eds.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Haq, Z.; Saleem, A.; Khan, A.A.; Dar, M.A.; Ganaie, A.M.; Beigh, Y.A.; Hamadani, H.; Ahmad, S.M. Nutrigenomics in livestock sector and its human-animal interface-a review. Vet. Anim. Sci. 2022, 17, 100262. [Google Scholar] [CrossRef]
- Hafez, M.H.; El-Kazaz, S.E.; Alharthi, B.; Ghamry, H.I.; Alshehri, M.A.; Sayed, S.; Shukry, M.; El-Sayed, Y.S. The Impact of Curcumin on Growth Performance, Growth-Related Gene Expression, Oxidative Stress, and Immunological Biomarkers in Broiler Chickens at Different Stocking Densities. Animals 2022, 12, 958. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Moustafa, A.; Metwally, A.S.; Nassan, M.A.; Abdallah, K.; Eldemery, F.; Tufarelli, V.; Laudadio, V.; Kishawy, A.T.Y. Potential Application of Cornelian Cherry Extract on Broiler Chickens: Growth, Expression of Antioxidant Biomarker and Glucose Transport Genes, and Oxidative Stability of Frozen Meat. Animals 2021, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Kishawy, A.T.Y.; Al-Khalaifah, H.S.; Nada, H.S.; Roushdy, E.M.; Zaglool, A.W.; Ismail, T.A.; Ibrahim, S.M.; Ibrahim, D. Black Pepper or Radish Seed Oils in a New Combination of Essential Oils Modulated Broiler Chickens’ Performance and Expression of Digestive Enzymes, Lipogenesis, Immunity, and Autophagy-Related Genes. Vet. Sci. 2022, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Ghavami, N.; Nosrati, M.; Rasouli, B.; Kadim, I.T.; Ramírez, L.S.; Gorlov, I.; Slozhenkina, M.; Mosolov, A.; Seidavi, A.; et al. The effects of peppermint (Mentha piperita L.) and chicory (Cichorium intybus L.) in comparison with a prebiotic on productive performance, blood constituents, immunity and intestinal microflora in broiler chickens. Anim. Biotechnol. 2022, 34, 3046–3052. [Google Scholar] [CrossRef]
- Shang, R.; He, C.; Chen, J.; Pu, X.; Liu, Y.; Hua, L.; Wang, L.; Liang, J. Hypericum perforatum extract therapy for chickens experimentally infected with infectious bursal disease virus and its influence on immunity. Can. J. Vet. Res. 2012, 76, 180–185. [Google Scholar]
- Erimbetov, K.T.; Obvintseva, O.V.; Solov’eva, A.G.; Mikhailov, V.V. Effects of low protein diets enriched with essential aminoacids and Leuzea extract in the growing pigs. Probl. Product. Anim. Biol. 2019, 4, 73–80. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Shahir, M.H.; Kheiry, A. Effects of thyme, garlic, echinacea and galbanum on performance, cecal microbiota and immune function of native ducks. J. Anim. Sci. 2022, 2, 119–130. [Google Scholar] [CrossRef]
- Efimov, D.N.; Egorova, A.V.; Emanuylova, J.V.; Ivanov, A.V.; Konopleva, A.P.; Zotov, A.A.; Lukashenko, V.S.; Komarov, A.A.; Egorov, I.A.; Egorova, T.A.; et al. Manual on Work with Poultry of Meat Cross ‘Smena-9’ with Autosex Maternal Parental Form: (Breeding Work; Egg, Incubation; Technology of Growing, Housing; Feeding; Health and Biosecurity); VNITIP: Sergiev Posad, Russia, 2021. [Google Scholar]
- Laptev, G.Y.; Filippova, V.A.; Kochish, I.I.; Yildirim, E.A.; Ilina, L.A.; Dubrovin, A.V.; Brazhnik, E.A.; Novikova, N.I.; Novikova, O.B.; Dmitrieva, M.E.; et al. Examination of the expression of immunity genes and bacterial profiles in the caecum of growing chickens infected with Salmonella enteritidis and fed a phytobiotic. Animals 2019, 9, 615. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Laptev, G.Y.; Yildirim, E.A.; Ilyina, L.A.; Filippova, V.A.; Kalitkina, K.A.; Ponomareva, E.S.; Dubrovin, A.V.; Tyurina, D.G.; Fisinin, V.I.; Egorov, I.A.; et al. Expression of genes of immune response and adaptation and cecal microbiome composition in males and females of chickens (Gallus gallus L.) in CM5 and CM9 preparental lines of Smena 9 cross. Agric. Biol. 2023, 58, 313–332. [Google Scholar] [CrossRef]
- Majeed, M.F.; Al-Asadi, F.S.; Nassir, A.A.; Rahi, E.H. The morphological and histological study of ِthe caecum in broiler chicken. Bas. J. Vet. Res. 2009, 8, 19–25. [Google Scholar] [CrossRef]
- Tyurina, D.G.; Laptev, G.Y.; Yildirim, E.A.; Ilina, L.A.; Filippova, V.A.; Brazhnik, E.A.; Tarlavin, N.V.; Gorfunkel, E.P.; Dubrovin, A.V.; Novikova, N.I.; et al. The impact of virginiamicin and probiotics on intestinal microbiome and growth performance traits of chicken (Gallus gallus L.) broilers. Agric. Biol. 2020, 55, 1220–1232. [Google Scholar] [CrossRef]
- Laptev, G.Y.; Turina, D.G.; Morozov, V.Y.; Yildirim, E.A.; Gorfunkel, E.P.; Ilina, L.A.; Filippova, V.A.; Brazhnik, E.A.; Novikova, N.I.; Melikidi, V.K.; et al. Changes in Expression of Key Genes in Ceca of Chicken Broilers as Affected by Glyphosate, Antibiotics and a Coccidiostat. Animals 2024, 14, 3544. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, X.; Li, H.; Su, P.; Lin, L.; Liu, L.; Li, X. The correlated expression of immune and energy metabolism related genes in the response to Salmonella enterica serovar Enteritidis inoculation in chicken. BMC Vet. Res. 2020, 16, 257. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef]
- Sugihara, K.; Morhardt, T.L.; Kamada, N. The Role of Dietary Nutrients in Inflammatory Bowel Disease. Front. Immunol. 2019, 9, 3183. [Google Scholar] [CrossRef]
- Saleh, H.; Mirakzehi, M.T.; Agah, M.J.; Baranzehi, T.; Saleh, H. The Effects of Saccharomyces Cerevisiae and Citric Acid on Productive Performance, Egg Quality Parameters, Small Intestinal Morphology, and Immune-Related Gene Expression in Laying Japanese Quails. Braz. J. Poult. Sci. 2022, 24, eRBCA-2022. [Google Scholar] [CrossRef]
- Shimizu, M.; Nii, T.; Isobe, N.; Yoshimura, Y. Effects of avian infectious bronchitis with Newcastle disease and Marek’s disease vaccinations on the expression of toll-like receptors and avian β-defensins in the kidneys of broiler chicks. Poult. Sci. 2020, 99, 7092–7100. [Google Scholar] [CrossRef]
- Yang, Q.; Fong, L.A.; Lyu, W.; Sunkara, L.T.; Xiao, K.; Zhang, G. Synergistic Induction of Chicken Antimicrobial Host Defense Peptide Gene Expression by Butyrate and Sugars. Front. Microbiol. 2021, 12, 781649. [Google Scholar] [CrossRef]
- Redondo, E.A.; Redondo, L.M.; Bruzzone, O.A.; Diaz-Carrasco, J.M.; Cabral, C.; Garces, V.; Liñeiro, M.; Fernandez-Miyakawa, M.E. Tannins and gut health in broilers: Effects of a blend of chestnut and quebracho tannins on gut health and performance of broiler chickens. PLoS ONE 2022, 17, e0254679. [Google Scholar] [CrossRef]
- Li, X.; Sun, R.; Liu, Q.; Gong, Y.; Ou, Y.; Qi, Q.; Xie, Y.; Wang, X.; Hu, C.; Jiang, S.; et al. Effects of dietary supplementation with dandelion tannins or soybean isoflavones on growth performance, antioxidant function, intestinal morphology, and microbiota composition in Wenchang chickens. Front. Vet. Sci. 2023, 9, 1073659. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, K.; Lin, S.; Zhang, Z.; Cheng, M.; Hu, S.; Hu, H.; Xiang, J.; Chen, F.; Li, G.; et al. Comparison of the Effects between Tannins Extracted from Different Natural Plants on Growth Performance, Antioxidant Capacity, Immunity, and Intestinal Flora of Broiler Chickens. Antioxidants 2023, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-H.; Song, W.; Lee, S.H.; Lillehoj, H. Differential gene expression profiles of β-defensins in the crop, intestine, and spleen using a necrotic enteritis model in 2 commercial broiler chicken lines. Poultr. Sci. 2012, 91, 1081–1088. [Google Scholar] [CrossRef]
- Yildirim, E.A.; Grozina, A.A.; Vertiprakhov, V.G.; Ilyina, L.A.; Filippova, V.A.; Laptev, G.Y.; Brazhnik, E.A.; Kalitkina, K.A.; Tarlavin, N.V.; Dubrovin, A.V.; et al. Effect of T-2 toxin on expression of genes associated with immunity in tissues of the blind processes of the intestinal and pancreas of broilers (Gallus gallus L.). Agric. Biol. 2021, 56, 664–681. [Google Scholar] [CrossRef]
- Verma, H.; Chandra, G.; Jaiswal, V.; Maurya, P.S.; Sahu, S. A comprehensive report on poultry intestinal microbiota. J. Entomol. Zool. Stud. 2020, 8, 192–197. [Google Scholar]
- Zhang, J.; Shen, Y.; Yang, G.; Sun, J.; Tang, C.; Liang, H.; Ma, J.; Wu, X.; Cao, H.; Wu, M.; et al. Commensal microbiota modulates phenotypic characteristics and gene expression in piglet Peyer’s patches. Front. Physiol. 2023, 14, 1084332. [Google Scholar] [CrossRef]
- Patel, R.P.; Shah, P.; Barve, K.; Patel, N.; Gandhi, J. Peyer’s Patch: Targeted Drug Delivery for Therapeutics Benefits. In Novel Drug Delivery Technologies. Innovative Strategies for Drug Repositioning; Misra, A., Shahiwala, A., Eds.; Springer: Singapore, 2019; pp. 121–149. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Yang, H.-S.; Ra, K.S.; Park, S.S.; Yu, K.-W. Intestinal immune system-modulating activity through peyer’s patch of flavonoid glycoside purified from citrus unshiu peel. J. Food Biochem. 2013, 37, 151–160. [Google Scholar] [CrossRef]
- Jha, R.; Mishra, P. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: A review. J. Anim. Sci. Biotechnol. 2021, 12, 51. [Google Scholar] [CrossRef]
- Dokou, S.; Mellidou, I.; Savvidou, S.; Stylianaki, I.; Panteli, N.; Antonopoulou, E.; Wang, J.; Grigoriadou, K.; Tzora, A.; Jin, L.; et al. A phytobiotic extract, in an aqueous or in a cyclodextrin encapsulated form, added in diet affects meat oxidation, cellular responses and intestinal morphometry and microbiota of broilers. Front. Anim. Sci. 2023, 4, 1050170. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, R.; Ma, X.; Wu, W.; Huang, Q.; Ye, W.; Wu, C.; Yao, B.; Xu, J.; Qian, L. A Multi-Enzyme Complex That Mitigates Hepatotoxicity, Improves Egg Production and Quality, and Enhances Gut and Liver Health in Laying Hens Exposed to Trace Aflatoxin B1. Toxins 2024, 16, 517. [Google Scholar] [CrossRef]
- Wall, D.C.; Malheiros, R.D.; Anderson, K.; Anthony, N. Comparative Intestinal Histological Features Observed in 1940 Leghorn vs. 2016 Leghorn-Based Commercial Laying Hens Fed Representative Diets. Int. J. Plant Anim. Environ. Sci. 2023, 13, 116–125. [Google Scholar] [CrossRef]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Ahmad, I.; Naz, S.; Alonaizan, R.; Al-akeel, R.K.; Khan, R.U.; Tufarelli, V. Effect of Lemon (Citrus limon, L.) Peel Powder on Oocyst Shedding, Intestinal Health, and Performance of Broilers Exposed to E. tenella Challenge. Animals 2023, 13, 3533. [Google Scholar] [CrossRef] [PubMed]
- Al-Garadi, M.A.; Al-Baadani, H.H.; Alqhtani, A.H. Growth Performance, Histological Changes and Functional Tests of Broiler Chickens Fed Diets Supplemented with Tribulus Terrestris Powder. Animals 2022, 12, 1930. [Google Scholar] [CrossRef]
- Oretomiloye, F.; Adewole, D. Exploring the modulatory effects of brown seaweed meal and extracts on intestinal microbiota and morphology of broiler chickens challenged with heat stress. Poult. Sci. 2024, 103, 103562. [Google Scholar] [CrossRef]
- Doneria, R.; Dubey, M.; Gendley, M.K.; Chourasia, D.; Pathak, R.; Ramteke, R.C.; Prusty, S.; Parmar, M.S. Impact of dietary supplementation of cinnamon oil on the oxidative stress indices, immune response and intestinal morphology in broiler chickens. Indian J. Anim. Sci. 2022, 92, 991–994. [Google Scholar] [CrossRef]
- Song, J.; Li, Q.; Everaert, N.; Liu, R.; Zheng, M.; Zhao, G.; Wen, J. Dietary Inulin Supplementation Modulates Short-Chain Fatty Acid Levels and Cecum Microbiota Composition and Function in Chickens Infected with Salmonella. Front. Microbiol. 2020, 11, 584380. [Google Scholar] [CrossRef]
- Xia, Y.; Kong, J.; Zhang, G.; Zhang, X.; Seviour, R.; Kong, Y. Effects of dietary inulin supplementation on the composition and dynamics of cecal microbiota and growth-related parameters in broiler chickens. Poult. Sci. 2019, 98, 6942–6953. [Google Scholar] [CrossRef]
- Shu, G.; Kong, F.; Xu, D.; Yin, L.; He, C.; Lin, J.; Fu, H.; Wang, K.; Tian, Y.; Zhao, X. Bamboo leaf flavone changed the community of cecum microbiota and improved the immune function in broilers. Sci. Rep. 2020, 10, 12324. [Google Scholar] [CrossRef]
- Gao, Y.-Y.; Liu, X.-P.; Zhou, Y.-H.; He, J.-Y.; Di, B.; Zheng, X.-Y.; Guo, P.-T.; Zhang, J.; Wang, C.-K.; Jin, L. The Addition of Hot Water Extract of Juncao-Substrate Ganoderma lucidum Residue to Diets Enhances Growth Performance, Immune Function, and Intestinal Health in Broilers. Animals 2024, 14, 2926. [Google Scholar] [CrossRef]
- Naji, T.A.A.; Amadou, I.; Zhao, R.Y.; Tang, X.; Shi, Y.-H.; Le, G.-W. Effects of Phytosterol in Feed on Growth and Related Gene Expression in Muscles of Broiler Chickens. Trop. J. Pharm. Res. 2014, 13, 9–16. [Google Scholar] [CrossRef]
- Bagno, O.A.; Prokhorov, O.N.; Shevchenko, S.A.; Shevchenko, A.I.; Dyadichkina, T.V. Use of phytobioticts in farm animal feeding (review). Agric. Biol. 2018, 53, 687–697. [Google Scholar] [CrossRef]
- Kishnyaykina, E.A.; Zhuchaev, K.V.; Bagno, O.A.; Prokhorovt, O.H.; Ulrich, E.V.; Izhmulkina, E.A. Slaughter Qualities and Chemical Composition of the Meat of the Broiler Chickens Fed with Thyme Extract. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 4113–4117. [Google Scholar] [CrossRef]
- Gebru, G.; Belay, G.; Dessie, T.; Kelkay, M.Z.; Dagnhegn, M.B.; Hanotte, O. Morphological and osteological characterization of indigenous domestic chickens (Gallus gallus domesticus): Validation of Rensch’s, Bergmann’s and Allen’s rules. Front. Ecol. Evol. 2023, 11, 1032082. [Google Scholar] [CrossRef]
| Groups | Number of Animals in Each Group (n) | Broiler Chicken Feeding Program |
|---|---|---|
| CON | 36 | Basic diet (BD) for broiler chickens: Starter, Grower, and Finisher feeds without phytochemicals. |
| INUL | 36 | BD + INUL: 405.0 g/t in Starter/Grower feeds, 504.0 g/t in Finisher feed |
| FLAV | 36 | BD + FLAV: 22.6 g/t in Starter/Grower feeds, 31.4 g/t in Finisher feed |
| ECDS | 36 | BD + ECDS: 0.9 g/t in Starter/Grower feeds, 1.1 g/t in Finisher feed |
| FLAV + TANN | 36 | BD + FLAV: 36.8 g/t in Starter/Grower feeds, 46.0 g/t in Finisher feed + TANN 7.2 g/t in Starter/Grower feeds, 9.0 g/t Finisher feed |
| Nutrients | Age of Poultry (Days) | ||
|---|---|---|---|
| 0–10 (Starter) | 11–22 (Grower) | 23–35 (Finisher) | |
| Nutritional Value (%) | |||
| Metabolic energy (ME) (kcal/100 g) | 305.00 | 299.00 | 303.00 |
| Crude protein | 23.00 | 21.50 | 18.50 |
| Assimilable lysine | 1.44 | 1.33 | 1.20 |
| Assimilable methionine | 0.67 | 0.67 | 0.63 |
| Assimilable methionine + cystine | 1.04 | 1.03 | 0.94 |
| Assimilable threonine | 0.97 | 0.91 | 0.81 |
| Assimilable tryptophan | 0.26 | 0.22 | 0.21 |
| Crude fiber | 3.69 | 4.75 | 5.00 |
| Essential extract | 3.73 | 5.38 | 5.50 |
| Linoleic acid | 1.74 | 2.63 | 2.81 |
| Calcium | 1.10 | 0.87 | 0.78 |
| Total phosphorus | 0.69 | 0.67 | 0.61 |
| Assimilable phosphorus | 0.58 | 0.42 | 0.51 |
| Sodium | 0.17 | 0.17 | 0.18 |
| Chlorine | 0.17 | 0.19 | 0.20 |
| Vitamin B4 (mg/kg) | 1568.00 | 1568.00 | 1540.00 |
| Gene | Primers | Author |
|---|---|---|
| ACTB (β-actin) | F: CTGTGCCCATCTATGAAGGCTA R: ATTTCTCTCTCGGCTGTGGTG | Laptev G.Yu. et al., 2023 [22] |
| IL8L2 (interleukin 8-like 2) | F: GGAAGAGAGGTGTGCTTGGA R: TAACATGAGGCACCGATGTG | |
| AvBD9 (β-defensin 9) | F: AACACCGTCAGGCATCTTCACA R: CGTCTTCTTGGCTGTAAGCTGGA | |
| AvBD10 (β-defensin 10) | F: GCTCTTCGCTGTTCTCCTCT R: CCAGAGATGGTGAAGGTG | |
| IRF7 (interferon regulatory factor 7) | F: ATCCCTTGGAAGCACAACGCC R: CTGAGGCAACCGCGTAGACCTT |
| Parameters | Group | p-Value | ||||
|---|---|---|---|---|---|---|
| CON | INUL | FLAV | ECDS | FLAV + TANN | ||
| Epithelium height | 40.42 ± 0.360 a | 15.64 ± 0.396 b | 16.15 ± 0.445 b | 20.92 ± 0.395 c | 13.08 ± 0.193 d | 0.000 |
| Crypt depth | 443.58 ± 5.931 a | 273.38 ± 3.754 b | 300.39 ± 4.238 c | 217.98 ± 2.103 d | 206.62 ± 2.342 d | 0.000 |
| Thickness of muscularis mucosae | 17.51 ± 0.231 a | 18.66 ± 0.193 b | 18.75 ± 0.383 bc | 12.40 ± 0.241 d | 17.87 ± 0.323 ab | 0.000 |
| Thickness of submucosa | 47.64 ± 0.818 a | 40.80 ± 0.465 b | 51.11 ± 0.998 a | 47.75 ± 1.005 a | 113.29 ± 3.410 c | 0.000 |
| Thickness of muscular layer | 283.61 ± 3.377 a | 579.87 ± 5.567 b | 430.99 ± 4.819 c | 151.59 ± 2.845 d | 291.59 ± 3.032 a | 0.000 |
| Parameters | Group | p-Value | ||||
|---|---|---|---|---|---|---|
| CON | INUL | FLAV | ECDS | FLAV + TANN | ||
| Initial number of birds | 36 | 36 | 36 | 36 | 36 | - |
| Live weight at 35 days, g * | 2061.9 ± 51.62 | 2204.6 ± 50.38 | 2167.3 ± 55.63 | 2216.1 ± 48.91 | 2146.8 ± 44.50 | 0.233 |
| Average daily gain, g | 57.7 | 61.8 | 60.7 | 62.1 | 60.1 | - |
| Livability of birds, % | 91.7 | 100.0 | 100.0 | 97.2 | 97.2 | - |
| Feed consumption per 1 kg of gain, kg | 1.58 | 1.63 | 1.66 | 1.60 | 1.66 | - |
| EPEF, units | 341.9 | 386.4 | 373.0 | 384.7 | 359.2 | - |
| Parameters | Groups | p-Value | ||||
|---|---|---|---|---|---|---|
| CON | INUL | FLAV | ECDS | FLAV + TANN | ||
| Total sample | ||||||
| Number of individuals | 6 | 6 | 6 | 6 | 6 | - |
| Weight of gutted carcass, g | 1393.9 ± 50.05 | 1513.4 ± 61.78 | 1499.7 ± 43.10 | 1524.6 ± 55.71 | 1532.3 ± 66.63 | 0.417 |
| Slaughter yield, % | 70.2 ± 1.60 | 73.0 ± 0.65 | 72.3 ± 1.04 | 73.2 ± 1.33 | 73.4 ± 0.82 | 0.291 |
| Weight of breast muscles, g | 440.8 ± 5.43 | 482.7 ± 19.87 | 463.4 ± 21.36 | 491.8 ± 17.20 | 495.4 ± 26.39 | 0.270 |
| Breast muscle yield, % | 22.3 ± 0.88 | 23.3 ± 0.43 | 22.3 ± 0.45 | 23.6 ± 0.31 | 23.7 ± 0.52 | 0.208 |
| Fat weight, g | 25.4 ± 1.59 a | 38.9 ± 4.54 ab | 52.7 ± 5.44 b | 36.3 ± 2.88 a | 32.8 ± 2.52 a | 0.001 |
| Fat yield, % | 1.3 ± 0.12 a | 1.9 ± 0.18 ab | 2.6 ± 0.31 b | 1.7 ± 0.10 a | 1.6 ± 0.16 a | 0.001 |
| ♂ | ||||||
| Number of individuals | 3 | 3 | 3 | 3 | 3 | - |
| Weight of gutted carcass, g | 1491.2 ± 31.14 | 1645.3 ± 40.69 | 1586.8 ± 35.51 | 1628.8 ± 54.92 | 1674.9 ± 33.71 | 0.063 |
| Slaughter yield, % | 68.7 ± 1.24 a | 72.4 ± 1.34 ab | 70.3 ± 0.97 ab | 72.3 ± 1.75 ab | 74.8 ± 0.71 b | 0.052 |
| Weight of breast muscles, g | 446.3 ± 7.79 a | 521.8 ± 18.34 bc | 502.2 ± 17.71 ac | 527.0 ± 13.82 bc | 549.6 ± 18.54 bc | 0.009 |
| Breast muscle yield, % | 20.6 ± 0.59 a | 23.0 ± 0.70 ab | 22.2 ± 0.56 ab | 23.4 ± 0.45 ab | 24.5 ± 0.66 b | 0.052 |
| Fat weight, g | 23.0 ± 0.85 | 44.5 ± 7.95 | 49.1 ± 8.41 | 41.7 ± 1.61 | 29.8 ± 4.57 | 0.042 |
| Fat yield, % | 1.1 ± 0.05 | 2.0 ± 0.36 | 2.2 ± 0.36 | 1.9 ± 0.08 | 1.3 ± 0.22 | 0.054 |
| ♀ | ||||||
| Number of individuals | 3 | 3 | 3 | 3 | 3 | - |
| Weight of gutted carcass, g | 1296.7 ± 45.81 | 1381.5 ± 4.02 | 1412.6 ± 21.12 | 1420.4 ± 40.57 | 1389.6 ± 26.51 | 0.112 |
| Slaughter yield, % | 71.7 ± 3.00 | 73.5 ± 0.21 | 74.3 ± 0.73 | 74.2 ± 2.21 | 72.1 ± 0.95 | 0.767 |
| Weight of breast muscles, g | 435.2 ± 7.49 | 443.7 ± 10.50 | 424.6 ± 21.53 | 456.5 ± 6.77 | 441.3 ± 14.41 | 0.566 |
| Breast muscle yield, % | 24.1 ± 0.71 | 23.6 ± 0.58 | 22.3 ± 0.82 | 23.8 ± 0.48 | 22.9 ± 0.47 | 0.312 |
| Fat weight, g | 27.9 ± 2.43 a | 33.3 ± 2.93 a | 56.2 ± 8.04 b | 31.0 ± 3.16 a | 35.8 ± 1.27 a | 0.006 |
| Fat yield, % | 1.5 ± 0.12 a | 1.8 ± 0.15 a | 3.0 ± 0.46 b | 1.6 ± 0.19 a | 1.9 ± 0.09 ab | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selionova, M.I.; Trukhachev, V.I.; Zagarin, A.Y.; Kulikov, E.I.; Belyaeva, N.P. Effects of Dietary Supplementation Using Phytobiotics with Different Functional Properties on Expression of Immunity Genes, Intestinal Histology, Growth, and Meat Productivity of Broiler Chickens. Vet. Sci. 2025, 12, 302. https://doi.org/10.3390/vetsci12040302
Selionova MI, Trukhachev VI, Zagarin AY, Kulikov EI, Belyaeva NP. Effects of Dietary Supplementation Using Phytobiotics with Different Functional Properties on Expression of Immunity Genes, Intestinal Histology, Growth, and Meat Productivity of Broiler Chickens. Veterinary Sciences. 2025; 12(4):302. https://doi.org/10.3390/vetsci12040302
Chicago/Turabian StyleSelionova, Marina I., Vladimir I. Trukhachev, Artem Yu. Zagarin, Egor I. Kulikov, and Nina P. Belyaeva. 2025. "Effects of Dietary Supplementation Using Phytobiotics with Different Functional Properties on Expression of Immunity Genes, Intestinal Histology, Growth, and Meat Productivity of Broiler Chickens" Veterinary Sciences 12, no. 4: 302. https://doi.org/10.3390/vetsci12040302
APA StyleSelionova, M. I., Trukhachev, V. I., Zagarin, A. Y., Kulikov, E. I., & Belyaeva, N. P. (2025). Effects of Dietary Supplementation Using Phytobiotics with Different Functional Properties on Expression of Immunity Genes, Intestinal Histology, Growth, and Meat Productivity of Broiler Chickens. Veterinary Sciences, 12(4), 302. https://doi.org/10.3390/vetsci12040302







