Lead, Cadmium, and Arsenic in Edible Tissues of Guinea Pigs Raised in the Central Andes of Peru: Potential Human Health Risk?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Study Location
2.3. Animals and Breeding System
2.4. Sample Preparation and Metal and Metalloid Determination
2.4.1. Sampling of Guinea Pig Meat and Offal
2.4.2. Preparation of Biological Samples for Toxic Metal and Metalloid Analysis
2.4.3. Metal and Metalloid Determination in Guinea Pig Meat and Offal for Human Consumption
2.4.4. Precision and Accuracy
2.5. Maximum Limits of Pb, Cd, and As in Meat and Meat Products
2.6. Risk Assessment
- -
- Lead: 0.0035 mg/kg of body weight/day
- -
- Cadmium: 0.001 mg/kg of body weight/day
- -
- Arsenic: 0.003 mg/kg of body weight/day
2.7. Data Processing
3. Results
3.1. Edible Components of the Guinea Pig Carcass
3.2. Lead Levels in Meat, Kidneys, Lungs, Heart, and Liver of Guinea Pigs
3.3. Cadmium Levels in Meat, Kidneys, Lungs, Heart, and Liver of Guinea Pigs
3.4. Arsenic Levels in Meat, Kidneys, Lungs, Heart, and Liver of Guinea Pigs
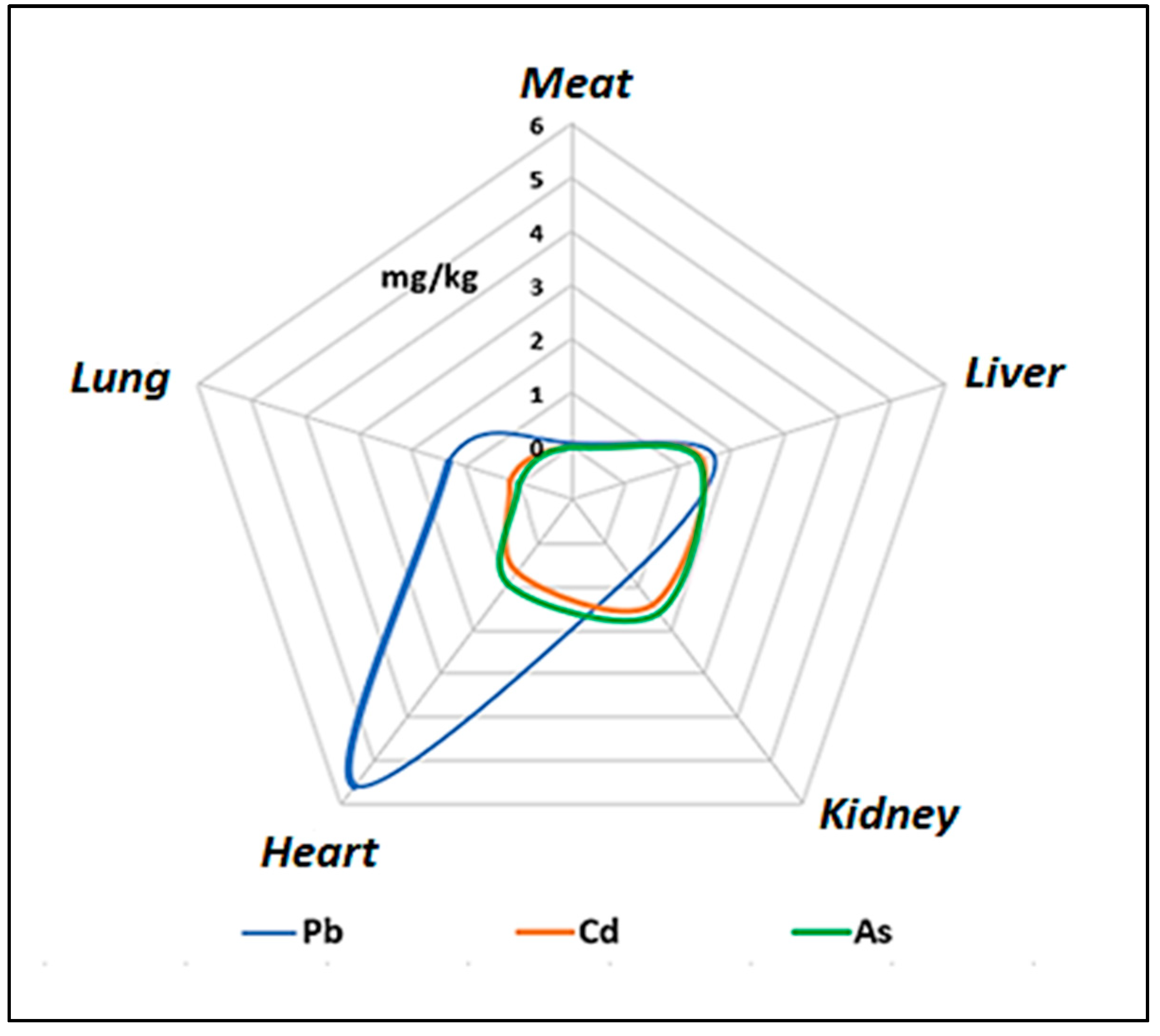
3.5. Comparison of Lead, Cadmium, and Arsenic Concentrations in Guinea Pig Meat and Offal
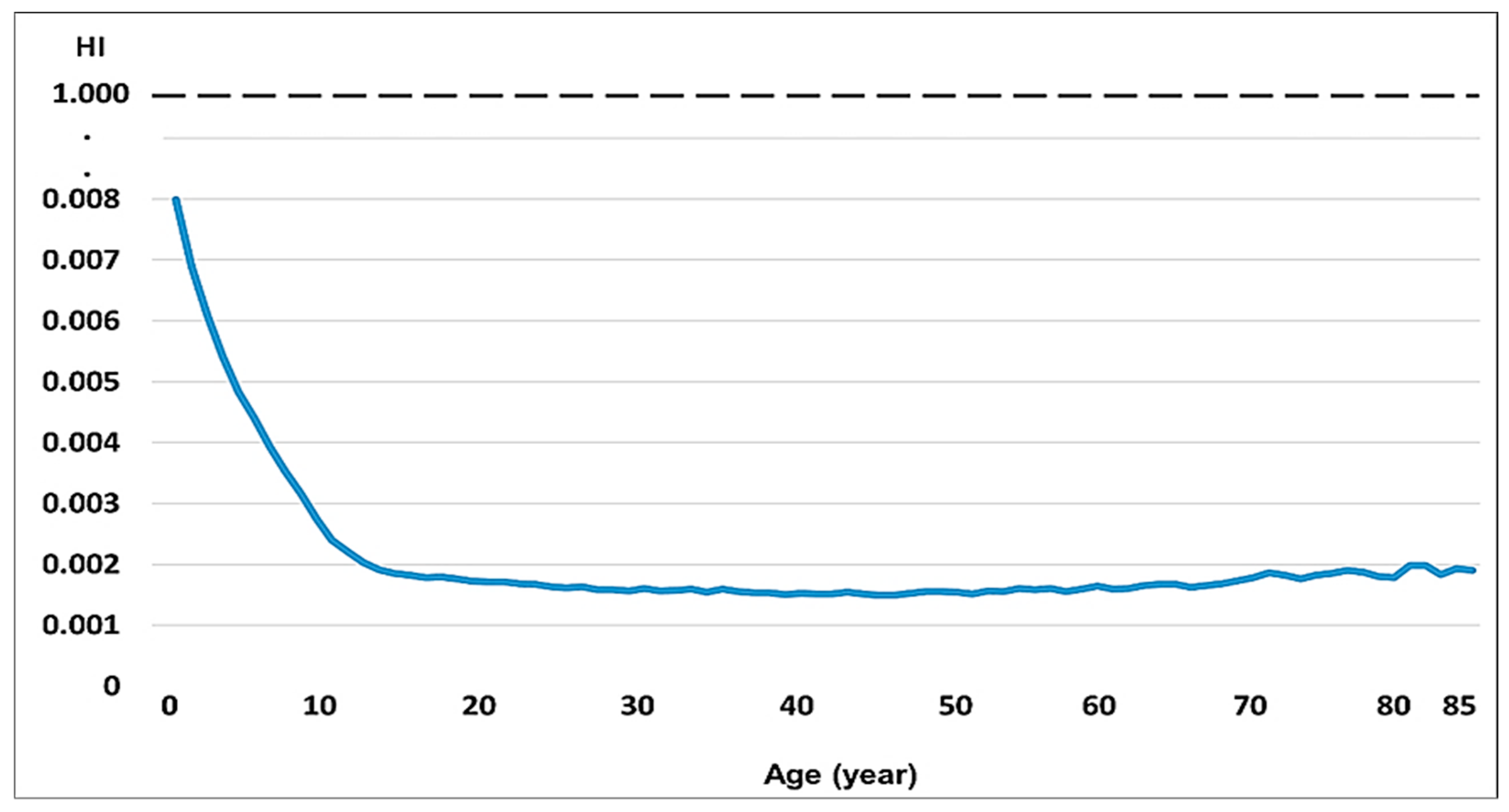
3.6. Potential Health Risks
4. Discussion
4.1. Edible Components of the Guinea Pig Carcass
4.2. Lead, Cadmium, and Arsenic Levels in Guinea Pig Meat, Liver, Kidney, Heart, and Lungs
4.3. Health Risk in the Peruvian Population Aged 3–85 Years Due to Consumption of Guinea Pig Meat
4.4. Implication of Pb, Cd, and As Intake on Health
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suneeta, C.; Prachi, D. Heavy Metal Content of Foods and Health Risk Assessment in the Study Population of Vadodara. Curr. World Environ. J. 2013, 8, 291–297. [Google Scholar] [CrossRef]
- Paredes, M.; Cerquín, M. Efectos de la suplementación de treonina sobre el rendimiento productivo, carcasa y pesos de órganos de cuyes de engorde con alimentación mixta por 72 días. Rev. Investig. Vet. Perú 2021, 32, e21701. [Google Scholar] [CrossRef]
- Castro-Bedriñana, J.; Chirinos-Peinado, D.; García-Olarte, E.; Quispe-Ramos, R.; Gordillo-Espinal, S. Lead transfer in the soil-root-plant system in a highly contaminated Andean area. PeerJ 2021, 9, e10624. [Google Scholar] [CrossRef] [PubMed]
- Chirinos-Peinado, D.; Castro-Bedriñana, J.; Ríos-Ríos, E.; Mamani-Gamarra, G.; Quijada-Caro, E.; Huacho-Jurado, A.; Nuñez-Rojas, W. Lead and Cadmium Bioaccumulation in Fresh Cow’s Milk in an Intermediate Area of the Central Andes of Peru and Risk to Human Health. Toxics 2022, 10, 317. [Google Scholar] [CrossRef]
- Chirinos-Peinado, D.; Castro-Bedriñana, J.; Ríos-Ríos, E.; Castro-Chirinos, G.; Quispe-Poma, Y. Lead, Cadmium, and Arsenic in Raw Milk Produced in the Vicinity of a Mini Mineral Concentrator in the Central Andes and Health Risk. Biol. Trace Elem. Res. 2024, 202, 2376–2390. [Google Scholar] [CrossRef]
- Ali, A.S.; Bayih, A.A.; Gari, S.R. Meta-analysis of public health risks of lead accumulation in wastewater, irrigated soil, and crops nexus. Front. Public Health 2022, 10, 3723. [Google Scholar] [CrossRef]
- Njoga, E.O.; Ezenduka, E.V.; Ogbodo, C.G.; Ogbonna, C.U.; Jaja, I.F.; Ofomatah, A.C.; Okpala, C.O.R. Detection, Distribution and Health Risk Assessment of Toxic Heavy Metals/Metalloids, Arsenic, Cadmium, and Lead in Goat Carcasses Processed for Human Consumption in South-Eastern Nigeria. Foods 2021, 10, 798. [Google Scholar] [CrossRef]
- Ullah, A.K.M.; Maksud, M.A.; Khan, S.R.; Lutfa, L.N.; Shamshad, B.Q. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 2017, 4, 574–579. [Google Scholar] [CrossRef]
- Muñoz, O.; Zamorano, P.; Garcia, O.; Bastias, J.M. Arsenic, cadmium, mercury, sodium, and potassium concentrations in common foods and estimated daily intake of the population in Valdivia (Chile) using a total diet study. Food Chem. Toxicol. 2017, 109 Pt 2, 1125–1134. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Raknuzzaman, M. The concentration, source and potential human health risk of heavy metals in the commonly consumed foods in Bangladesh. Ecotoxicol. Environ. Saf. 2015, 122, 462–469. [Google Scholar] [CrossRef]
- Pinchao-Pinchao, Y.; Serna-Cock, L.; Osorio-Mora, O.; Tirado, D.F. Guinea pig breeding and its relation to sustainable food security and sovereignty in South America: Nutrition, health, and production challenges. CyTA—J. Food 2024, 22, 2392886. [Google Scholar] [CrossRef]
- Sánchez-Macías, D.; Barba-Maggi, L.; Morales-DelaNuez, A.; Palmay-Paredes, J. Guinea pig for meat production: A systematic review of factors affecting the production, carcass and meat quality. Meat Sci. 2018, 143, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ngoula, F.; Guemdjo Tekam, M.; Kenfack, A.; Tadondjou Tchingo, C.; Nouboudem, S.; Tsafack, B.; Teguia, A. Effects of heat stress on some reproductive parameters of male cavie (Cavia porcellus) and mitigation strategies using guava (Psidium guajava) leaves essential oil. J. Therm. Biol. 2017, 64, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Spotorno, A.; Marin, J.; Manriquez, G.; Valladares, J.; Rico, E.; Rivas, C. Ancient and modern steps during the domestication of Guinea pigs (Cavia porcellus L.). J. Zool. 2006, 270, 57–62. [Google Scholar] [CrossRef]
- Irshad, M.; Malik, H.A.; Shaukat, S.; Mushtaq, S.; Ashraf, M. Characterization of heavy metals in livestock manures. Pol. J. Environ. Stud. 2013, 22, 1257–1262. [Google Scholar]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.W. Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- Khoshakhlagh, A.H.; Mohammadzadeh, M.; Gruszecka-Kosowska, A. The preventive and carcinogenic effect of metals on cancer: A systematic review. BMC Public Health 2024, 24, 2079. [Google Scholar] [CrossRef]
- Aderemi, T.A.; Adenuga, A.A.; Oyekunle, J.A.O.; Ogunfowokan, A.O. High level leaching of heavy metals from colorful ceramic foodwares: A potential risk to human. Environ. Sci. Pollut. Res. 2017, 24, 17116–17126. [Google Scholar] [CrossRef]
- Cui, J.; Wu, B.; Halbrook, R.S.; Zang, S. Age-dependent accumulation of heavy metals in liver, kidney and lung tis-sues of homing pigeons in Beijing, China. Ecotoxicology 2013, 22, 1490–1497. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef]
- Lukáčová, A.; Binkowski, Ł.; Golian, J. Comparision of mercury concentration in meat products of different origin. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 31–33. [Google Scholar]
- Damerau, A.; Venäläinen, E.R.; Peltonen, K. Heavy metals in meat of Finnish city rabbits. Food Addit. Contam. Part B Surveill. 2012, 5, 246–250. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils, Revision 2. Washington, DC. 1996. Available online: https://www.epa.gov/esam/epa-method-3050b-acid-digestion-sediments-sludges-and-soils (accessed on 2 June 2023).
- Thirumdas, R.; Janve, M.; Siliveru, K.; Kothakota, A. Chapter 10: Determination of food quality using atomic emission spectroscopy. In Evaluation Technologies for Food Quality; Zhong, J., Wang, X., Eds.; Woodhead Publishing: Kidlington, UK, 2019; pp. 175–192. [Google Scholar]
- Ramos, N.C.; Lamorena, R.B. Detection of Copper, Cadmium, Manganese, Lead, and Zinc Content in Milled Rice Using Microwave Plasma Atomic Emission Spectroscopy. Philipp. J. Sci. 2021, 150, 765–776. [Google Scholar] [CrossRef]
- USEPA. Method 200.7: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry. 1994. Revision 4.4. Cincinnati, OH. Available online: https://www.epa.gov/esam/method-2007-determination-metals-and-trace-elements-water-and-wastes-inductively-coupled (accessed on 2 June 2023).
- Alvira, M.L.; Rada-Mendoza, M.D.P.; Hoyos, S.O.; Villada, C.H. Cuantificación de arsénico por absorción atómica en termoformados y películas flexibles y biodegradables. Biotecnol. Sect. Agropecu. Agroind. 2012, 10, 157–165. [Google Scholar]
- USDA China Releases the Standard for Maximum Levels of Contaminants in Foods. National Food Safety Standard Maximum Levels of Contaminants in Foods. GAIN Report Number: CH2023-0040. 2023. Available online: https://fas.usda.gov/data/china-china-releases-standard-maximum-levels-contaminants-foods-0 (accessed on 12 July 2024).
- Zheng, S.; Wang, Q.; Yuan, Y.; Sun, W. Human health risk assessment of heavy metals in soil and food crops in the Pearl River Delta urban agglomeration of China. Food Chem. 2020, 316, 126213. [Google Scholar] [CrossRef]
- Shaheen, N.; Irfan, N.M.; Khan, I.N.; Islam, S.; Islam, M.S.; Ahmed, M.K. Presence of heavy metals in fruits and vegetables: Health risk implications in Bangladesh. Chemosphere 2016, 152, 431–438. [Google Scholar] [CrossRef]
- MIDAGRI Cadena Productiva de Cuy. Viceministerio de Políticas y Supervisión del Desarrollo Agrario Dirección General de Políticas Agrarias. Ministerio de Agricultura del Perú. 2023. Available online: https://cdn.www.gob.pe/uploads/document/file/4061856/Cadena%20productiva%20de%20cuy.pdf (accessed on 11 July 2024).
- CENAN-INEI Estado Nutricional en el Perú. Componente Nutricional ENAHO-CENAN-INS; Ministerio de Salud: Lima, Perú, 2011. Available online: https://bvs.minsa.gob.pe/local/MiNSA/1843.pdf (accessed on 3 June 2023).
- FAO; WHO. Foodborne Antimicrobial Resistance-Compendium of Codex Standards; Codex Alimentarius Commissio: Rome, Italy, 2023. [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar] [CrossRef]
- European Food Safety Authority. EFSA. Lead dietary exposure in the European population. EFSA J. 2012, 10, 2831–2859. [Google Scholar]
- Aditi Singh, A.; Kostova, I. Health effects of heavy metal contaminants Vis-à-Vis microbial response in their bioremediation. Inorg. Chim. Acta 2024, 568, 122068. [Google Scholar] [CrossRef]
- Rahmani, J.; Fakhri, Y.; Shahsavani, A.; Bahmani, Z.; Urbina, M.A.; Chirumbolo, S.; Keramati, H.; Moradi, B.; Bay, A.; Bjørklund, G. A systematic review and meta-analysis of metal concentrations in canned tuna fish in Iran and human health risk assessment. Food Chem. Toxicol. 2018, 118, 753–765. [Google Scholar] [CrossRef]
- Suren, B.; Bandara, K.M.; Towle, A.; Monnot, D. A human health risk assessment of heavy metal ingestion among consumers of protein powder supplements. Toxicol. Rep. 2020, 7, 1255–1262. [Google Scholar] [CrossRef]
- Riaz, R.M.Y.; Murtaza, G.; Farooqi, Z.U.R.; Ali, S.; Aziz, H.; Mahboob, S.; Al-Ghanim, K.A.; Owens, G.; Ahmad, H.R.; Riaz, U. Assessment of Arsenic Contamination in Groundwater and Associated Human Health Risk. Sustainability 2022, 14, 12460. [Google Scholar] [CrossRef]
- Khan, M.U.; Malik, R.N.; Muhammad, S.; Ullah, F.; Qadir, A. Health Risk Assessment of Consumption of Heavy Metals in Market Food Crops from Sialkot and Gujranwala Districts, Pakistan. Hum. Ecol. Risk Assess. Int. J. 2014, 21, 327–337. [Google Scholar] [CrossRef]
- Sultana, M.S.; Rana, S.; Yamazaki, S.; Aono, T.; Yoshida, S. Health risk assessment for carcinogenic and noncarcinogenic heavy metal exposures from vegetables and fruits of Bangladesh. Cogent Environ. Sci. 2017, 3, 1291107. [Google Scholar] [CrossRef]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef]
- Ayaz, H.; Nawaz, R.; Nasim, I.; Irshad, M.A.; Irfan, A.; Khurshid, I.; Okla, M.K.; Wondmie, G.F.; Ahmed, Z.; Bourhia, M. Comprehensive human health risk assessment of heavy metal contamination in urban soils: Insights from selected metropolitan zones. Front. Environ. Sci. 2023, 11, 1260317. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, A. Quantification of metal uptake in Spinacia oleracea irrigated with water containing a mixture of CuO and ZnO nanoparticles. Chemosphere 2020, 243, 125239. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Risk Assessment Guidance for Superfund: Volume III—Part A, Process for Conducting Probabilistic Risk Assessment EPA 540-R-02-002; USEPA: Washington, DC, USA, 2001.
- Chauca, L. Producción de Cuyes (Cavia porcellus). Cap.6 Comercialización de Productor. Mercadeo de Carcasas; Organización de las Naciones Unidas para la Agricultura y la Alimentación: Roma, Italy, 1997. Available online: https://www.fao.org/4/W6562s/w6562s06.htm (accessed on 12 July 2024).
- Stan, F.G.; Martonos, C.; Dezdrobitu, C.; Damina, A.; Gudea, A. Detailed Morphological Description of the Liver and Hepatic Ligaments in the Guinea Pig (Cavia porcellus). Sci. Works Ser. C Vet. Med. 2017, 63, 35–42. [Google Scholar]
- Paredes-Vilca, O.J.; Jimenes, D.L.; Dávila, G.J.; Apaza, C.J. Contaminación y pérdida de biodiversidad por actividades mineras y agropecuarias. estado del arte. Rev. Investig. Altoandinas 2024, 26, 56–66. [Google Scholar] [CrossRef]
- Goyer, R.A.; Clarkson, T.W. Toxic Effects of Metals. In Casarett and Doullis Toxicology: The Basic Science of Poisons, 6th ed.; McGraw-Hill: New York, NY, USA, 2001; pp. 811–867. [Google Scholar]
- Rudy, M. The analysis of correlations between the age and the level of bioaccumulation of heavy metals in tissues and the chemical composition of sheep meat from the region in SE Poland. Food Chem. Toxicol. 2009, 47, 1117–1122. [Google Scholar] [CrossRef]
- Thompson, L.J. Chapter 37. Lead. In Veterinary Toxicology: Basic and Clinical Principles, 2nd ed.; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 522–526. [Google Scholar]
- Skerfving, S.; Bergdahl, I. Chapter 31: Lead. In Handbook on the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 599–643. [Google Scholar]
- Pareja-Carrera, J.; Mateo, R.; Rodríguez-Estival, J. Lead (Pb) in sheep exposed to mining pollution: Implications for animal and human health. Ecotoxicol. Environ. Saf. 2014, 108, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Kia, S.A.; Aslani, R.; Khaniki, G.J.; Shariatifar, N.; Molaee-Aghaee, E. Determination and health risk assessment of heavy metals in chicken meat and edible giblets in Tehran, Iran. J. Trace Elem. Miner. 2024, 7, 100117. [Google Scholar] [CrossRef]
- JECFA. Codex General Standard for Contaminants and Toxins in Food and Feed. In Proceedings of the 64th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Rome, Italy, 8–17 February 2005. JECFA/64/CAC/RCP49-2001. [Google Scholar]
- FAO/WHO. Codex Alimentarius—General Standards for Contaminants and Toxins in Food. Schedule 1: Maximum and Guideline Levels for Contaminants and Toxins in Food; Joint FAO/WHO Food Standards Programme, Codex Committee: Rome, Italy, 2002.
- Salim, S.A.; Ov, N.S.; Dana, Z.; Hashami, Z.; Afrah, A.; Sadeghi, E.; Bashiry, M. A comprehensive image of environmental toxic heavy metals in red meat: A global systematic review and meta-analysis and risk assessment study. Sci. Total Environ. 2023, 889, 164100. [Google Scholar] [CrossRef] [PubMed]
- Agnieszka, A.; Barszcz, S.M. Impacts of water management in aquaculture on heavy metal accumulation in rainbow trout muscles and associated health risks from consumption. Food Control 2024, 164, 110617. [Google Scholar] [CrossRef]
- López-Alonso, M.; Miranda, M.; Castillo, C.; Hernández, J.; García-Vaquero, M.; Benedito, J.L. Metales tóxicos y esenciales en hígado, riñón y músculo de cerdos en matadero en Galicia, noroeste de España. Food Addit. Contam. 2007, 24, 943–954. [Google Scholar] [CrossRef]
- Restrepo, D.A.; López, J.H.; Berdugo, J.A.; Gallo-Ortiz, A.; Duarte-Correa, Y. Residues of veterinary drugs and heavy metals in bovine meat from Urabá (Antioquia, Colombia), a promising step forward towards international commercialization. Vet. Anim. Sci. 2021, 13, 100192. [Google Scholar] [CrossRef]
- EU. Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, L15, 1–72. [Google Scholar]
- Al-Zuhairi, W.A.; Farham, M.A.; Ahemd, M.A. Determine of Heavy Metals in the Heart, Kidney and Meat of Beef, Mutton, and Chicken from Baquba and Howaydir Market in Baquba, Diyala Province, Irak. Int. J. Recent Sci. Res. 2015, 6, 5965–5967. [Google Scholar] [CrossRef]
- FAO/WHO. Report of the 32nd Session of the Codex Committee of the Food Additives Contaminants; FAO/WHO: Beijing, China, 2000.
- Hashemi, M. Heavy metal concentrations in bovine tissues (muscle, liver and kidney) and their relationship with heavy metal contents in consumed feed. Ecotoxicol. Environ. Saf. 2018, 154, 263–267. [Google Scholar] [CrossRef]
- USEPA. USEPA Region III Risk-Based Concentration Table: Technical Background Information; United States Environmental Protection Agency: Washington, DC, USA, 2006.
- JECFA. Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants. 73 Report, 2010, Geneva, Switzerland; WHO Technical Report Series, no. 960. 2011; 237p. Available online: https://iris.who.int/bitstream/handle/10665/44515/WHO_TRS_960_eng.pdf (accessed on 24 October 2023).
- Castro-Bedriñana, J.; Chirinos-Peinado, D.; Ríos-Ríos, E.; Castro-Chirinos, G.; Chagua-Rodríguez, P.; De La Cruz-Calderón, G. Lead, Cadmium, and Arsenic in Raw Cow’s Milk in a Central Andean Area and Risks for the Peruvian Populations. Toxics 2023, 11, 809. [Google Scholar] [CrossRef]
- Castro-Gonzalez, N.P.; Moreno-Rojas, R.; Calderón-Sánchez, F.; Moreno, O.A.; Meneses, J.M. Assessment risk to children’s health due to consumption of cow’s milk in polluted areas in Puebla and Tlaxcala, Mexico. Food Addit. Contam. Part B 2017, 10, 200–207. [Google Scholar] [CrossRef]
- Castro-González, N.P.; Calderón-Sánchez, F.; Pérez-Sato, M.; Soní-Guillermo, E.; Reyes-Cervantes, E. Health risk due to chronic heavy metal consumption via cow’s milk produced in Puebla, Mexico, in irrigated wastewater areas. Food Addit. Contam. Part B Surveill 2019, 12, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Chirinos-Peinado, D.; Castro-Bedrinana, J.; Barnes, E.P.G.; Ríos-Ríos, E.; García-Olarte, E.; Castro-Chirinos, G. Assessing the Health Risk and Trophic Transfer of Lead and Cadmium in Dairy Farming Systems in the Mantaro Catchment, Central Andes of Peru. Toxics 2024, 12, 308. [Google Scholar] [CrossRef]
- Castro-Bedriñana, J.; Chirinos-Peinado, D.; Ríos-Ríos, E.; Machuca-Campuzano, M.; Gómez-Ventura, E. Dietary risk of milk contaminated with lead and cadmium in areas near mining-metallurgical industries in the Central Andes of Peru. Ecotoxicol. Environ. Saf. 2021, 220, 112382. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Liu, H.; Qu, X.; Zhou, X.; Gao, Y.; Yang, H.; Zheng, N.; Wang, J. Heavy Metals in Raw Milk and Dietary Exposure Assessment in the Vicinity of Leather-Processing Plants. Biol. Trace Elem. Res. 2021, 199, 3303–3311. [Google Scholar] [CrossRef]
- ATSDR. The ATSDR 2022 Substance Priority List; ATSDR: Atlanta, GA, USA, 2024. Available online: https://www.atsdr.cdc.gov/programs/substance-priority-list.html (accessed on 6 February 2025).
- Ramirez, O.D.; Gonzalez, E.D.F.; Blanco, A.T.; Pineda, B.; Gomez, M.S.; Quino, M.J.; Carrillo, M.P.; Perez de la Cruz, V. Cognitive impairment induced by lead exposure during lifespan: Mechanisms of lead neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef]
- Zaw, Y.H.; Taneepanichskul, N. Blood heavy metals and brain-derived neurotrophic factor in the first trimester of pregnancy among migrant workers. PLoS ONE 2019, 14, e0218409. [Google Scholar] [CrossRef]
- Astete, J.; Gastanaga, M.C.; Fiestas, V.; Oblitas, T.; Sabastizagal, I.; Lucero, M.; Abadie, J.M.; Munoz, M.E.; Valverde, A.; Suarez, M. Comunicable diseases, mental health and exposure to environmental pollutants in population living near Las Bambas mining project before exploitation phase, Peru 2006. Rev. Peru Med. Exp. Salud Publica 2010, 27, 512–519. [Google Scholar]
- Ayuso-Alvarez, A.; Simon, L.; Nunez, O.; Rodriguez-Blazquez, C.; Martin-Mendez, I.; Bel-Lan, A. Association between heavy metals and metalloids in topsoil and mental health in the adult population of Spain. Environ. Res. 2019, 179, 108784. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Andreazza, A.C.; Pasco, J.A.; Dodd, S.; Jacka, F.N. Heavy metal and the blues: Secondary analysis of persistent organic pollutants (POP), heavy metals and depressive symptoms in the NHANES National Epidemiological Survey. BMJ Open 2014, 4, e005142. [Google Scholar] [CrossRef]
- Jurczak, A.; Brodowska, A.; Szkup, M.; Prokopowicz, A.; Karakiewicz, B.; Łój, B.; Kotwas, A.; Brodowska, A.; Grochans, E. Influence of Pb and Cd levels in whole blood of postmenopausal women on the incidence of anxiety and depressive symptoms. Ann. Agric. Environ. Med. 2018, 25, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Lopes de Andrade, V.; Marreilha Dos Santos, A.P.; Aschner, M. Neurotoxicity of metal mixtures. Adv. Neurotoxicol. 2021, 5, 329–364. [Google Scholar] [CrossRef] [PubMed]
- Weider, S.; Laerum, A.M.W.; Evensen, K.A.I.; Reitan, S.K.; Lydersen, S.; Brubakk, A.M.; Indredavik, M.S. Neurocognitive function and associations with mental health in adults born preterm with very low birthweight or small for gestational age at term. Front. Psychol. 2023, 13, 1078232. [Google Scholar] [CrossRef]
- Althomali, R.H.; Abbood, M.A.; Saleh, E.A.M.; Djuraeva, L.; Abdullaeva, B.S.; Habash, R.T.; Alhassan, M.S.; Alawady, A.H.; Alsaalamy, A.H.; Najafi, M.L. Exposure to heavy metals and neurocognitive function in adults: A systematic review. Environ. Sci. Eur. 2024, 36, 18. [Google Scholar] [CrossRef]


| Element | Wavelength (nm) | Nebulizer Flow (L/min) | Replicas | Pump Speed (rpm) | Reading Time (s) |
|---|---|---|---|---|---|
| Pb | 220.353 | 15.0 | 3 | 15 | 3 |
| Cd | 226.502 | 15.0 | 3 | 15 | 3 |
| As | 228.812 | 15.0 | 3 | 15 | 3 |
| Meats and Meat Products | Pb | Cd | As |
|---|---|---|---|
| Meats (excluding organ meats from livestock and poultry and their products) | 0.2 | 0.1 | 0.5 |
| Organ meats from livestock and poultry | 0.5 | - | 0.5 |
| Meat products (excluding organ meats from livestock and poultry) | 0.3 | 0.1 | 0.5 |
| Liver from livestock and poultry | 0.5 | 0.5 | 0.5 |
| Kidney from livestock and poultry | 0.5 | 1.0 | 0.5 |
| Edible Component | Grams | % |
|---|---|---|
| Meat | 749.5 | 93.69 |
| Liver | 30.86 | 3.86 |
| Kidneys | 10.47 | 1.31 |
| Heart | 4.85 | 0.61 |
| Lungs | 4.32 | 0.54 |
| Total | 800 | 100.00 |
| Samples | n | Mean | Standard Deviation | 95% Confidence Interval for the Mean | Min | Max | |
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||
| Meat | 10 | 0.069 b | 0.060 | 0.025 | 0.112 | <LOD | 0.183 |
| Liver | 10 | 1.669 b | 0.355 | 1.415 | 1.923 | 1.232 | 2.356 |
| Kidneys | 10 | 0.765 b | 0.289 | 0.559 | 0.972 | 0.294 | 1.183 |
| Heart | 10 | 5.583 a | 3.593 | 3.013 | 8.153 | 1.249 | 11.946 |
| Lungs | 10 | 1.312 b | 0.684 | 0.823 | 1.802 | 0.341 | 2.010 |
| Samples | n | Mean | Standard Deviation | 95% Confidence Interval for the Mean | Min | Max | |
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||
| Meat | 10 | 0.002 b | 0.006 | 0.002 | 0.007 | <LOD | 0.020 |
| Liver | 10 | 1.445 a | 0.730 | 0.923 | 1.967 | 0.684 | 2.745 |
| Kidneys | 10 | 1.420 a | 1.002 | 0.703 | 2.137 | <LOD | 3.096 |
| Heart | 10 | 0.652 ab | 1.960 | 0.749 | 2.055 | <LOD | 6.227 |
| Lungs | 10 | 0.158 ab | 0.282 | 0.044 | 0.359 | <LOD | 0.941 |
| Samples | n | Mean | Standard Deviation | 95% Confidence Interval for the Mean | Min | Max | |
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||
| Meat | 10 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Liver | 10 | 1.385 ab | 0.574 | 0.975 | 1.795 | 0.775 | 2.409 |
| Kidneys | 10 | 1.607 a | 1.231 | 0.726 | 2.487 | <LOD | 3.358 |
| Heart | 10 | 0.934 ab | 2.207 | 0.645 | 2.513 | <LOD | 6.783 |
| Lungs | 10 | <LOD | <LOD | <LOD | <LOD | <LOD | - |
| Age | THQ-Pb | Age | THQ-Pb | Age | THQ-Pb | Age | THQ-Pb |
|---|---|---|---|---|---|---|---|
| 2 | 0.0013 | 25 | 0.0003 | 48 | 0.0002 | 71 | 0.0003 |
| 3 | 0.0011 | 26 | 0.0003 | 49 | 0.0002 | 72 | 0.0003 |
| 4 | 0.0010 | 27 | 0.0003 | 50 | 0.0002 | 73 | 0.0003 |
| 5 | 0.0009 | 28 | 0.0003 | 51 | 0.0002 | 74 | 0.0003 |
| 6 | 0.0008 | 29 | 0.0003 | 52 | 0.0002 | 75 | 0.0003 |
| 7 | 0.0007 | 30 | 0.0003 | 53 | 0.0002 | 76 | 0.0003 |
| 8 | 0.0006 | 31 | 0.0002 | 54 | 0.0002 | 77 | 0.0003 |
| 9 | 0.0006 | 32 | 0.0003 | 55 | 0.0002 | 78 | 0.0003 |
| 10 | 0.0005 | 33 | 0.0002 | 56 | 0.0003 | 79 | 0.0003 |
| 11 | 0.0004 | 34 | 0.0002 | 57 | 0.0003 | 80 | 0.0003 |
| 12 | 0.0004 | 35 | 0.0003 | 58 | 0.0003 | 81 | 0.0003 |
| 13 | 0.0004 | 36 | 0.0002 | 59 | 0.0002 | 82 | 0.0003 |
| 14 | 0.0003 | 37 | 0.0003 | 60 | 0.0003 | 83 | 0.0003 |
| 15 | 0.0003 | 38 | 0.0002 | 61 | 0.0003 | 84 | 0.0003 |
| 16 | 0.0003 | 39 | 0.0002 | 62 | 0.0003 | 85 | 0.0003 |
| 17 | 0.0003 | 40 | 0.0002 | 63 | 0.0003 | ||
| 18 | 0.0003 | 41 | 0.0002 | 64 | 0.0003 | ||
| 19 | 0.0003 | 42 | 0.0002 | 65 | 0.0003 | ||
| 20 | 0.0003 | 43 | 0.0002 | 66 | 0.0003 | ||
| 21 | 0.0003 | 44 | 0.0002 | 67 | 0.0003 | ||
| 22 | 0.0003 | 45 | 0.0002 | 68 | 0.0003 | ||
| 23 | 0.0003 | 46 | 0.0002 | 69 | 0.0003 | ||
| 24 | 0.0003 | 47 | 0.0002 | 70 | 0.0003 |
| Age | THQ-Cd | Age | THQ-Cd | Age | THQ-Cd | Age | THQ-Cd |
|---|---|---|---|---|---|---|---|
| 2 | 0.0011 | 25 | 0.0002 | 48 | 0.0002 | 71 | 0.0002 |
| 3 | 0.0009 | 26 | 0.0002 | 49 | 0.0002 | 72 | 0.0002 |
| 4 | 0.0008 | 27 | 0.0002 | 50 | 0.0002 | 73 | 0.0002 |
| 5 | 0.0007 | 28 | 0.0002 | 51 | 0.0002 | 74 | 0.0002 |
| 6 | 0.0006 | 29 | 0.0002 | 52 | 0.0002 | 75 | 0.0002 |
| 7 | 0.0006 | 30 | 0.0002 | 53 | 0.0002 | 76 | 0.0002 |
| 8 | 0.0005 | 31 | 0.0002 | 54 | 0.0002 | 77 | 0.0003 |
| 9 | 0.0005 | 32 | 0.0002 | 55 | 0.0002 | 78 | 0.0003 |
| 10 | 0.0004 | 33 | 0.0002 | 56 | 0.0002 | 79 | 0.0002 |
| 11 | 0.0004 | 34 | 0.0002 | 57 | 0.0002 | 80 | 0.0002 |
| 12 | 0.0003 | 35 | 0.0002 | 58 | 0.0002 | 81 | 0.0003 |
| 13 | 0.0003 | 36 | 0.0002 | 59 | 0.0002 | 82 | 0.0003 |
| 14 | 0.0003 | 37 | 0.0002 | 60 | 0.0002 | 83 | 0.0002 |
| 15 | 0.0003 | 38 | 0.0002 | 61 | 0.0002 | 84 | 0.0003 |
| 16 | 0.0002 | 39 | 0.0002 | 62 | 0.0002 | 85 | 0.0003 |
| 17 | 0.0002 | 40 | 0.0002 | 63 | 0.0002 | ||
| 18 | 0.0002 | 41 | 0.0002 | 64 | 0.0002 | ||
| 19 | 0.0002 | 42 | 0.0002 | 65 | 0.0002 | ||
| 20 | 0.0002 | 43 | 0.0002 | 66 | 0.0002 | ||
| 21 | 0.0002 | 44 | 0.0002 | 67 | 0.0002 | ||
| 22 | 0.0002 | 45 | 0.0002 | 68 | 0.0002 | ||
| 23 | 0.0002 | 46 | 0.0002 | 69 | 0.0002 | ||
| 24 | 0.0002 | 47 | 0.0002 | 70 | 0.0002 |
| Age | THQ-As | Age | THQ-As | Age | THQ-As | Age | THQ-As |
|---|---|---|---|---|---|---|---|
| 2 | 0.0056 | 25 | 0.0012 | 48 | 0.0011 | 71 | 0.0013 |
| 3 | 0.0049 | 26 | 0.0012 | 49 | 0.0011 | 72 | 0.0013 |
| 4 | 0.0043 | 27 | 0.0011 | 50 | 0.0011 | 73 | 0.0013 |
| 5 | 0.0038 | 28 | 0.0012 | 51 | 0.0011 | 74 | 0.0012 |
| 6 | 0.0034 | 29 | 0.0011 | 52 | 0.0011 | 75 | 0.0013 |
| 7 | 0.0031 | 30 | 0.0011 | 53 | 0.0011 | 76 | 0.0013 |
| 8 | 0.0028 | 31 | 0.0011 | 54 | 0.0011 | 77 | 0.0013 |
| 9 | 0.0025 | 32 | 0.0011 | 55 | 0.0011 | 78 | 0.0013 |
| 10 | 0.0022 | 33 | 0.0011 | 56 | 0.0011 | 79 | 0.0013 |
| 11 | 0.0019 | 34 | 0.0011 | 57 | 0.0011 | 80 | 0.0013 |
| 12 | 0.0017 | 35 | 0.0011 | 58 | 0.0011 | 81 | 0.0014 |
| 13 | 0.0016 | 36 | 0.0011 | 59 | 0.0011 | 82 | 0.0014 |
| 14 | 0.0014 | 37 | 0.0011 | 60 | 0.0011 | 83 | 0.0013 |
| 15 | 0.0014 | 38 | 0.0011 | 61 | 0.0012 | 84 | 0.0014 |
| 16 | 0.0013 | 39 | 0.0011 | 62 | 0.0011 | 85 | 0.0013 |
| 17 | 0.0013 | 40 | 0.0011 | 63 | 0.0011 | ||
| 18 | 0.0013 | 41 | 0.0011 | 64 | 0.0012 | ||
| 19 | 0.0013 | 42 | 0.0011 | 65 | 0.0012 | ||
| 20 | 0.0012 | 43 | 0.0011 | 66 | 0.0012 | ||
| 21 | 0.0012 | 44 | 0.0011 | 67 | 0.0011 | ||
| 22 | 0.0012 | 45 | 0.0011 | 68 | 0.0012 | ||
| 23 | 0.0012 | 46 | 0.0011 | 69 | 0.0012 | ||
| 24 | 0.0012 | 47 | 0.0011 | 70 | 0.0012 |
| Age | HI | Age | HI | Age | Hi | Age | HI |
|---|---|---|---|---|---|---|---|
| 2 | 0.0080 | 25 | 0.0017 | 48 | 0.0015 | 71 | 0.0018 |
| 3 | 0.0069 | 26 | 0.0016 | 49 | 0.0015 | 72 | 0.0019 |
| 4 | 0.0061 | 27 | 0.0016 | 50 | 0.0016 | 73 | 0.0018 |
| 5 | 0.0054 | 28 | 0.0016 | 51 | 0.0016 | 74 | 0.0018 |
| 6 | 0.0048 | 29 | 0.0016 | 52 | 0.0015 | 75 | 0.0018 |
| 7 | 0.0044 | 30 | 0.0016 | 53 | 0.0015 | 76 | 0.0019 |
| 8 | 0.0039 | 31 | 0.0016 | 54 | 0.0016 | 77 | 0.0019 |
| 9 | 0.0035 | 32 | 0.0016 | 55 | 0.0016 | 78 | 0.0019 |
| 10 | 0.0032 | 33 | 0.0016 | 56 | 0.0016 | 79 | 0.0018 |
| 11 | 0.0027 | 34 | 0.0016 | 57 | 0.0016 | 80 | 0.0018 |
| 12 | 0.0024 | 35 | 0.0016 | 58 | 0.0016 | 81 | 0.0020 |
| 13 | 0.0022 | 36 | 0.0015 | 59 | 0.0016 | 82 | 0.0020 |
| 14 | 0.0020 | 37 | 0.0016 | 60 | 0.0016 | 83 | 0.0018 |
| 15 | 0.0019 | 38 | 0.0016 | 61 | 0.0016 | 84 | 0.0019 |
| 16 | 0.0019 | 39 | 0.0015 | 62 | 0.0016 | 85 | 0.0019 |
| 17 | 0.0018 | 40 | 0.0015 | 63 | 0.0016 | ||
| 18 | 0.0018 | 41 | 0.0015 | 64 | 0.0017 | ||
| 19 | 0.0018 | 42 | 0.0015 | 65 | 0.0017 | ||
| 20 | 0.0018 | 43 | 0.0015 | 66 | 0.0017 | ||
| 21 | 0.0017 | 44 | 0.0015 | 67 | 0.0016 | ||
| 22 | 0.0017 | 45 | 0.0015 | 68 | 0.0017 | ||
| 23 | 0.0017 | 46 | 0.0015 | 69 | 0.0017 | ||
| 24 | 0.0017 | 47 | 0.0015 | 70 | 0.0017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirinos-Peinado, D.; Castro-Bedriñana, J.; Rivera-Parco, F.; Ríos-Ríos, E. Lead, Cadmium, and Arsenic in Edible Tissues of Guinea Pigs Raised in the Central Andes of Peru: Potential Human Health Risk? Vet. Sci. 2025, 12, 292. https://doi.org/10.3390/vetsci12040292
Chirinos-Peinado D, Castro-Bedriñana J, Rivera-Parco F, Ríos-Ríos E. Lead, Cadmium, and Arsenic in Edible Tissues of Guinea Pigs Raised in the Central Andes of Peru: Potential Human Health Risk? Veterinary Sciences. 2025; 12(4):292. https://doi.org/10.3390/vetsci12040292
Chicago/Turabian StyleChirinos-Peinado, Doris, Jorge Castro-Bedriñana, Fiorela Rivera-Parco, and Elva Ríos-Ríos. 2025. "Lead, Cadmium, and Arsenic in Edible Tissues of Guinea Pigs Raised in the Central Andes of Peru: Potential Human Health Risk?" Veterinary Sciences 12, no. 4: 292. https://doi.org/10.3390/vetsci12040292
APA StyleChirinos-Peinado, D., Castro-Bedriñana, J., Rivera-Parco, F., & Ríos-Ríos, E. (2025). Lead, Cadmium, and Arsenic in Edible Tissues of Guinea Pigs Raised in the Central Andes of Peru: Potential Human Health Risk? Veterinary Sciences, 12(4), 292. https://doi.org/10.3390/vetsci12040292







