Comparison of Medical Imaging Quality Related to Embalming Solutions in Canine Cadavers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Embalming Solutions and Protocol
2.3. Imaging Protocol and Assessment Criteria
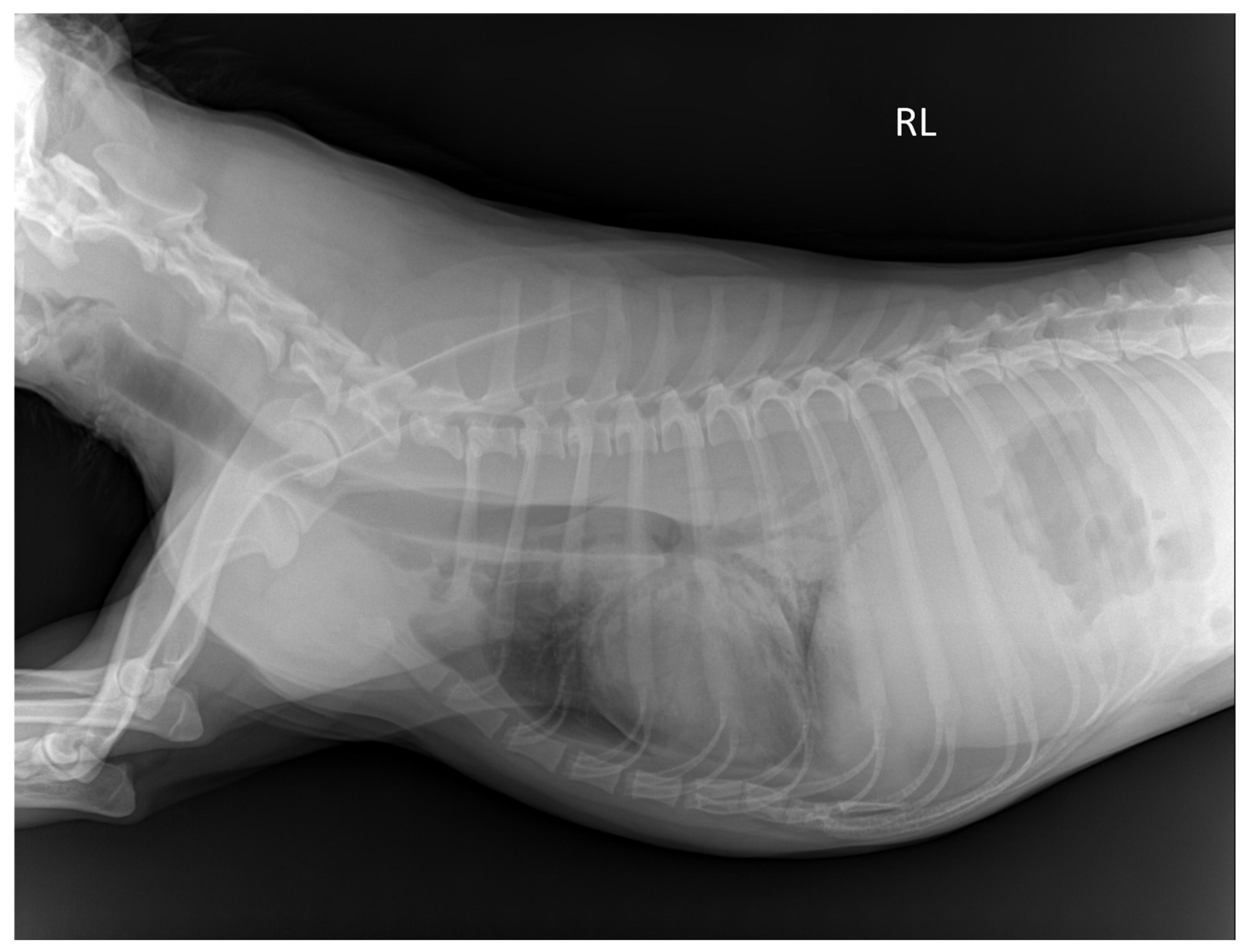
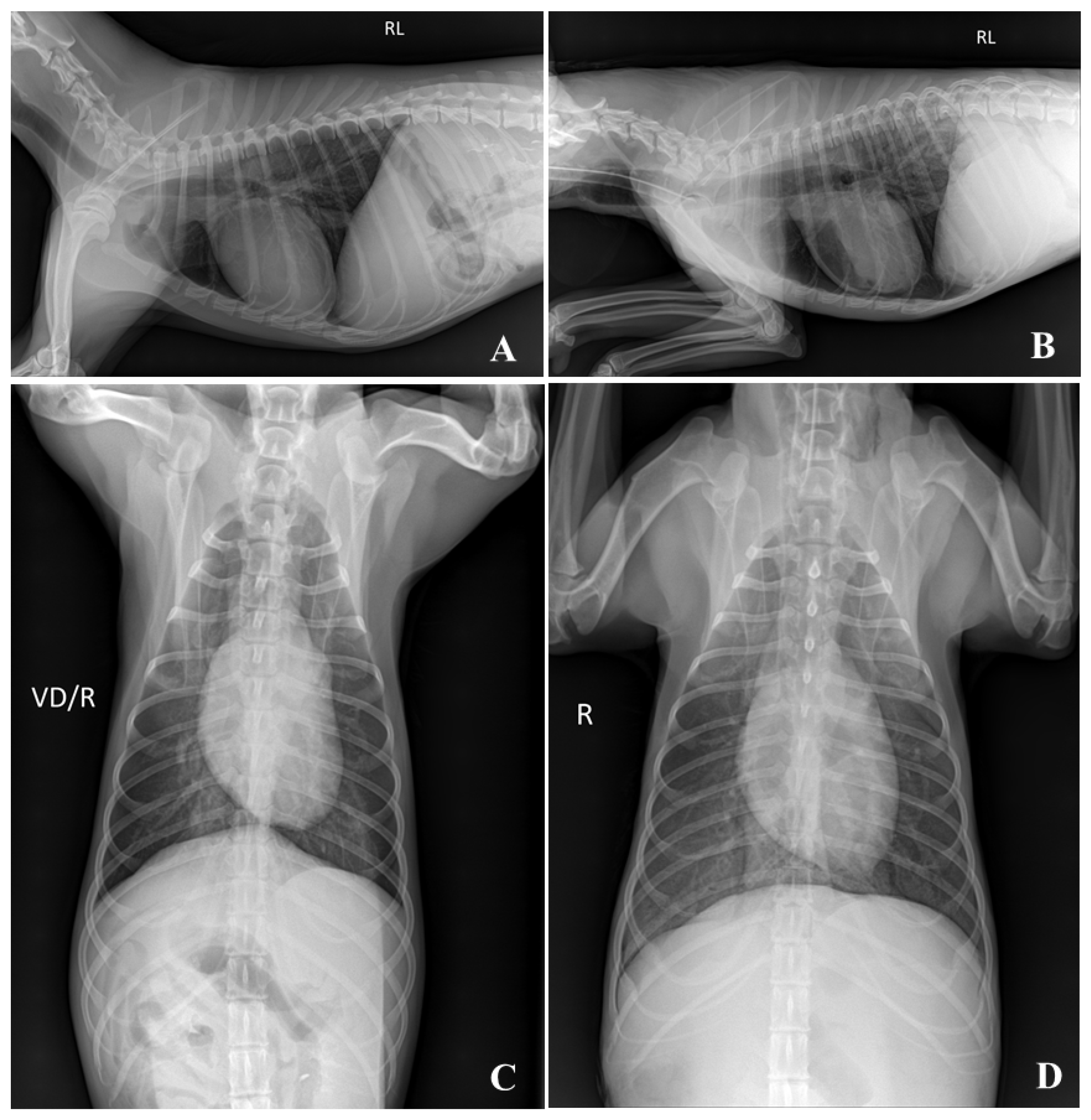
2.4. Plain Radiographic Examination of Thoracic and Abdominal Cavity
2.5. Ultrasonographic Examination of Abdominal Organ
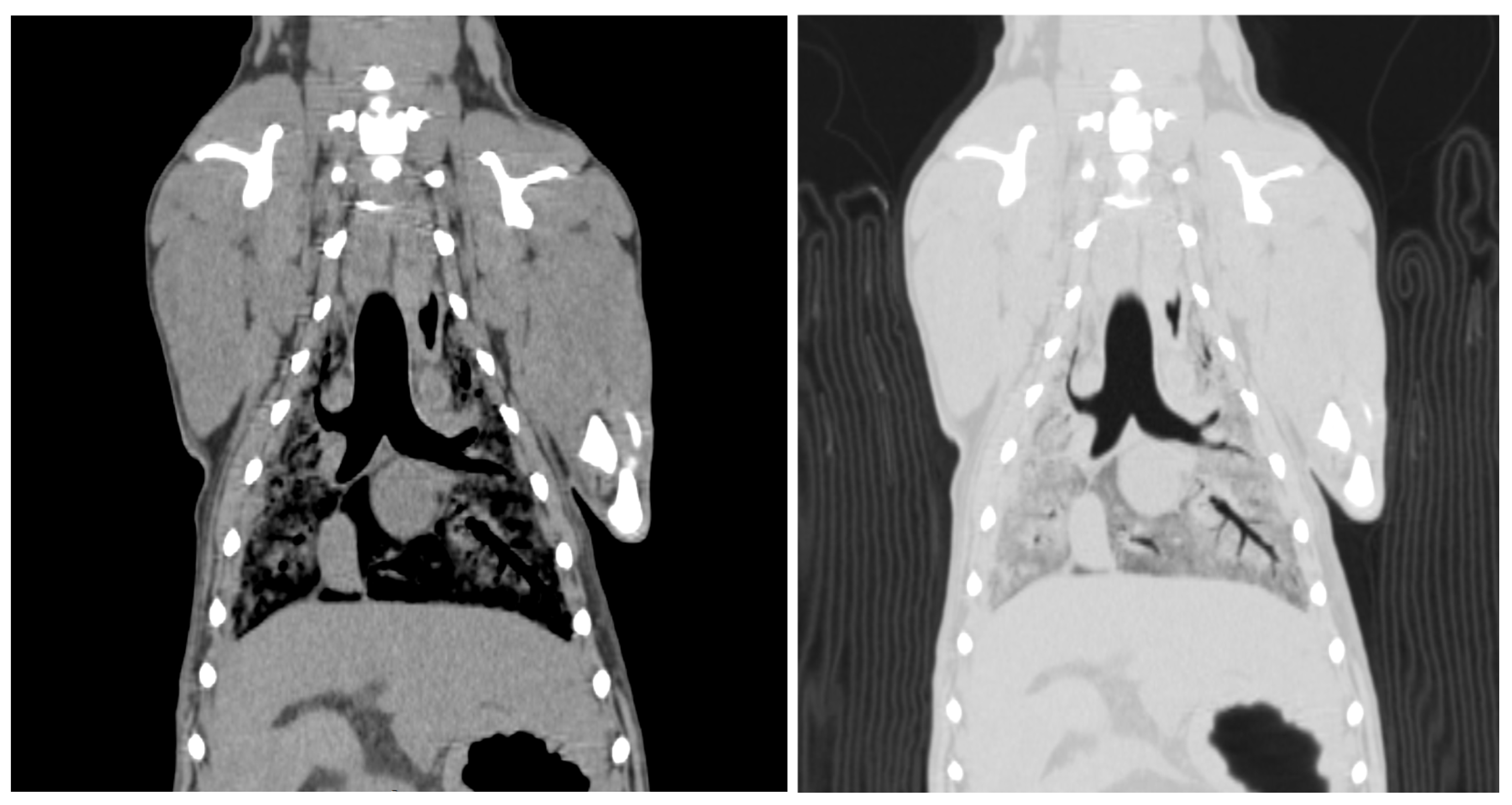
2.6. Computed Tomographic Examination of Whole Body
2.7. Statistical Methods
3. Results
3.1. Subsection
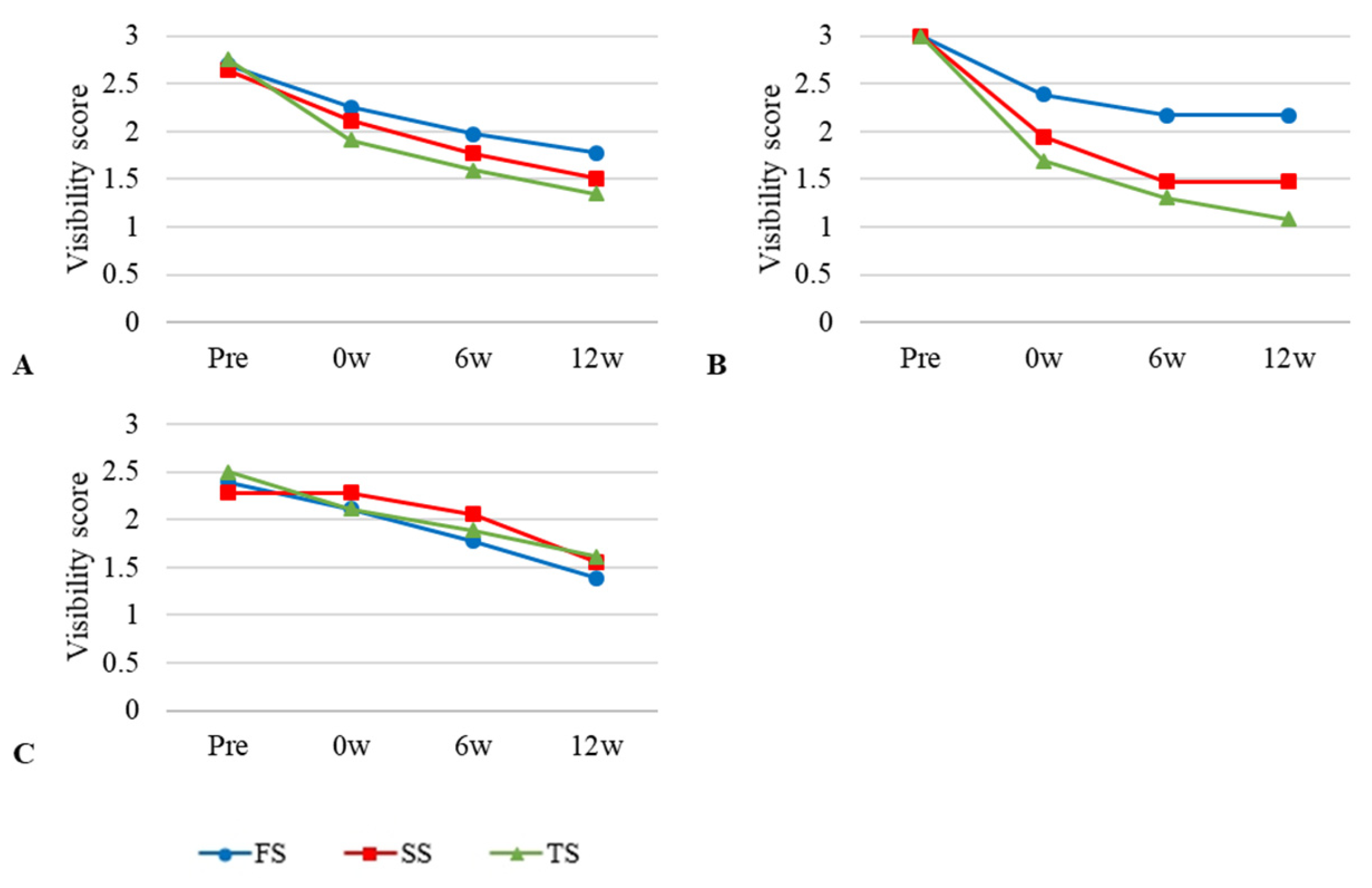
3.2. Visibility of Plain Radiographic Examination
3.3. Visibility of Ultrasonographic Examination
3.4. Visibility of Computed Tomographic Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLeod, G.; Eisma, R.; Schwab, A.; Corner, G.; Soames, R.; Cochran, S. An evaluation of Thiel-embalmed cadavers for ultrasound-based regional anaesthesia training and research. Ultrasound 2010, 18, 125–129. [Google Scholar] [CrossRef]
- Eisma, R.; Lamb, C.; Soames, R.W. From formalin to Thiel embalming: What changes? One anatomy department’s experiences. Clin. Anat. 2013, 26, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Healy, S.E.; Rai, B.P.; Biyani, C.S.; Eisma, R.; Soames, R.W.; Nabi, G. Thiel embalming method for cadaver preservation: A review of new training model for urologic skills training. Urology 2015, 85, 499–504. [Google Scholar] [CrossRef]
- Oliver, S.W.; Patel, R.K.; Ali, K.A.; Geddes, C.C.; MacKinnon, B. Teaching percutaneous renal biopsy using unfixed human cadavers. BMC Nephrol. 2015, 16, 209–212. [Google Scholar] [CrossRef][Green Version]
- Hoyer, R.; Means, R.; Robertson, J.; Rappaport, D.; Schmier, C.; Jones, T.; Stolz, L.A.; Kaplan, S.J.; Adamas-Rappaport, W.J.; Amini, R. Ultrasound-guided procedures in medical education: A fresh look at cadavers. Intern. Emerg. Med. 2016, 11, 431–436. [Google Scholar] [CrossRef]
- Balta, J.Y.; Twomey, M.; Moloney, F.; O’Connor, O.J.; Murphy, K.P.; Cronin, M.; Cryan, J.F.; Maher, M.M.; O’Mahony, S.M. Assessing radiological images of human cadavers: Is there an effect of different embalming solutions? J. Forensic Radiol. Imaging 2017, 11, 40–46. [Google Scholar] [CrossRef]
- Nam, S.M.; Moon, J.S.; Yoon, H.Y.; Chan, B.J.; Nahm, S.S. Comparative evaluation of canine cadaver embalming methods for veterinary anatomy education. Anat. Sci. Int. 2020, 95, 498–507. [Google Scholar] [CrossRef]
- Törnqvist, E.; Annas, A.; Granath, B.; Jalkesten, E.; Cotgreave, I.; Öberg, M. Strategic focus on 3R principles reveals major reductions in the use of animals in pharmaceutical toxicity testing. PLoS ONE 2014, 9, e101638. [Google Scholar] [CrossRef]
- Chew, F.S.; Relyea-Chew, A.; Ochoa, E.R. Postmortem computed tomography of cadavers embalmed for use in teaching gross anatomy. J. Comput. Assist. Tomogr. 2006, 30, 949–954. [Google Scholar] [CrossRef]
- Slon, V.; Hershkovitz, I.; May, H. The value of cadaver CT scans in gross anatomy laboratory. Anat. Sci. Educ. 2014, 7, 80–82. [Google Scholar] [CrossRef]
- Balta, J.Y.; Clare, L.; Soames, R.W. A pilot study comparing the use of Thiel-and formalin-embalmed cadavers in the teaching of human anatomy. Anat. Sci. Educ. 2015, 8, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, S.A.; Hoshino, K. An improved embalming procedure for long-lasting preservation of the cadaver for anatomical study. Acta Anat. 1978, 101, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Janczyk, P.; Weigner, J.; Luebke-Becker, A.; Kaessmeyer, S.; Plendl, J. Nitrite pickling salt as an alternative to formaldehyde for embalming in veterinary anatomy—A study based on histo-and microbiological analyses. Ann. Anat. 2011, 193, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Homma, H.; Naito, M.; Oda, J.; Nishiyama, T.; Kawamoto, A.; Kawata, S.; Sato, N.; Fukuhara, T.; Taguchi, H.; et al. Saturated salt solution method: A useful cadaver embalming for surgical skills training. Medicine 2014, 93, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Lombardero, M.; Yllera, M.M.; Costa-E-Silva, A.; Oliveira, M.J.; Ferreira, P.G. Saturated salt solution: A further step to a formaldehyde-free embalming method for veterinary gross anatomy. J. Anat. 2017, 231, 309–317. [Google Scholar] [CrossRef]
- Turan, E.; Gules, O.; Kilimci, F.S.; Kara, M.E.; Dilek, O.G.; Sabanci, S.S.; Tatar, M. The mixture of liquid foam soap, ethanol and citric acid as a new fixative–preservative solution in veterinary anatomy. Ann. Anat. 2017, 209, 11–17. [Google Scholar] [CrossRef]
- Brenner, E. Human body preservation–old and new techniques. J. Anat. 2014, 224, 316–344. [Google Scholar] [CrossRef]
- Hayashi, S.; Naito, M.; Kawata, S.; Qu, N.; Hatayama, N.; Hirai, S.; Itoh, M. History and future of human cadaver preservation for surgical training: From formalin to saturated salt solution method. Anat. Sci. Int. 2016, 91, 1–7. [Google Scholar] [CrossRef]
- Balta, J.Y.; Cronin, M.; Cryan, J.F.; O’mahony, S.M. Human preservation techniques in anatomy: A 21st century medical education perspective. Clin. Anat. 2015, 28, 725–734. [Google Scholar] [CrossRef]
- Entius, C.A.C.; Rijn, R.R.V.; Zwamborn, A.W.; Kleinrensink, G.J.; Robben, S.G.F. Influence of formaldehyde/phenol fixation on MRI of the stifle joint and correlation with plastinated slices. J. Int. Soc. Plast. 2004, 19, 26–32. [Google Scholar]
- Wantke, F.; Focke, M.; Hemmer, W.; Bracun, R.; Wolf-Abdolvahab, S.; GoÈtz, M.; Jarisch, R.; GoÈtz, M.; Tschabitscher, M.; Gann, M.; et al. Exposure to formaldehyde and phenol during an anatomy dissecting course: Sensitizing potency of formaldehyde in medical students. Allergy 2000, 55, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Villacorta, P.R.F.; Hernando, H.C.T.; Abdulla, A.J.A.; Barroa, J.K.H. A Comparative Study of Thiel Soft-embalmed and Formalin Preserved Cadavers for Anatomy Dissection. Acta Med. Philipp. 2019, 53, 12–20. [Google Scholar] [CrossRef]
- Haizuka, Y.; Nagase, M.; Takashino, S.; Kobayashi, Y.; Fujikura, Y.; Matsumura, G. A new substitute for formalin: Application to embalming cadavers. Clin. Anat. 2018, 31, 90–98. [Google Scholar] [CrossRef]
- Kennel, L.; Martin, D.M.A.; Shaw, H.; Wilkinson, T. Learning anatomy through Thiel- vs. formalin-embalmed cadavers: Student perceptions of embalming methods and effect on functional anatomy knowledge. Anat. Sci. Educ. 2018, 11, 166–174. [Google Scholar] [CrossRef]
- Balta, J.Y.; Twomey, M.; Moloney, F.; Duggan, O.; Murphy, K.P.; O’Connor, O.J.; Cronin, M.; Cryan, J.F.; Maher, M.M.; O’Mahony, S.M. A comparison of embalming fluids on the structures and properties of tissue in human cadavers. Anat. Histol. Embryol. 2019, 48, 64–73. [Google Scholar] [CrossRef]
- Joy, J.; McLeod, G.; Lee, N.; Munirama, S.; Corner, G.; Eisma, R.; Cochran, S. Quantitative assessment of Thiel soft-embalmed human cadavers using shear wave elastography. Ann. Anat. 2015, 202, 52–56. [Google Scholar] [CrossRef]
- American Institute of Ultrasound in Medicine. AIUM Practice parameter for the performance of an ultrasound examination of the abdomen and/or retroperitoneum. J. Ultrasound Med. 2022, 41, E1–E8. [Google Scholar]
- Leonardi, M.; van Meerten, E.v.P.; Geleijns, J.; Jessen, K.A.; Panzer, W.; Shrimpton, P.C.; Tosi, G. European Guidelines on Quality Criteria for Computed Tomography; Office for Official Publications of the European Communities: Luxembourg, 2000. [Google Scholar]
- Benkhadra, M.; Faust, A.; Ladoire, S.; Trost, O.; Trouilloud, P.; Girard, C.; Anderhuber, F.; Feigl, G. Comparison of fresh and Thiel’s embalmed cadavers according to the suitability for ultrasound-guided regional anesthesia of the cervical region. Surg. Radiol. Anat. 2009, 31, 531–536. [Google Scholar] [CrossRef]
- Schramek, G.G.R.; Stoevesandt, D.; Reising, A.; Kielstein, J.T.; Hiss, M.; Kielstein, H. Imaging in anatomy: A comparison of imaging techniques in embalmed human cadavers. BMC Med. Educ. 2013, 13, 143–149. [Google Scholar] [CrossRef]
- Ishida, M.; Gonoi, W.; Hagiwara, K.; Okuma, H.; Shirota, G.; Shintani, Y.; Abe, H.; Takazawa, Y.; Fukayama, M.; Ohtomo, K. Early postmortem volume reduction of adrenal gland: Initial longitudinal computed tomographic study. Radiol. Med. 2015, 120, 662–669. [Google Scholar] [CrossRef]



| Formalin Solution | Saturated Salt Solution | Thiel’s Solution | |||
|---|---|---|---|---|---|
| Solution Formula | Amount | Solution Formula | Amount | Solution Formula | Amount |
| 36–40% Formalin | 0.25 L | Sodium chloride | 4 kg | 1. Stem solution A | |
| Ethanol | 1.5 L | 36–40% Formalin | 0.134 L | 4-Chloro-3-methylphenol | 26.4 g |
| Phenol | 0.3 L | Isopropyl alcohol | 0.8 L | Propylene glycol | 0.264 L |
| Glycerol | 0.1 L | Phenol | 0.04 L | 2. Stem solution B | |
| Water | 3.05 L | Glycerol | 0.1 L | Ammonium nitrate | 1 kg |
| Total | 5 L | Water | 3.86 + 0.66 L | Hot water | 1.6 L |
| Total | 5 L | 3. Stem solution C | |||
| Boric acid | 148 g | ||||
| Potassium nitrate | 248 g | ||||
| Hot water | 2 L | ||||
| 4. Stock solution | |||||
| Stem solution A | 0.264 L | ||||
| Stem solution B | 1.6 L | ||||
| Stem solution C | 2 L | ||||
| Propylene glycol | 1.48 L | ||||
| Hot water | 1.32 L | ||||
| 5. Final solution | |||||
| Stock solution | 6.664 L | ||||
| Sodium sulfate | 320 g | ||||
| 36–40% Formalin | 0.16 L | ||||
| Morpholine | 0.12 L | ||||
| Ethanol | 0.52 L | ||||
| Total | 7.544 L | ||||
| Modality | ICC | Interval for 95% ICC | p-Value |
|---|---|---|---|
| Plain radiography | 0.893 | 0.629–0.969 | <0.001 |
| CT | 0.978 | 0.925–0.994 | <0.001 |
| Embalming Solution | Pre | 0 w | 6 w | 12 w |
|---|---|---|---|---|
| FS | 2.69 ± 0.12 | 2.25 ± 0.35 a | 1.97 ± 0.40 a | 1.77 ± 0.23 a |
| TS | 2.75 ± 0.25 | 1.90 ± 0.02 b | 1.59 ± 0.25 b | 1.34 ± 0.27 b |
| SS | 2.64 ± 0.19 | 2.11 ± 0.43 | 1.76 ± 0.30 | 1.51 ± 0.37 |
| Embalming Solution | Pre | 0 w | 6 w | 12 w |
|---|---|---|---|---|
| FS | 2.93 ± 0.12 | 2.15 ± 0.35 a | 1.44 ± 0.50 a | 1.33 ± 0.57 a |
| TS | 2.94 ± 0.04 | 2.07 ± 0.10 | 1.36 ± 0.15 | 1.26 ± 0.34 |
| SS | 2.90 ± 0.10 | 1.79 ± 0.52 b | 0.82 ± 0.33 b | 0.64 ± 0.38 b |
| Embalming Solution | Pre | 0 w | 6 w | 12 w |
|---|---|---|---|---|
| FS | 2.90 ± 0.02 | 2.60 ± 0.02 a | 2.47 ± 0.02 a | 2.42 ± 2.04 a |
| TS | 2.90 ± 0.02 | 1.95 ± 0.12 b | 1.70 ± 0.06 b | 1.62 ± 0.10 b |
| SS | 2.89 ± 0.02 | 2.24 ± 0.15 | 2.01 ± 0.19 | 1.89 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, A.; Nam, S.-M.; Nahm, S.-S.; Eom, K.-D.; Kim, W. Comparison of Medical Imaging Quality Related to Embalming Solutions in Canine Cadavers. Vet. Sci. 2025, 12, 188. https://doi.org/10.3390/vetsci12020188
Oh A, Nam S-M, Nahm S-S, Eom K-D, Kim W. Comparison of Medical Imaging Quality Related to Embalming Solutions in Canine Cadavers. Veterinary Sciences. 2025; 12(2):188. https://doi.org/10.3390/vetsci12020188
Chicago/Turabian StyleOh, Ahsa, Sung-Min Nam, Sang-Soep Nahm, Ki-Dong Eom, and Woosuk Kim. 2025. "Comparison of Medical Imaging Quality Related to Embalming Solutions in Canine Cadavers" Veterinary Sciences 12, no. 2: 188. https://doi.org/10.3390/vetsci12020188
APA StyleOh, A., Nam, S.-M., Nahm, S.-S., Eom, K.-D., & Kim, W. (2025). Comparison of Medical Imaging Quality Related to Embalming Solutions in Canine Cadavers. Veterinary Sciences, 12(2), 188. https://doi.org/10.3390/vetsci12020188







