The Use of Grape By-Products as a Feed Additive Enhances the Oxidative Stability of Rabbit Meat
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Measurement and Sample Collection
2.3. Laboratory Analysis
2.3.1. Feed Analysis
2.3.2. Chemical Analysis of LD
- A is the absorbance of the sample;
- m is the slope of the calibration curve;
- 72.063 is the molecular weight of malondialdehyde;
- E is the sample weight equivalent.
2.4. Statistical Analysis
3. Results
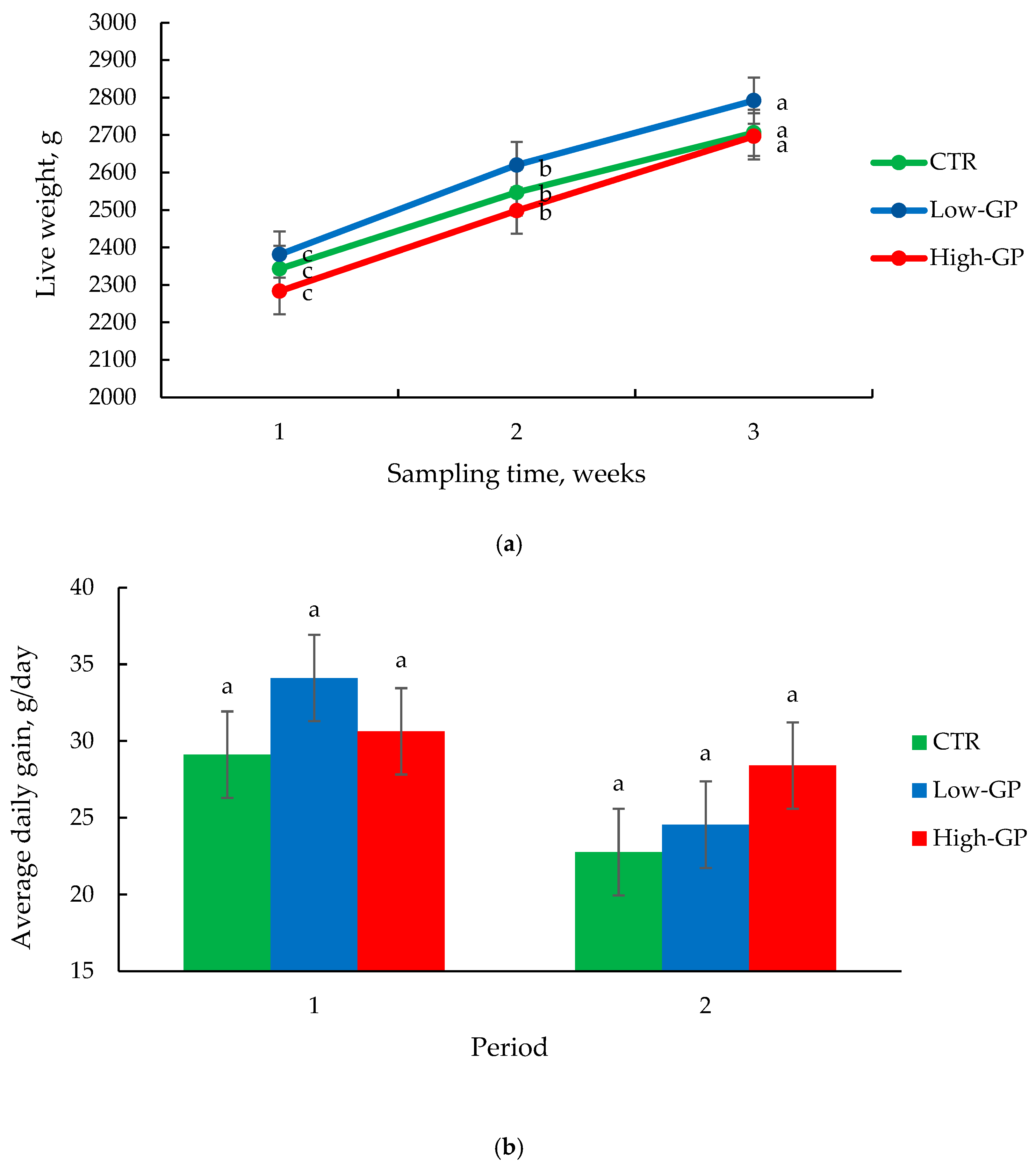
3.1. Animal Performance and Carcass Traits
3.2. Proximate Composition and Fatty Acid Profile
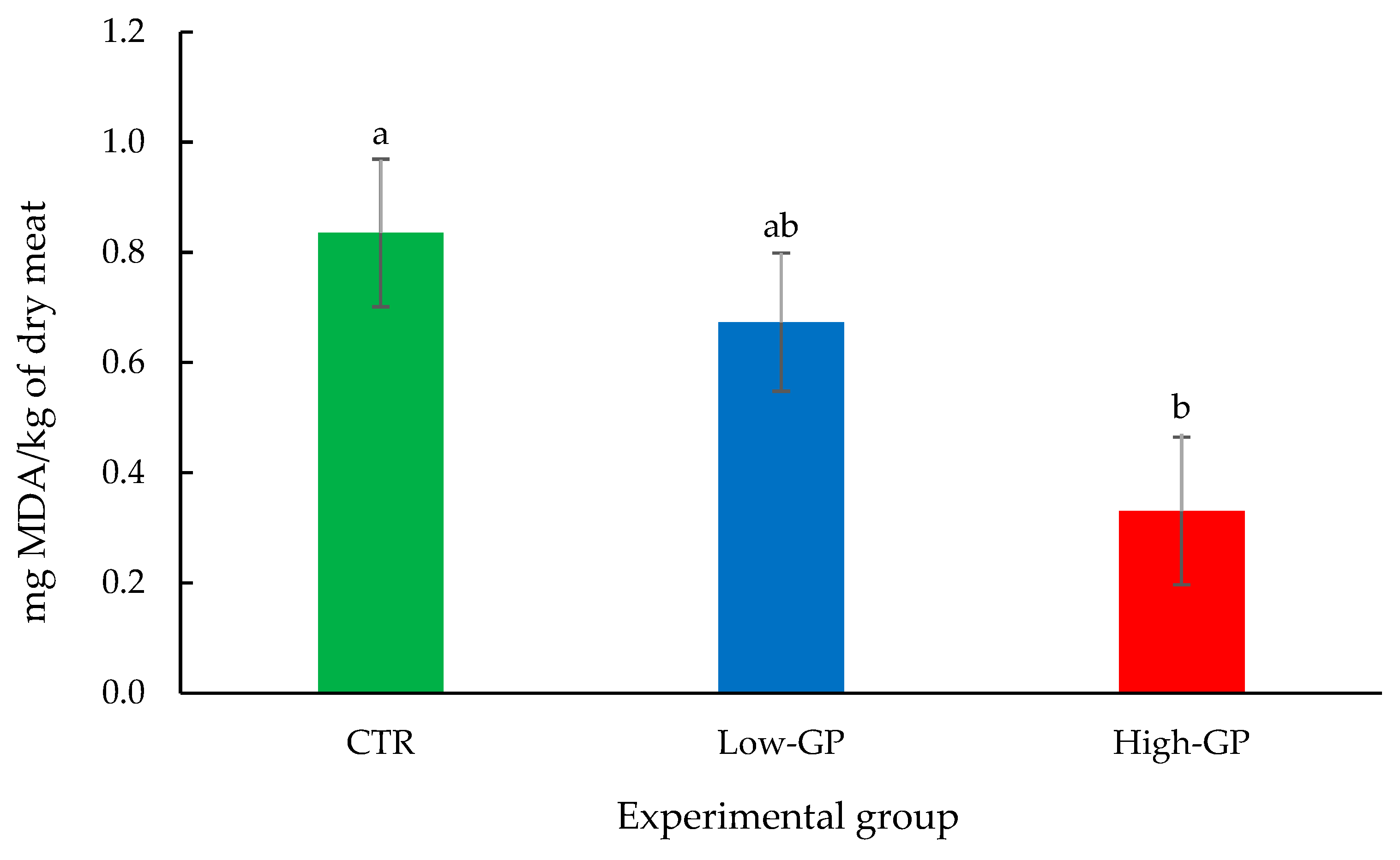
3.3. TBARs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalle Zotte, A.; Szendrő, Z. The role of rabbit meat as functional food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Yao, K.; Zhang, X.; Zhao, S.; Sun, Z.; Tian, G.; You, B.; Lin, J.; Zhu, B.; Jia, G.; et al. Nutrition and health relevant regulation of intestinal sulfur amino acid metabolism. Amino Acids 2010, 39, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of skeletal muscle function by amino acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zeng, X.; Qiao, S.; Wu, G.; Li, D. Specific roles of threonine in intestinal mucosal integrity and barrier function. Front. Biosci. 2011, 3, 200. [Google Scholar] [CrossRef]
- Hernández, P.; Zotte, A.D. Influence of diet on rabbit meat quality. In Nutrition of the Rabbit, 2nd ed.; de Blas, C., Wiseman, J., Eds.; CAB International: Oxfordshire, UK, 2010; pp. 163–178. [Google Scholar]
- Ryan-Harshman, M.; Aldoori, W. Vitamin B12 and health. Can. Fam. Physician 2008, 54, 536–541. [Google Scholar] [PubMed]
- Mahmood, L. The metabolic processes of folic acid and Vitamin B12 deficiency. J. Health Res. Rev. 2014, 1, 5–9. [Google Scholar] [CrossRef]
- Società Italiana di Nutrizione Umana. Livelli di Assunzione Raccomandati di Energia e Nutrienti per la Popolazione Italiana (LARN); S.I.N.U.: Roma, Italy, 1996. [Google Scholar]
- Salma, U.; Miah, A.G.; Maki, T.; Nishimura, M.; Tsujii, H. Effect of dietary Rhodobacter capsulatus on cholesterol concentration and fatty acid composition in broiler meat. Poult. Sci. 2007, 86, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, S.; Samaraweera, H.; Lee, E.J.; Ahn, D.U. Improving functional value of meat products. Meat Sci. 2010, 86, 15–31. [Google Scholar] [CrossRef]
- Buffa, G.; Tsiplakou, E.; Mitsiopoulou, C.; Pulina, G.; Nudda, A. Supplementation of by-products from grape, tomato and myrtle affects antioxidant status of dairy ewes and milk fatty acid profile. J. Anim. Physiol. Anim. Nutr. 2020, 104, 493–506. [Google Scholar] [CrossRef]
- Nudda, A.; Buffa, G.; Atzori, A.S.; Cappai, M.G.; Caboni, P.; Fais, G.; Pulina, G. Small amounts of agro-industrial byproducts in dairy ewes diets affects milk production traits and hematological parameters. Anim. Feed Sci. Technol. 2019, 251, 76–85. [Google Scholar] [CrossRef]
- Directive, E. Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Legislative Decree No 146, implementing Directive 98/58/EC. Gazzetta Ufficiale della Repubblica Italiana, No. 95. 26 March 2001.
- AOAC. Official Methods of Analysis, 17th ed.; AOAC: Arlington, VA, USA, 2000. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Robertson, J.B.; Van Soest, P.J. The detergent system of analysis and its application to human foods. In The Analysis of Dietary Fiber in Food; James, W.P.T., Thean-der, O., Eds.; Marcel Dekker: New York, NY, USA, 1981; p. 123. [Google Scholar]
- Correddu, F.; Nudda, A.; Battacone, G.; Boe, R.; Francesconi, A.H.D.; Pulina, G. Effects of grape seed supplementation, alone or associated with linseed, on ruminal metabolism in Sarda dairy sheep. Anim. Feed Sci. Technol. 2015, 199, 61–72. [Google Scholar] [CrossRef]
- Nudda, A.; Palmquist, D.L.; Battacone, G.; Fancellu, S.; Rassu, S.P.G.; Pulina, G. Relationships between the contents of vaccenic acid CLA and n-3 fatty acids of goat milk and the muscle of their suckling kids. Livest. Sci. 2008, 118, 195–203. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Nudda, A.; Correddu, F.; Atzori, A.S.; Marzano, A.; Battacone, G.; Nicolussi, P.; Bonelli, P.; Pulina, G. Whole exhausted berries of Myrtus communis L. supplied to dairy ewes: Effects on milk production traits and blood metabolites. Small Rumin. Res. 2017, 155, 33–38. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Nudda, A.; McGuire, M.A.; Battacone, G.; Pulina, G. Seasonal variation in conjugated linoleic acid and vaccenic acid in milk fat of sheep and its transfer to cheese and ricotta. J. Dairy Sci. 2005, 88, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Raharjo, S.; Sofos, J.N.; Schmidt, G.R. Improved speed, specificity, and limit of determination of an aqueous acid extraction thiobarbituric acid-C18 method for measuring lipid peroxidation in beef. J. Agric. Food Chem. 1992, 40, 2182–2185. [Google Scholar] [CrossRef]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Evaluation of three modified TBA methods for measuring lipid oxidation in chicken meat. J. Agric. Food Chem. 1989, 37, 1309–1313. [Google Scholar] [CrossRef]
- Bouzaida, M.D.; Resconi, V.C.; Gimeno, D.; Romero, J.V.; Calanche, J.B.; Barahona, M.; Olleta, J.L.; María, G.A. Effect of dietary grape pomace on fattening rabbit performance, fatty acid composition, and shelf life of meat. Antioxidants 2021, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Hulot, F.; Ouhayoun, J. Muscular pH and related traits in rabbits: A review. World Rabbit Sci. 1999, 7, 15–36. [Google Scholar] [CrossRef]
- Koné, A.P.; Cinq-Mars, D.; Desjardins, Y.; Guay, F.; Gosselin, A.; Saucier, L. Effects of plant extracts and essential oils as feed supplements on quality and microbial traits of rabbit meat. World Rabbit Sci. 2016, 24, 107–119. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Castellini, C.; Bianchi, L.; Mugnai, C. Effect of dietary α-linolenic acid and vitamin E on the fatty acid composition, storage stability and sensory traits of rabbit meat. Meat Sci. 2004, 66, 407–413. [Google Scholar] [CrossRef]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 2017, 12, 246–255. [Google Scholar] [CrossRef]
- Trombetta, F.; Fruet, A.P.B.; Stefanello, F.S.; Fonseca, P.A.F.; De Souza, A.N.M.; Tonetto, C.J.; Rosado, A.G.J.; Nörnberg, J.L. Effects of the dietary inclusion of linseed oil and grape pomace on weight gain, carcass characteristics, and meat quality of swine. Int. Food Res. J. 2019, 26, 1741–1749. [Google Scholar]
- Smink, W.; Verstegen, M.W.A.; Gerrits, W.J.J. Effect of intake of linoleic acid and α-linolenic acid levels on conversion into long-chain polyunsaturated fatty acids in backfat and in intramuscular fat of growing pigs. J. Anim. Physiol. Anim. Nutr. 2013, 97, 558–565. [Google Scholar] [CrossRef]
- Komprda, T.; Zelenka, J.; Fajmonova, E.; Fialova, M.; Kladroba, D. Arachidonic acid and long-chain n-3 polyunsaturated fatty acid contents in meat of selected poultry and fish species in relation to dietary fat sources. J. Agric. Food Chem. 2005, 53, 6804–6812. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Rebollar, P.G.; Mattioli, S.; Castellini, C. n-3 PUFA sources (precursor/products): A review of current knowledge on rabbit. Animals 2019, 9, 806. [Google Scholar] [CrossRef]
- Bennato, F.; Di Luca, A.; Martino, C.; Ianni, A.; Marone, E.; Grotta, L.; Ramazzotti, S.; Cichelli, A.; Martino, G. Influence of grape pomace intake on nutritional value, lipid oxidation and volatile profile of poultry meat. Foods 2020, 9, 508. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Brenes, A.; Viveros, A.; Goñi, I. Antioxidative effect of dietary grape pomace concentrate on lipid oxidation of chilled and long-term frozen stored chicken patties. Meat Sci. 2009, 83, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Vizzarri, F.; Chiapparini, S.; Ratti, S.; Casamassima, D.; Palazzo, M.; Corino, C. Effects of dietary levels of brown seaweeds and plant polyphenols on growth and meat quality parameters in growing rabbit. Meat Sci. 2020, 161, 107987. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, D.; Tong, J.; Vaddella, V. Influence of chestnut tannins on welfare, carcass characteristics, meat quality, and lipid oxidation in rabbits under high ambient temperature. Meat Sci. 2012, 90, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, J.; Serrano, R.; Jordán, M.J.; Bañón, S. Shelf life of meat from lambs given essential oil-free rosemary extract containing carnosic acid plus carnosol at 200 or 400 mg kg−1. Meat Sci. 2014, 96, 1452–1459. [Google Scholar] [CrossRef]
- Andrés, S.; Huerga, L.; Mateo, J.; Tejido, M.L.; Bodas, R.; Morán, L.; Prieto, N.; Rotolo, L.; Giráldez, F.J. The effect of quercetin dietary supplementation on meat oxidation processes and texture of fattening lambs. Meat Sci. 2014, 96, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
| Items | Concentrate | Grape Pomace |
|---|---|---|
| Chemical composition (% of DM unless otherwise noted) | ||
| DM, % | 88.16 | 25.35 |
| NDF | 32.84 | 50.97 |
| ADF | 19.04 | 45.09 |
| ADL | 4.84 | 32.10 |
| CP | 17.76 | 13.23 |
| Ash | 7.81 | 7.00 |
| EE | 3.70 | 5.05 |
| Total polyphenols, g GAE/100 of DM | - | 1.48 |
| FAME, g/100 g of total FAME | ||
| C14:0 | 0.23 | 0.29 |
| C16:0 | 17.44 | 14.06 |
| C18:0 | 3.25 | 4.02 |
| C18:1 cis-9 | 25.90 | 17.18 |
| C18:1 cis-11 | 1.13 | 1.02 |
| C18:2 n-6 (LA) | 44.27 | 55.35 |
| C18:3 n-6 | 0.08 | 0.00 |
| C18:3 n-3 (LNA) | 4.49 | 2.94 |
| C22:0 | 0.44 | 0.96 |
| C20:4 n-6 (ARA) | 0.14 | 0.26 |
| C24:0 | 0.55 | 0.45 |
| C22:5 n-3 (DPA) | 0.13 | 0.66 |
| Groups of FA (g/100 g of total FAME) | ||
| SCFA | 0.00 | 0.08 |
| MCFA | 18.15 | 15.32 |
| LCFA | 81.85 | 84.60 |
| SFA | 22.54 | 21.09 |
| MUFA | 28.08 | 19.28 |
| PUFA | 49.38 | 59.63 |
| UFA | 77.46 | 78.91 |
| PUFA n-6 | 44.71 | 55.81 |
| PUFA n-3 | 4.62 | 3.68 |
| n-6/n-3 | 9.67 | 15.16 |
| n-3/n-6 | 0.10 | 0.07 |
| Diet | SEM | p-Value | |||
|---|---|---|---|---|---|
| CTR | Low-GP | High-GP | |||
| Slaughter weight, g | 2706.25 | 2791.88 | 2696.88 | 37.25 | 0.84 |
| Hot Carcass weight, g | 1604.38 | 1628.13 | 1588.13 | 26.35 | 0.84 |
| Cold carcass weight, g | 1563.13 | 1600.63 | 1558.75 | 25.40 | 0.78 |
| Hot carcass yield, % | 59.23 | 58.31 | 58.91 | 0.53 | 0.79 |
| Cold carcass yield, % | 57.74 | 57.32 | 57.81 | 0.52 | 0.93 |
| pH LD, 45 min | 6.51 | 6.38 | 6.41 | 0.05 | 0.57 |
| pH LD, 24 h | 5.74 | 5.66 | 5.68 | 0.03 | 0.59 |
| pH stomach | 1.61 | 1.61 | 1.62 | 0.04 | 0.99 |
| pH cecum | 5.94 | 5.85 | 5.88 | 0.04 | 0.74 |
| Items | Diet | SEM | p-Value | ||
|---|---|---|---|---|---|
| CTR | Low-GP | High-GP | |||
| Moisture, % | 75.33 | 75.45 | 75.65 | 0.133 | 0.63 |
| Protein, % | 22.62 | 22.23 | 22.11 | 0.130 | 0.25 |
| Fat, % | 0.91 | 0.99 | 0.93 | 0.037 | 0.67 |
| Individual FA (g/100 g of FA) | |||||
| C14:0 | 1.17 | 1.07 | 1.23 | 0.098 | 0.80 |
| isoC15:0 | 0.03 | 0.04 | 0.04 | 0.002 | 0.88 |
| anteisoC15:0 | 0.06 | 0.06 | 0.06 | 0.003 | 0.98 |
| C15:0 | 0.51 | 0.51 | 0.50 | 0.007 | 0.67 |
| isoC16:0 | 0.13 | 0.13 | 0.13 | 0.007 | 0.99 |
| C16:0 | 29.06 | 28.64 | 28.78 | 0.231 | 0.76 |
| isoC17:0 | 0.02 | 0.02 | 0.02 | 0.001 | 0.04 |
| C16:1 cis-7 | 0.35 | 0.37 | 0.38 | 0.015 | 0.74 |
| anteisoC17:0 | 0.05 | 0.04 | 0.05 | 0.003 | 0.42 |
| C16:1 cis-9 | 1.20 | 1.38 | 1.53 | 0.198 | 0.81 |
| C17:0 | 0.63 | 0.61 | 0.60 | 0.010 | 0.38 |
| C18:0 | 9.07 | 8.90 | 8.75 | 0.159 | 0.74 |
| C18:1 cis-9 | 23.24 | 22.74 | 23.04 | 0.476 | 0.92 |
| C18:1 cis-11 | 1.45 | 1.58 | 1.46 | 0.031 | 0.19 |
| C18:2 n-6 (LA) | 23.80 | 24.55 | 24.48 | 0.255 | 0.44 |
| C20:0 | 0.09 | 0.09 | 0.09 | 0.004 | 0.69 |
| C18:3 n-6 | 0.07 | 0.08 | 0.07 | 0.002 | 0.49 |
| C20:1 cis-11 | 0.18 | 0.20 | 0.19 | 0.007 | 0.67 |
| C18:3 n-3 (LNA) | 0.94 | 0.93 | 1.00 | 0.073 | 0.92 |
| C20:2 n-6 | 0.30 | 0.33 | 0.35 | 0.015 | 0.42 |
| C20:3 n-6 | 0.60 | 0.63 | 0.62 | 0.032 | 0.94 |
| C20:4 n-6 (ARA) | 5.92 | 5.96 | 5.59 | 0.423 | 0.93 |
| C20:5 n-3 (EPA) | 0.26 | 0.29 | 0.27 | 0.016 | 0.77 |
| C22:5 n-3 (DPA) | 0.71 | 0.71 | 0.64 | 0.052 | 0.84 |
| C22:6 n-3 (DHA) | 0.11 | 0.12 | 0.11 | 0.010 | 0.91 |
| Groups of FA (g/100 g of FA) | |||||
| MCFA | 33.20 | 32.85 | 33.31 | 0.327 | 0.85 |
| LCFA | 66.80 | 67.15 | 66.69 | 0.327 | 0.85 |
| SFA | 40.81 | 40.10 | 40.24 | 0.272 | 0.55 |
| MUFA | 26.42 | 26.26 | 26.60 | 0.621 | 0.98 |
| PUFA | 32.76 | 33.64 | 33.16 | 0.586 | 0.84 |
| UFA | 59.19 | 59.90 | 59.76 | 0.272 | 0.55 |
| OCFA | 1.14 | 1.12 | 1.09 | 0.015 | 0.46 |
| BCFA | 0.29 | 0.28 | 0.29 | 0.013 | 0.99 |
| OBCFA | 1.43 | 1.40 | 1.38 | 0.021 | 0.70 |
| PUFA n-6 | 30.70 | 31.55 | 31.11 | 0.578 | 0.85 |
| PUFA n-3 | 2.02 | 2.04 | 2.02 | 0.033 | 0.96 |
| n-6/n-3 | 15.27 | 15.50 | 15.47 | 0.333 | 0.96 |
| n-3/n-6 | 0.07 | 0.07 | 0.07 | 0.001 | 0.98 |
| Nutritional indices | |||||
| AI | 0.57 | 0.55 | 0.57 | 0.009 | 0.65 |
| TI | 0.87 | 0.85 | 0.86 | 0.012 | 0.68 |
| h/H | 1.89 | 1.94 | 1.91 | 0.024 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carta, S.; Chessa, R.; Rubattu, R.; Nudda, A.; Battacone, G. The Use of Grape By-Products as a Feed Additive Enhances the Oxidative Stability of Rabbit Meat. Vet. Sci. 2025, 12, 148. https://doi.org/10.3390/vetsci12020148
Carta S, Chessa R, Rubattu R, Nudda A, Battacone G. The Use of Grape By-Products as a Feed Additive Enhances the Oxidative Stability of Rabbit Meat. Veterinary Sciences. 2025; 12(2):148. https://doi.org/10.3390/vetsci12020148
Chicago/Turabian StyleCarta, Silvia, Riccardo Chessa, Roberto Rubattu, Anna Nudda, and Gianni Battacone. 2025. "The Use of Grape By-Products as a Feed Additive Enhances the Oxidative Stability of Rabbit Meat" Veterinary Sciences 12, no. 2: 148. https://doi.org/10.3390/vetsci12020148
APA StyleCarta, S., Chessa, R., Rubattu, R., Nudda, A., & Battacone, G. (2025). The Use of Grape By-Products as a Feed Additive Enhances the Oxidative Stability of Rabbit Meat. Veterinary Sciences, 12(2), 148. https://doi.org/10.3390/vetsci12020148








