Effects of Dietary Supplementation of Omega-3 PUFA Enriched Fish Oil During Late-Pregnancy and Lactation on Reproductive Performance, Immune Activity and Fecal Microbiota Composition in Postpartum Sows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Care
2.2. Data Recording
2.3. Estrus Identification
2.4. Sample Collection
2.5. Hormonal Assay
2.6. 16S rRNA Sequencing
2.7. Statistical Analysis
3. Results
3.1. Dietary Administration of Fish Oil Shortened the Weaning-Estrous Interval in Sows
3.2. Dietary Administration of Fish Oil-Altered Estrus-Related Factors in Lactating Sows
3.3. Dietary Administration of Fish Oil Enhanced Immune Activity and Antioxidant Capacity in Lactating Sows
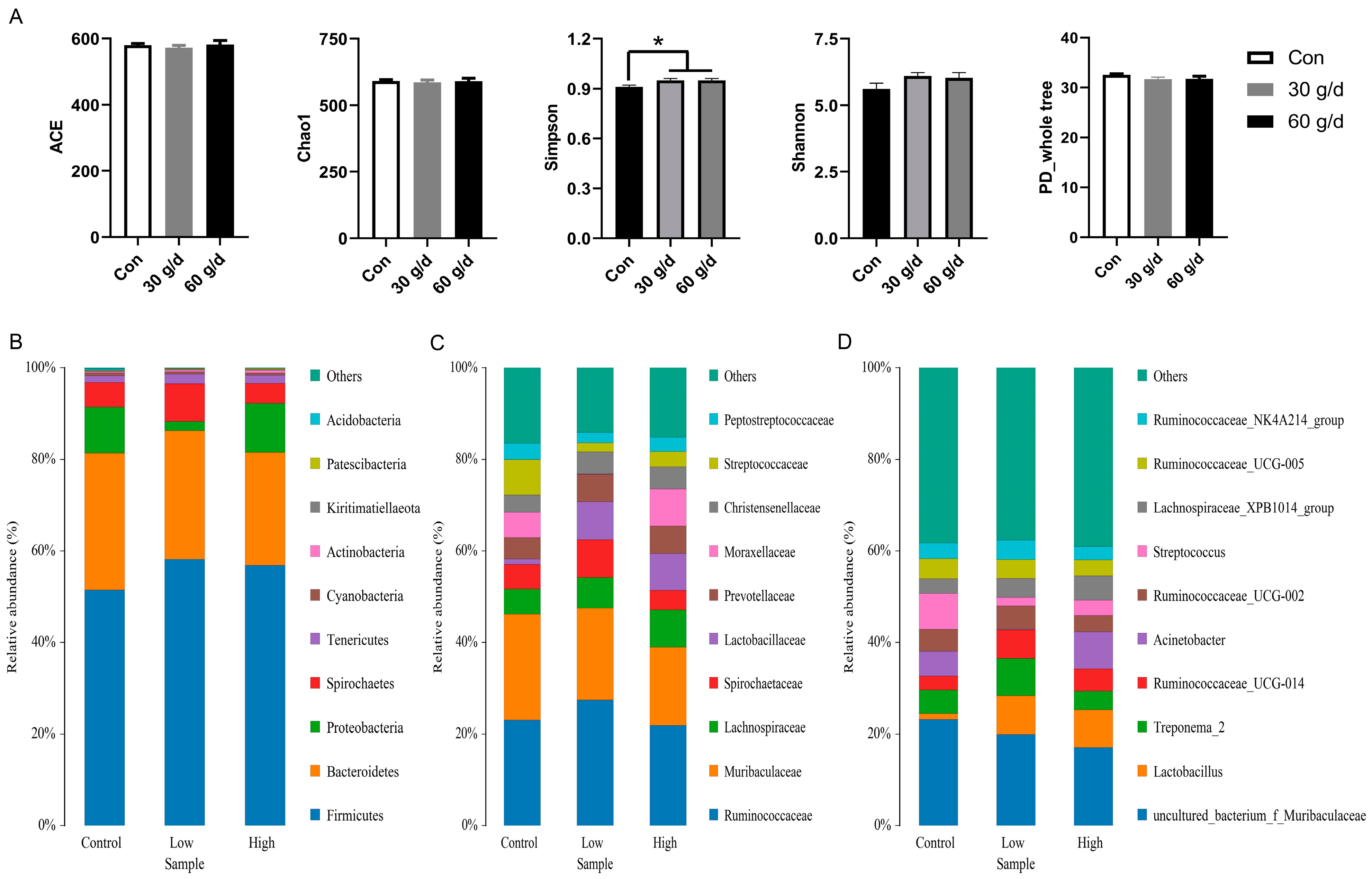
3.4. Dietary Administration of Fish Oil Improved Fecal Health in Lactating Sows
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Item | Gestation Diet | Lactation Diet |
|---|---|---|
| Corn (%) | 48.80 | 63.20 |
| 46 Soybean meal (%) | - | 20.00 |
| 43 Soybean meal (%) | 12.70 | - |
| Wheat flour (%) | - | 5.00 |
| Wheat bran (%) | 20.00 | 3.00 |
| Beet pulp (%) | 8.00 | - |
| Alfalfa meal (%) | 4.00 | - |
| Oil (%) | 1.00 | 3.00 |
| Yeast culture (%) | 1.00 | - |
| Import fish powder (%) | - | 2.00 |
| CaHPO4 (%) | 1.30 | 1.20 |
| Mineral feed (%) | 0.80 | 1.20 |
| Salt (%) | 0.40 | 0.40 |
| Premix (%) | 2.00 | 1.00 |
| Digestible energy (MJ/kg) | 12.77 | 13.82 |
| Crude protein (%) | 16.49 | 18.39 |
| Coarse ash (%) | 5.49 | 5.08 |
| Calcium (%) | 0.99 | 0.62 |
| Total phosphorus (%) | 0.64 | 0.58 |
| Lysine (%) | 0.6 | 1.1 |
| Methionine (%) | 0.19 | 0.27 |
| Threonine (%) | 0.48 | 0.64 |
| Name | Abbreviation | The Concentration (μg/mL) |
|---|---|---|
| Eicosapentaenoate (EPA) | C20:5N3 | 5936.289 |
| Docosahexaenoate (DHA) | C22:6N3 | 13,333.422 |
| Caproate | C6:0 | 27.627 |
| Caprylate | C8:0 | 31.908 |
| Caprate | C10:0 | 23.095 |
| Unndecanoate | C11:0 | 31.923 |
| Laurate | C12:0 | 133.506 |
| Tridecanoate | C13:0 | 74.947 |
| Myristate | C14:0 | 3575.214 |
| Myristelaidate | C14:1T | 74.888 |
| Myristoleate | C14:1 | 31.249 |
| Pentadecanoate | C15:0 | 642.742 |
| 10-Transpentadecenoate | C15:1T | 30.084 |
| 10-Pentadecenoate | C15:1 | 28.121 |
| Palmitate | C16:0 | 14,792.208 |
| Palmitelaidate | C16:1T | 288.805 |
| Palmitoleate | C16:1 | 4494.199 |
| Heptadecanoate | C17:0 | 693.754 |
| 10-Transsheptadecenoate | C17:1T | 116.88 |
| 10-Heptadecenoate | C17:1 | 305.8 |
| Stearate | C18:0 | 3929.074 |
| Petroselaidate | C18:1N12T | 47.085 |
| Elaidate | C18:1N9T | 32.811 |
| Transvaccenate | C18:1N7T | 207.986 |
| Petroselinate | C18:1N12 | 599.501 |
| Oleate | C18:1N9C | 11,906.25 |
| Vaccenate | C18:1N7 | 2023.044 |
| Linoelaidate | C18:2N6T | 34.346 |
| 7-Transnonadecenoate | C19:1N12T | 85.128 |
| 10-Transnonadecenoate | C19:1N9T | 42.827 |
| Linoleate | C18:2N6 | 6162.907 |
| Arachidate | C20:0 | 312.336 |
| Gamma Linolenate | C18:3N6 | 153.646 |
| Trans 11-Eicosenoate | C20:1T | 34.057 |
| 11-Eicosenoate | C20:1 | 792.849 |
| Alpha Linolenate | C18:3N3 | 1502.661 |
| Heneicosanoate | C21:0 | 92.092 |
| 11-14 Eicosadienoate | C20:2 | 207.826 |
| Behenate | C22:0 | 172.247 |
| Homogamma Linolenate | C20:3N6 | 116.971 |
| Brassidate | C22:1N9T | 25.023 |
| Erucate | C22:1N9 | 1078.639 |
| 11-14-17 Eicosatrienoate | C20:3N3 | 470.27 |
| Arachidonate | C20:4N6 | 1031.33 |
| Tricosanoate | C23:0 | 103.727 |
| Docosadienoate | C22:2 | 43.381 |
| Lignocerate | C24:0 | 131.36 |
| Nervonoate | C24:1 | 255.125 |
| Docosatetraenoate | C22:4 | 160.603 |
| Docosapentaenoate | C22:5N6 | 873.062 |
| Docosapentaenoate | C22:5N3 | 1049.842 |
| %CV | ||
|---|---|---|
| Intra-Assay | Inter-Assay | |
| Pig PROG ELISA Kit (HS034-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <4.2% | <6.3% |
| Pig OT ELISA Kit (HS304-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <4.3% | <6.7% |
| Pig E2 ELISA Kit (HS316-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <5.7% | <8.9% |
| Pig PRL ELISA Kit (HS303-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <6.7% | <8.5% |
| Pig IgA ELISA Kit (HS175-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <5.2% | <7.3% |
| Pig IgG ELISA Kit (HS173-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <4.9% | <8.5% |
| Pig IgM ELISA Kit (HS170-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <5.4% | <7.3% |
| Pig IL-1β ELISA Kit (HS350-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <5.9% | <8.2% |
| Pig IL-6 ELISA Kit (HS343-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <5.3% | <8.7% |
| Pig IL-10 ELISA Kit (HS355-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <6.1% | <8.9% |
| Pig zonulin ELISA Kit (HS427-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <5.8% | <8.3% |
| Pig TNF-α ELISA Kit (HS015-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <5.1% | <7.8% |
| Pig IFN-γ ELISA Kit (HS359-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <4.3% | <9.5% |
| Pig T3 ELISA Kit (HS139-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <5.3% | <7.2% |
| Pig T4 ELISA Kit (HS419-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <6.1% | <7.8% |
| Pig IGF1 ELISA Kit (HS052-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <6.8% | <9.6% |
| Pig INS ELISA Kit (HS057-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <6.7% | <8.3% |
| Pig Cortisol ELISA Kit (HS157-Pg; Shanghai Hengyuan Biological, Shanghai, China) | <6.3% | <7.8% |
| Pig T-AOC ELISA Kit (A015; Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) | <6.83% | <3.2% |
| Pig SOD ELISA Kit (A001; Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) | <3.52% | <1.7% |
| Pig GSH-Px ELISA Kit (A005; Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) | <4.34% | <3.1% |
| Pig CAT ELISA Kit (A007; Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) | <4.94% | <1.9% |
| Pig MDA ELISA Kit (A003-1; Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) | <4.11% | <3.5% |
| Name | Abbreviation | Fish Oil, g per Day per Sow | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| 0 | 30 g/d | 60 g/d | ||||
| Caproate | C6:0 | 0.09 a | 0.06 ab | 0.02 b | 0.029 | 0.022 |
| Caprylate | C8:0 | 0.15 a | 0.13 b | 0.13 b | 0.008 | 0.025 |
| Caprate | C10:0 | 0.14 | 0.11 | 0.12 | 0.022 | 0.221 |
| Unndecanoate | C11:0 | 0.230 a | 0.23 ab | 0.22 b | 0.005 | 0.065 |
| Laurate | C12:0 | 0.333 | 0.29 | 0.38 | 0.084 | 0.361 |
| Tridecanoate | C13:0 | 0.59 | 0.57 | 0.57 | 0.016 | 0.263 |
| Myristate | C14:0 | 2.54 | 2.40 | 3.63 | 0.880 | 0.151 |
| Myristelaidate | C14:1 T | 0.96 a | 0.92 ab | 0.76 b | 0.125 | 0.102 |
| Pentadecanoate | C15:0 | 2.42 | 2.42 | 2.49 | 0.302 | 0.918 |
| 10-Transpentadecenoate | C15:1T | 2.21 a | 1.96 ab | 1.86 b | 0.184 | 0.068 |
| 10-Pentadecenoate | C15:1 | 5.14 | 4.60 | 4.44 | 0.443 | 0.116 |
| Palmitate | C16:0 | 152.64 | 139.68 | 160.26 | 38.689 | 0.756 |
| Palmitelaidate | C16:1T | 1.06 | 1.01 | 1.05 | 0.119 | 0.849 |
| Palmitoleate | C16:1 | 6.30 | 4.62 | 5.66 | 2.926 | 0.723 |
| Heptadecanoate | C17:0 | 5.32 | 5.34 | 4.68 | 1.155 | 0.663 |
| 10-Transsheptadecenoate | C17:1T | 2.11 a | 1.96 ab | 1.84 b | 0.147 | 0.076 |
| 10-Heptadecenoate | C17:1 | 2.71 | 2.51 | 2.25 | 0.454 | 0.388 |
| Stearate | C18:0 | 131.04 | 98.626 | 106.24 | 45.814 | 0.596 |
| Petroselaidate | C18:1N12T | 3.15 a | 2.47 ab | 1.99 b | 0.511 | 0.031 |
| Elaidate | C18:1N9T | 1.23 | 1.07 | 1.05 | 0.118 | 0.110 |
| Transvaccenate | C18:1N7T | 5.57 a | 4.85 ab | 4.55 b | 0.508 | 0.016 |
| Petroselinate | C18:1N12 | 50.05 | 37.97 | 51.33 | 19.408 | 0.581 |
| Oleate | C18:1N9C | 87.10 | 58.95 | 62.83 | 44.684 | 0.642 |
| Vaccenate | C18:1N7 | 7.99 | 5.99 | 7.13 | 3.447 | 0.722 |
| Linoelaidate | C18:2N6T | 0.96 a | 0.82 ab | 0.73 b | 0.114 | 0.050 |
| 7-Transnonadecenoate | C19:1N12T | 1.54 a | 1.44 ab | 1.24 b | 0.138 | 0.034 |
| 10-Transnonadecenoate | C19:1N9T | 2.59 a | 2.63 ab | 4.04 b | 0.620 | 0.014 |
| Arachidate | C20:0 | 1.46 | 1.30 | 1.15 | 0.206 | 0.156 |
| Trans 11-Eicosenoate | C20:1T | 1.96 a | 1.74 ab | 1.50 b | 0.197 | 0.028 |
| 11-Eicosenoate | C20:1 | 2.48 | 2.21 | 2.52 | 0.517 | 0.669 |
| Heneicosanoate | C21:0 | 1.12 a | 1.07 ab | 0.97 b | 0.052 | 0.009 |
| 11-14 Eicosadienoate | C20:2 | 2.29 | 2.15 | 2.02 | 0.364 | 0.614 |
| Behenate | C22:0 | 0.69 | 0.66 | 0.64 | 0.067 | 0.518 |
| Homogamma Linolenate | C20:3N6 | 2.39 | 2.15 | 1.95 | 0.621 | 0.622 |
| Brassidate | C22:1N9T | 1.75 a | 1.57 ab | 1.36 b | 0.195 | 0.055 |
| Erucate | C22:1N9 | 3.02 a | 2.56 ab | 2.21 b | 0.320 | 0.018 |
| 11-14-17 Eicosatrienoate | C20:3N3 | 20.43 | 13.92 | 14.06 | 11.548 | 0.673 |
| Tricosanoate | C23:0 | 1.63 | 1.62 | 1.61 | 0.019 | 0.155 |
| Docosadienoate | C22:2 | 1.57 a | 1.45 ab | 1.24 b | 0.160 | 0.047 |
| Lignocerate | C24:0 | 0.78 | 0.77 | 0.74 | 0.070 | 0.688 |
| Nervonoate | C24:1 | 1.67 a | 1.55 ab | 1.37 b | 0.176 | 0.104 |
| Docosatetraenoate | C22:4 | 2.29 | 1.64 | 1.41 | 0.634 | 0.181 |
| Docosapentaenoate | C22:5N6 | 1.32 | 9.19 | 13.95 | 15.397 | 0.528 |
| Docosapentaenoate | C22:5N3 | 5.72 | 5.08 | 4.01 | 2.827 | 0.700 |
| Fish Oil, g per Day per Sow | SEM | p-Value | ||||
|---|---|---|---|---|---|---|
| 0 | 30 g/d | 60 g/d | ||||
| Body weight | Day 90 of gestation, kg | 212.00 | 212.33 | 212.73 | 11.061 | 0.984 |
| Day 110 of gestation, kg | 226.07 | 228.33 | 227.93 | 11.448 | 0.846 | |
| Day 21 of lactation, kg | 200.20 | 205.27 | 207.07 | 13.000 | 0.334 | |
| Loss during lactation, mm | 25.93 | 23.07 | 20.87 | 10.646 | 0.433 | |
| Feed intake, kg | Gestation | 66.62 | 66.32 | 65.69 | 1.392 | 0.190 |
| Lactation | 98.75 | 100.91 | 97.67 | 6.093 | 0.339 | |
| Total (during late gestation and lactation) | 165.37 | 167.24 | 163.36 | 6.460 | 0.270 | |
| Backfat thickness | Day 110 of gestation, mm | 22.80 | 21.60 | 22.60 | 2.276 | 0.312 |
| Day 21 of lactation, mm | 18.93 | 18.33 | 18.60 | 2.785 | 0.840 | |
| Loss during lactation, mm | 3.87 | 3.27 | 4.00 | 2.313 | 0.655 | |
| Percentage of Loss during lactation, % | 16.95 | 15.06 | 17.53 | 10.075 | 0.783 | |
| EPA + DHA, % of Diet | SEM | p-Value | |||
|---|---|---|---|---|---|
| 0 | 0.2 | 0.4 | |||
| Glutamate transaminase, U/L | 18.14 | 24.43 | 19.14 | 6.456 | 0.281 |
| Glutathane transaminase, U/L | 21.71 | 21.29 | 14.71 | 12.119 | 0.601 |
| Alkaline phosphatase, U/L | 27.43 | 35.14 | 30.14 | 9.580 | 0.450 |
| Total protein, g/L | 29.83 | 38.49 | 33.94 | 9.718 | 0.390 |
| Albumin, g/L | 13.40 | 17.83 | 15.59 | 4.545 | 0.328 |
| Globulin, g/L | 16.43 | 20.66 | 18.36 | 5.332 | 0.470 |
| Albumin/Globulin | 0.83 | 0.87 | 0.86 | 0.106 | 0.786 |
| Lactate dehydrogenase, U/L | 339.29 | 268.43 | 229.43 | 136.554 | 0.451 |
| Creatine Kinase, U/L | 1065.57 | 706.14 | 524.57 | 675.109 | 0.451 |
| Urea, mmol/L | 2.51 | 2.94 | 2.73 | 0.710 | 0.648 |
| Creatinine, μmol/L | 62.46 | 81.86 | 79.41 | 20.890 | 0.302 |
| Glucose, mmol/L | 1.78 | 2.47 | 2.24 | 0.606 | 0.215 |
| Triglycerides, mmol/L | 0.19 | 0.23 | 0.29 | 0.108 | 0.349 |
| Total cholesterol, mmol/L | 1.03 | 1.39 | 1.14 | 0.368 | 0.305 |
| HDL-c, mmol/L | 0.34 | 0.53 | 0.43 | 0.149 | 0.194 |
| LDL-c, mmol/L | 0.40 | 0.56 | 0.44 | 0.158 | 0.294 |
References
- Sakali, A.K.; Bargiota, A.; Fatouros, I.G.; Jamurtas, A.; Macut, D.; Mastorakos, G.; Papagianni, M. Effects on Puberty of Nutrition-Mediated Endocrine Disruptors Employed in Agriculture. Nutrients 2021, 13, 4184. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shi, J.; Dong, Y.; Li, Z.; Wu, X.; Lin, Y.; Che, L.; Li, J.; Feng, B.; Fang, Z.; et al. Fecal bacteria and metabolite responses to dietary lysozyme in a sow model from late gestation until lactation. Sci. Rep. 2020, 10, 3210. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.D.; Lui, J.F.; Albuquerque, L.G.; Alves, D.N. Environmental and genetic factors affecting the weaning-estrus interval in sows. Genet. Mol. Res. GMR 2011, 10, 2692–2701. [Google Scholar] [CrossRef] [PubMed]
- Cerri, R.L.A.; Burnett, T.A.; Madureira, A.M.L.; Silper, B.F.; Denis-Robichaud, J.; LeBlanc, S.; Cooke, R.F.; Vasconcelos, J.L.M. Symposium review: Linking activity-sensor data and physiology to improve dairy cow fertility. J. Dairy Sci. 2021, 104, 1220–1231. [Google Scholar] [CrossRef]
- Politano, C.A.; Lopez-Berroa, J. Omega-3 Fatty Acids and Fecundation, Pregnancy and Breastfeeding. Rev. Bras. Ginecol. Obs. 2020, 42, 160–164. [Google Scholar] [CrossRef]
- Teeli, A.S.; Sheikh, P.A.; Patra, M.K.; Singh, D.; Kumar, B.; Kumar, H.; Singh, S.K.; Verma, M.R.; Krishnaswamy, N. Effect of dietary n-3 polyunsaturated rich fish oil supplementation on ovarian function and interferon stimulated genes in the repeat breeding cow. Anim. Reprod. Sci. 2019, 211, 106230. [Google Scholar] [CrossRef] [PubMed]
- Mahla, A.S.; Bunkar, S.K.; Kumawat, B.L.; Kumar Saxena, V.; Selvaraju, S.; Bhatt, R.S.; Singh, R.; Kumar, A. Dietary n-3 PUFA augments pre-ovulatory follicle turnover and prolificacy in well-fed ewes. Anim. Reprod. Sci. 2023, 252, 107231. [Google Scholar] [CrossRef]
- Yadav, D.; Singh, A.K.; Kumar, B.; Mahla, A.S.; Singh, S.K.; Patra, M.K.; Kumar, H.; Kumar, S.; Tyagi, B.; Verma, M.R.; et al. Effect of n-3 PUFA-rich fish oil supplementation during late gestation on kidding, uterine involution and resumption of follicular activity in goat. Reprod. Domest. Anim. 2019, 54, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Q.; Li, Y.; Tang, Z.; Sun, W.; Zhang, X.; Sun, J.; Sun, Z. Comparative effects of dietary supplementations with sodium butyrate, medium-chain fatty acids, and n-3 polyunsaturated fatty acids in late pregnancy and lactation on the reproductive performance of sows and growth performance of suckling piglets. J. Anim. Sci. 2019, 97, 4256–4267. [Google Scholar] [CrossRef]
- Lam, I.P.Y.; Fong, J.J. Are fecal samples an appropriate proxy for amphibian intestinal microbiota? Ecol. Evol. 2024, 14, e10862. [Google Scholar] [CrossRef]
- Yan, W.; Sun, C.; Zheng, J.; Wen, C.; Ji, C.; Zhang, D.; Chen, Y.; Hou, Z.; Yang, N. Efficacy of Fecal Sampling as a Gut Proxy in the Study of Chicken Gut Microbiota. Front. Microbiol. 2019, 10, 2126. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef]
- Chen, S.; Xue, X.; Zhang, H.; Huang, X.; Lin, X.; He, J.; Chen, L.; Luo, S.; Gao, J. Jianwei Shoutai Pills alleviates miscarriage by modulating gut microbial production of BAs and NLRP3-inflammasome at the maternal-fetal interface of rats. Phytomedicine 2024, 135, 156000. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, H.; Zhou, Y.; Yan, M.; Chen, D.; Yang, M.; Xiao, S.; Chen, C.; Huang, L. Identification of the gut microbiota biomarkers associated with heat cycle and failure to enter oestrus in gilts. Microb. Biotechnol. 2021, 14, 1316–1330. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Ke, S.; Huang, X.; Fang, S.; He, M.; Fu, H.; Chen, C.; Huang, L. Gut and Vagina Microbiota Associated With Estrus Return of Weaning Sows and Its Correlation With the Changes in Serum Metabolites. Front. Microbiol. 2021, 12, 690091. [Google Scholar] [CrossRef]
- Gu, X.; Chen, J.; Li, H.; Song, Z.; Chang, L.; He, X.; Fan, Z. Isomaltooligosaccharide and Bacillus regulate the duration of farrowing and weaning-estrous interval in sows during the perinatal period by changing the gut microbiota of sows. Anim. Nutr. 2021, 7, 72–83. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, R.; Kanis, E.; Verstegen, M.W.; Knol, E.F. Genetic parameters and predicted selection results for maternal traits related to lactation efficiency in sows. J. Anim. Sci. 2008, 86, 1067–1080. [Google Scholar] [CrossRef]
- Lawlor, P.G.; Lynch, P.B.; Gardiner, G.E.; Caffrey, P.J.; O′Doherty, J.V. Effect of liquid feeding weaned pigs on growth performance to harvest. J. Anim. Sci. 2002, 80, 1725–1735. [Google Scholar] [CrossRef]
- Chang, Z.; Bo, S.; Xiao, Q.; Wang, Y.; Wu, X.; He, Y.; Iqbal, M.; Ye, Y.; Shang, P. Remodeling of the microbiota improves the environmental adaptability and disease resistance in Tibetan pigs. Front. Microbiol. 2022, 13, 1055146. [Google Scholar] [CrossRef]
- Shen, Y.; Wan, H.; Zhu, J.; Fang, Z.; Che, L.; Xu, S.; Lin, Y.; Li, J.; Wu, D. Fish Oil and Olive Oil Supplementation in Late Pregnancy and Lactation Differentially Affect Oxidative Stress and Inflammation in Sows and Piglets. Lipids 2015, 50, 647–658. [Google Scholar] [CrossRef]
- Chen, B.; Ji, X.; Zhang, L.; Hou, Z.; Li, C.; Tong, Y. Fish oil supplementation improves pregnancy outcomes and size of the newborn: A meta-analysis of 21 randomized controlled trials. J. Matern. Fetal Neonatal Med. 2016, 29, 2017–2027. [Google Scholar] [CrossRef]
- Masina, M.; Medithi, S.; Muley, A. Impact of Maternal Essential Fatty Acid Intake on the Birth Weight of Infants. J. Mother. Child. 2023, 27, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Petrone, R.C.; Williams, K.A.; Estienne, M.J. Effects of dietary menhaden oil on growth and reproduction in gilts farrowed by sows that consumed diets containing menhaden oil during gestation and lactation. Animal 2019, 13, 1944–1951. [Google Scholar] [CrossRef]
- Lauridsen, C. Effects of dietary fatty acids on gut health and function of pigs pre- and post-weaning. J. Anim. Sci. 2020, 98, skaa086. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, X.; Song, Y.; Xiao, L.; Wen, J.; Ding, H.; Zhao, S.; Qiao, D.; Zhang, B.; Niu, A.; et al. Effect of altrenogest treatment before weaning on reproductive performance and production efficiency in primiparous and multiparous sows. Porc. Health Manag. 2024, 10, 25. [Google Scholar] [CrossRef]
- Thaker, M.Y.; Bilkei, G. Lactation weight loss influences subsequent reproductive performance of sows. Anim. Reprod. Sci. 2005, 88, 309–318. [Google Scholar] [CrossRef]
- Mendoza, S.M.; Boyd, R.D.; Remus, J.; Wilcock, P.; Martinez, G.E.; van Heugten, E. Sow performance in response to natural betaine fed during lactation and post-weaning during summer and non-summer months. J. Anim. Sci. Biotechnol. 2020, 11, 69. [Google Scholar] [CrossRef]
- Madej, A.; Lang, A.; Brandt, Y.; Kindahl, H.; Madsen, M.T.; Einarsson, S. Factors regulating ovarian function in pigs. Domest. Anim. Endocrinol. 2005, 29, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.M.; Ramirez, J.L.; Matamoros, I.A.; Bennett, W.A. Estrogen induces estrus unaccompanied by a preovulatory surge in luteinizing hormone in suckled sows. Biol. Reprod. 1988, 38, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Stopa, L.R.S.; de Souza, C.F.; Martins, A.B.; Lopes, G.M.; Costa, N.O.; Gerardin, D.C.C.; de Carvalho, G.G.; Zaia, D.A.M.; Zaia, C.; Uchoa, E.T.; et al. Neonatal overfeeding reduces estradiol plasma levels and disrupts noradrenergic-kisspeptin-GnRH pathway and fertility in adult female rats. Mol. Cell Endocrinol. 2021, 524, 111147. [Google Scholar] [CrossRef] [PubMed]
- Khedr, N.F. Fish oil and wheat-germ oil supplementation restores ovarian function in streptozotocin-diabetic rats. Reprod. Fertil. Dev. 2017, 29, 1689–1698. [Google Scholar] [CrossRef]
- Kouba, J.M.; Burns, T.A.; Webel, S.K. Effect of dietary supplementation with long-chain n-3 fatty acids during late gestation and early lactation on mare and foal plasma fatty acid composition, milk fatty acid composition, and mare reproductive variables. Anim. Reprod. Sci. 2019, 203, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Cools, A.; Maes, D.; Papadopoulos, G.; Vandermeiren, J.A.; Meyer, E.; Demeyere, K.; De Smet, S.; Janssens, G.P. Dose-response effect of fish oil substitution in parturition feed on erythrocyte membrane characteristics and sow performance. J. Anim. Physiol. Anim. Nutr. 2011, 95, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.J.; Binder, C.J. Malondialdehyde epitopes as mediators of sterile inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Morkl, S.; Lackner, S.; Meinitzer, A.; Mangge, H.; Lehofer, M.; Halwachs, B.; Gorkiewicz, G.; Kashofer, K.; Painold, A.; Holl, A.K.; et al. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur. J. Nutr. 2018, 57, 2985–2997. [Google Scholar] [CrossRef]
- Tanghe, S.; Millet, S.; De Smet, S. Echium oil and linseed oil as alternatives for fish oil in the maternal diet: Blood fatty acid profiles and oxidative status of sows and piglets. J. Anim. Sci. 2013, 91, 3253–3264. [Google Scholar] [CrossRef] [PubMed]
- Kazuo, M. Prevention of Fish Oil Oxidation. J. Oleo Sci. 2019, 68, 1–11. [Google Scholar] [CrossRef]
- Yin, J.; Lee, K.Y.; Kim, J.K.; Kim, I.H. Effects of different n-6 to n-3 polyunsaturated fatty acids ratio on reproductive performance, fecal microbiota and nutrient digestibility of gestation-lactating sows and suckling piglets. Anim. Sci. J. 2017, 88, 1744–1752. [Google Scholar] [CrossRef]
- Xu, K.; Bai, M.; Liu, H.; Duan, Y.; Zhou, X.; Wu, X.; Liao, P.; Li, T.; Yin, Y. Gut microbiota and blood metabolomics in weaning multiparous sows: Associations with oestrous. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Li, M.; Lei, H.; Jiang, X.; Tu, W.; Lu, Y.; Xia, D. Butyric acid regulates progesterone and estradiol secretion via cAMP signaling pathway in porcine granulosa cells. J. Steroid Biochem. Mol. Biol. 2017, 172, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef]

| Fish Oil, g per Day per Sow | SEM | p-Value | |||
|---|---|---|---|---|---|
| 0 | 30 g/d | 60 g/d | |||
| EPA, μg/mL | 3.34 | 4.97 | 6.47 | 1.916 | 0.082 |
| DHA, μg/mL | 3.49 b | 6.63 ab | 10.51 a | 2.965 | 0.014 |
| ALA, μg/mL | 3.31 | 3.16 | 3.95 | 1.082 | 0.502 |
| ω-3 PUFA, μg/mL | 10.14 | 14.76 | 20.94 | 5.914 | 0.052 |
| LA, μg/mL | 69.52 | 64.69 | 81.68 | 34.772 | 0.736 |
| GLA, μg/mL | 2.51 | 2.29 | 2.05 | 0.780 | 0.660 |
| AA, μg/mL | 23.63 | 16.56 | 14.91 | 14.183 | 0.618 |
| ω-6 PUFA, μg/mL | 95.50 | 83.55 | 98.65 | 49.210 | 0.879 |
| ω-6 PUFA/ω-3 PUFA | 8.72 a | 5.75 b | 4.94 b | 1.328 | 0.004 |
| Fish Oil, g per Day per Sow | SEM | p-Value | |||
|---|---|---|---|---|---|
| 0 | 30 g/d | 60 g/d | |||
| No. born per litter | 13.73 | 13.67 | 15.33 | 2.496 | 0.130 |
| No. born alive | 11.80 | 12.47 | 13.07 | 2.569 | 0.409 |
| No. healthy piglets | 10.67 | 11.53 | 11.60 | 2.303 | 0.471 |
| No. weak piglets | 1.13 | 0.93 | 1.47 | 1.095 | 0.411 |
| No. stillborn | 1.47 | 1.07 | 1.80 | 1.293 | 0.507 |
| Litter weight alive at parturition, kg | 15.31 | 16.70 | 17.16 | 3.948 | 0.419 |
| Average weight of piglets born alive, kg | 1.29 | 1.34 | 1.32 | 0.157 | 0.643 |
| Litter weight at weaning, kg | 73.72 | 80.53 | 81.58 | 10.581 | 0.110 |
| Duration of farrowing, min | 253.33 | 225.00 | 214.00 | 116.790 | 0.639 |
| WEI, day | 6.07 a | 4.27 b | 4.40 b | 1.486 | 0.003 |
| Lactation capacity | 58.44 | 63.98 | 65.45 | 8.711 | 0.121 |
| Lactation volume, kg | 233.76 | 255.92 | 261.80 | 34.842 | 0.121 |
| Fish Oil, g per Day per Sow | SEM | p-Value | |||
|---|---|---|---|---|---|
| 0 | 30 g/d | 60 g/d | |||
| Estradiol E2, pmol/L | 123.24 b | 142.15 a | 142.95 a | 13.10 | 0.018 |
| Prolactin, ng/L | 54.23 b | 61.52 b | 75.24 a | 6.84 | 0.001 |
| Progesterone, pmol/L | 1699.94 | 1593.37 | 1658.56 | 175.34 | 0.530 |
| Oxytocin, ng/L | 46.40 | 47.89 | 48.07 | 5.32 | 0.817 |
| IGF1, μg/L | 9.54 | 9.86 | 10.15 | 1.05 | 0.671 |
| Insulin, mIU/L | 55.64 | 50.30 | 51.06 | 4.19 | 0.059 |
| T3, pmol/L | 99.20 | 102.38 | 109.41 | 13.08 | 0.473 |
| T4, pmol/L | 560.32 | 484.03 | 563.71 | 56.84 | 0.080 |
| Cortisol, μg/L | 117.69 a | 88.84 b | 108.65 a | 13.26 | 0.014 |
| Fish Oil, g per Day per Sow | SEM | p-Value | |||
|---|---|---|---|---|---|
| 0 | 30 g/d | 60 g/d | |||
| IgA, μg/mL | 13.48 b | 14.67 ab | 16.98 a | 1.84 | 0.032 |
| IgG, μg/mL | 184.57 | 208.05 | 215.57 | 22.95 | 0.125 |
| IgM, μg/mL | 17.11 | 18.05 | 19.05 | 1.62 | 0.205 |
| Fish Oil, g per Day per Sow | SEM | p-Value | |||
|---|---|---|---|---|---|
| 0 | 30 g/d | 60 g/d | |||
| T-AOC, mmol/L | 0.23 | 0.31 | 0.34 | 0.10 | 0.147 |
| MDA, nmol/mL | 4.38 b | 7.44 a | 7.79 a | 1.63 | 0.002 |
| SOD, U/mL | 106.21 b | 157.10 a | 157.65 a | 25.02 | 0.001 |
| GSH-Px, U/mL | 388.42 b | 423.45 a | 395.88 ab | 25.38 | 0.045 |
| CAT, U/mL | 1.95 b | 3.02 b | 5.64 a | 1.65 | 0.002 |
| IL-1β, ng/L | 16.10 b | 19.34 ab | 21.14 a | 2.36 | 0.017 |
| IL-6, ng/L | 1056.37 b | 1181.68 a | 1182.50 a | 75.06 | 0.032 |
| IFN-γ, pg/mL | 1169.69 | 1187.18 | 1299.31 | 158.49 | 0.402 |
| TNF-α, pg/mL | 167.54 | 170.03 | 188.18 | 20.42 | 0.163 |
| IL-10, ng/L | 102.32 | 96.18 | 105.23 | 18.83 | 0.745 |
| CRP,/L | 2287.75 | 2126.03 | 2221.05 | 161.83 | 0.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Z.; An, Y.; Lan, W.; Li, X. Effects of Dietary Supplementation of Omega-3 PUFA Enriched Fish Oil During Late-Pregnancy and Lactation on Reproductive Performance, Immune Activity and Fecal Microbiota Composition in Postpartum Sows. Vet. Sci. 2025, 12, 139. https://doi.org/10.3390/vetsci12020139
Ge Z, An Y, Lan W, Li X. Effects of Dietary Supplementation of Omega-3 PUFA Enriched Fish Oil During Late-Pregnancy and Lactation on Reproductive Performance, Immune Activity and Fecal Microbiota Composition in Postpartum Sows. Veterinary Sciences. 2025; 12(2):139. https://doi.org/10.3390/vetsci12020139
Chicago/Turabian StyleGe, Zihao, Yalong An, Wei Lan, and Xiao Li. 2025. "Effects of Dietary Supplementation of Omega-3 PUFA Enriched Fish Oil During Late-Pregnancy and Lactation on Reproductive Performance, Immune Activity and Fecal Microbiota Composition in Postpartum Sows" Veterinary Sciences 12, no. 2: 139. https://doi.org/10.3390/vetsci12020139
APA StyleGe, Z., An, Y., Lan, W., & Li, X. (2025). Effects of Dietary Supplementation of Omega-3 PUFA Enriched Fish Oil During Late-Pregnancy and Lactation on Reproductive Performance, Immune Activity and Fecal Microbiota Composition in Postpartum Sows. Veterinary Sciences, 12(2), 139. https://doi.org/10.3390/vetsci12020139






