Swine-Derived Probiotics and Their Metabolites as an Alternative to Veterinary Antibiotics
Simple Summary
Abstract
1. Probiotics and Their Beneficial Effects
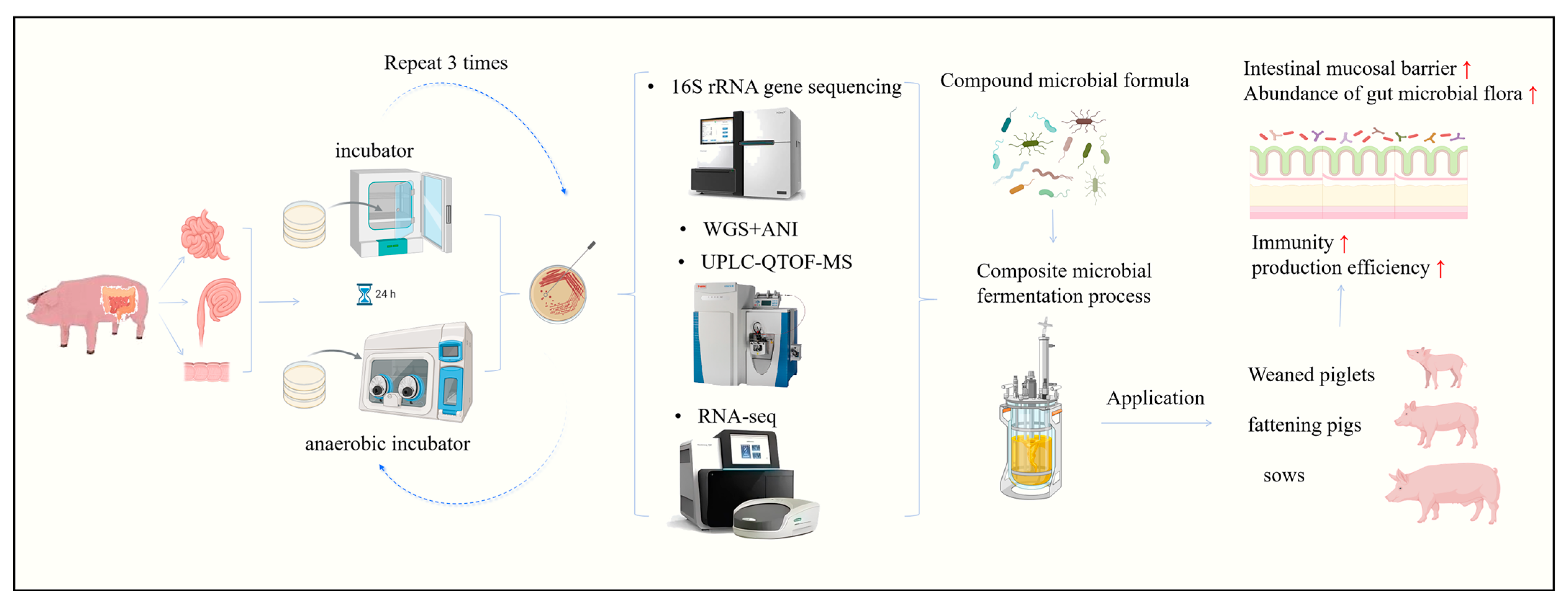
2. Isolation, Identification and Selection of Swine-Derived Beneficial Strains
3. Metabolites Derived from Beneficial Microorganisms of Swine Origin
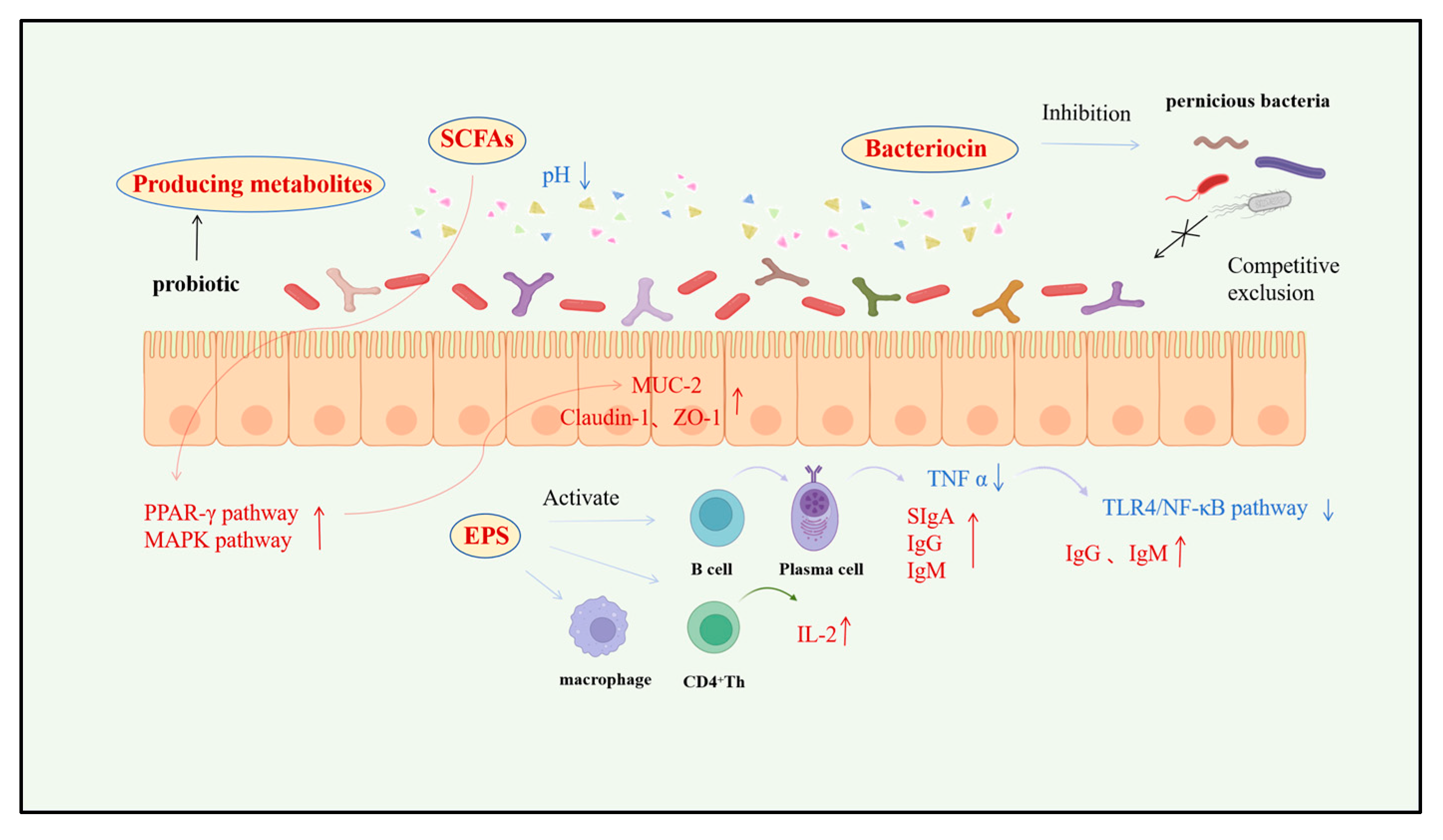
3.1. Short-Chain Fatty Acids (SCFAs)
| Compound | Mechanism | Effects |
|---|---|---|
| Acetic acid | Acetate serves as an energy source for colonic cells, mucosal epithelial cells, and muscle tissue. It also participates in hepatic metabolism and crosses the blood–brain barrier to regulate appetite through central mechanisms. | The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health [50] |
| Propionic acid | Propionate, absorbed in the colon, is primarily used for hepatic gluconeogenesis. It enhances intestinal epithelial activity and suppresses the synthesis of cholesterol and lipids. | Propionate alleviates palmitic acid-induced endoplasmic reticulum stress by enhancing autophagy in calf hepatic cells [51] |
| Butyric acid | Butyrate acts as the main energy substrate for the colon and cecum. It strengthens the intestinal barrier and exerts anti-inflammatory effects via HDAC inhibition, while also modulating immune cell differentiation. | Short-chain fatty acids and their producing organisms: An overlooked therapy for IBD? [52] |
| Pentoic acid | Valerate inhibits Th17 cell proliferation and IL-17A production, downregulates key Th17-related genes, and promotes IL-10 expression along with metabolic reprogramming, thereby reducing pathogenicity. | The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes [53] |
3.2. Antimicrobial Peptides
| Compound | Mechanism | Effect |
|---|---|---|
| Bacteriocins | Proteinaceous toxins that inhibit the growth of closely related bacterial strains and foodborne pathogens by forming pores in the cell membrane, disrupting cell wall synthesis, or inhibiting enzyme activity. | Bacteriocins: Properties and potential use as antimicrobials [68] |
| Bioactive Peptides | Peptides released during the fermentation of milk, legumes, or other proteins by probiotics. Exhibits antihypertensive (ACE-inhibition), antioxidant, antimicrobial, immunomodulatory, and opioid-like activities. | Bioactive peptides from meat: Current status on production, biological activity, safety, and regulatory framework [69] |
| Surface Layer Proteins | Crystalline protein structures coating the cell surface. Contribute to adhesion to the intestinal epithelium, competitive exclusion of pathogens, modulation of immune responses, and maintenance of epithelial barrier function. | Lactobacillus surface layer proteins: structure, function and applications [70] |
3.3. Exopolysaccharides (EPS)
| Compound | Mechanism | Effects |
|---|---|---|
| Exopolysaccharides (EPS) | High-molecular-weight sugar polymers secreted into the environment. Form a protective biofilm, aid in adhesion to surfaces, protect the producing bacterium from harsh conditions (acid, bile), and modulate the host immune system towards an anti-inflammatory state. | Exopolysaccharides from probiotic bacteria and their health potential [84] |
| Lipopolysaccharides (LPS) (from specific probiotic strains) | Component of the outer membrane of Gram-negative bacteria. Probiotic-derived LPS often has a less inflammatory structure (penta-acylated lipid A) that can act as an antagonist to more inflammatory LPS from pathogens, thereby training the immune system and reducing excessive inflammation. | The role of lipopolysaccharide in the development of atopy in humans [85] |
| Capsular Polysaccharides (CPS) | Tightly bound polysaccharide layer surrounding the cell. Provides physical protection against phagocytosis and antimicrobial peptides, facilitates biofilm formation, and can directly interact with host pattern-recognition receptors to elicit immunomodulatory effects. | The Intestinal Commensal, Bacteroides fragilis, Modulates Host Responses to Viral Infection and Therapy: Lessons for Exploration during Mycobacterium tuberculosis Infection [86] |
3.4. Vitamins and Coenzymes
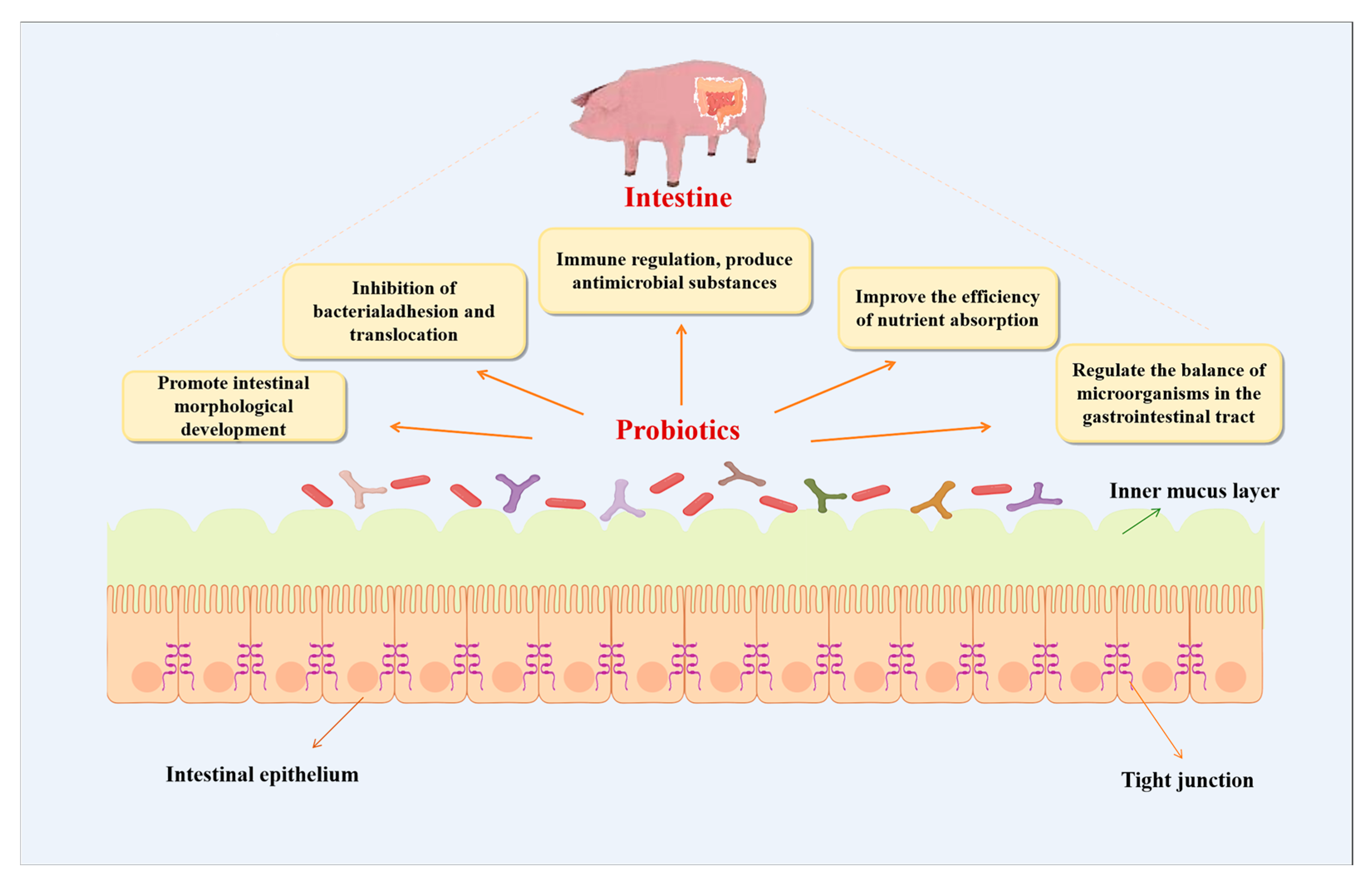
4. Mechanisms and Efficacy of Swine-Derived Probiotic Metabolites
4.1. Enhancement of Intestinal Morphological Development and Physical Barrier Function
4.2. Immunomodulation and Antimicrobial Substance Production
4.3. Enhanced Nutrient Absorption Efficiency and Metabolic Pathway Optimization
4.4. Modulation of Gastrointestinal Microbial Balance and Intestinal Health Maintenance
5. Application of Swine Beneficial Strains and Their Metabolites as Antibiotic Alternatives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leng, S.M. Formation and control of antibiotic resistance under intensive breeding mode. Chin. J. Anim. Husb. Vet. Med. 2024, 12, 25–27. [Google Scholar]
- Checcucci, A.; Trevisi, P.; Luise, D.; Modesto, M.; Blasioli, S.; Braschi, I.; Mattarelli, P. Exploring the Animal Waste Resistome: The Spread of Antimicrobial Resistance Genes Through the Use of Livestock Manure. Front. Microbiol. 2020, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X. The impact of antibiotic drug resistance on pig farming industry. China Anim. Health 2022, 24, 3–4. [Google Scholar]
- Li, C.Y. Five years of resistance: Challenges and innovations in China’s livestock industry. GDF 2024, 33, 15–17. [Google Scholar]
- Guo, H.W. Common feed replacement products: Effects and advantages and disadvantages. J. Anim. Sci. Vet. Sci. Inform. 2023, 3, 189–191. [Google Scholar]
- Zhang, J.S.; Zhu, Y.F.; Hai, L.; Nan, J.D.; Chen, G.W.; Hao, C.H.; Jia, B.; Zhang, B.; Wang, L.K.; Zhang, G.H.; et al. Advances in the Mechanism of Action and Application of Probiotics in Pig Production. J. Anim. Feed Sci. 2024, 45, 29–34. [Google Scholar]
- Kelsic, E.D.; Zhao, J.; Vetsigian, K.; Kishony, R. Counteraction of antibiotic production and degradation stabilizes microbial communities. Nature 2015, 521, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S.; Yang, Y.Y.; Zhang, Y.L.; Liu, M.Y.; Hou, Y.T.; Han, D.D.; Meng, X.B. The Application of Microecological Preparations in Livestock Production. Shandong J. Anim. Sci. Vet. Med. 2025, 46, 36–40. [Google Scholar]
- Cai, Y.; Wu, G.; Yang, H.; Fang, Y.; Cheng, Z.; Zhan, P. The Mechanism and Application Status of Probiotics in Animal Health Management. China Anim. Husb. Vet. Med. 2025, 52, 2101–2114. [Google Scholar]
- Wang, Q.; Sun, Q.; Qi, R.; Wang, J.; Qiu, X.; Liu, Z.; Huang, J. Effects of Lactobacillus plantarum on the intestinal morphology, intestinal barrier function and microbiota composition of suckling piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1908–1918. [Google Scholar] [CrossRef]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The Functional Roles of Lactobacillus acidophilus in Different Physiological and Pathological Processes. J. Microbiol. Biotechnol. 2022, 32, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.J.; Healy, S.; O’Toole, P.W.; Murphy, E.F.; Cotter, P.D. The probiotic L. casei LC-XCAL™ improves metabolic health in a diet-induced obesity mouse model without altering the microbiome. Gut Microbes. 2020, 12, 1704141. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Wang, M.F.; Chang, C.C.; Huang, S.Y.; Pan, C.H.; Yeh, Y.T.; Huang, C.H.; Chan, C.H.; Huang, H.Y. Lacticaseibacillus paracasei PS23 Effectively Modulates Gut Microbiota Composition and Improves Gastrointestinal Function in Aged SAMP8 Mice. Nutrients 2021, 13, 1116. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, X.; Hao, H.; Liu, Q.; Zhou, Z.; Liang, X.; Liu, T.; Gong, P.; Zhang, L.; Zhai, Z.; et al. Lactobacillus rhamnosus GG Derived Extracellular Vesicles Modulate Gut Microbiota and Attenuate Inflammatory in DSS-Induced Colitis Mice. Nutrients 2021, 13, 3319. [Google Scholar] [CrossRef]
- Martoni, C.J.; Srivastava, S.; Damholt, A.; Leyer, G.J. Efficacy and dose response of Lactiplantibacillus plantarum in diarrhea-predominant irritable bowel syndrome. World J. Gastroenterol. 2023, 29, 4451–4465. [Google Scholar] [CrossRef]
- Cheng, J.; Laitila, A.; Ouwehand, A.C. Bifidobacterium animalis subsp. lactis HN019 Effects on Gut Health: A Review. Front. Nutr. 2021, 8, 790561. [Google Scholar]
- Moreno-Muñoz, J.A.; Ojeda, J.D.; López, J.J. A Probiotic Bacterium with Activity against the Most Frequent Bacteria and Viruses Causing Pediatric Diarrhea: Bifidobacterium longum subsp. infantis CECT 7210 (B. infantis IM1). Microorganisms 2024, 12, 1183. [Google Scholar]
- Li, Y.; Li, Q.; Yuan, R.; Wang, Y.; Guo, C.; Wang, L. Bifidobacterium breve-derived indole-3-lactic acid ameliorates colitis-associated tumorigenesis by directing the differentiation of immature colonic macrophages. Theranostics 2024, 14, 2719–2735. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Zhang, J.; Sun, F.; Duan, L. Efficacy of Probiotics for Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 859967. [Google Scholar] [CrossRef]
- Stülke, J.; Grüppen, A.; Bramkamp, M.; Pelzer, S. Bacillus subtilis, a Swiss Army Knife in Science and Biotechnology. J. Bacteriol. 2023, 205, e0010223. [Google Scholar] [CrossRef]
- Acosta-Rodríguez-Bueno, C.P.; Abreu y Abreu, A.T.; Guarner, F.; Guno, M.J.V.; Pehlivanoğlu, E.; Perez, M., 3rd. Bacillus clausii for Gastrointestinal Disorders: A Narrative Literature Review. Adv. Ther. 2022, 39, 4854–4874. [Google Scholar]
- Zhou, X.; Willems, R.J.L.; Friedrich, A.W.; Rossen, J.W.A.; Bathoorn, E. Enterococcus faecium: From microbiological insights to practical recommendations for infection control and diagnostics. Antimicrob. Resist. Infect. Control 2020, 9, 130. [Google Scholar] [CrossRef]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef]
- Pang, Y.; Xu, X.; Li, D.; Ge, Y.; Liang, J.; Xiong, X.; Wen, S.; Li, X.; Wang, Y.; Li, X.; et al. Impact of Clostridium butyricum HADIG-CB003 dietary supplementation on the gut microbiota of Kunming mice. Appl. Microbiol. Biotechnol. 2025, 109, 237. [Google Scholar] [CrossRef] [PubMed]
- Aw, W.; Fukuda, S. Protective effects of bifidobacteria against enteropathogens. Microb. Biotechnol. 2019, 12, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.B.; Bertolli, S.K.; Mougous, J.D. The Central Role of Interbacterial Antagonism in Bacterial Life. Curr. Biol. 2020, 30, R1203–R1214. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, B.; Sun, J.; Liu, Z.; Chen, H.; Ge, L.; Chen, D. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 61. [Google Scholar] [CrossRef]
- Bao, G.; Yu, X.; Wang, X.; Ye, P.; Yin, H.; Hu, T.; Cao, Z. Study on the Isolation, Identification and Probiotic Characteristics of Lactic Acid Bacteria from Free-Range Local Pigs in Yunnan. Anim. Nutr. 2023, 35, 5418–5429. [Google Scholar]
- Dowarah, R.; Verma, A.K.; Agarwal, N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: A review. Anim. Nutr. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Qiao, S. Advances in research on solid-state fermented feed and its utilization: The pioneer of private customization for intestinal microorganisms. Anim. Nutr. 2021, 7, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, C.; Wang, J.; Hu, C.; Ji, F.; Xie, J.; Yang, Y.; Yu, X.; Diao, X.; Lv, R. Effects of Dietary Probiotics and Acidifiers on the Production Performance, Colostrum Components, Serum Antioxidant Activity and Hormone Levels, and Gene Expression in Mammary Tissue of Lactating Sows. Animals 2023, 13, 1536. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, G. Screening of Porcine Probiotics and research on their probiotic characteristics. Gansu Anim. Vet. Sci. 2024, 54, 77–80. [Google Scholar]
- Marchwińska, K.; Gwiazdowska, D. Isolation and probiotic potential of lactic acid bacteria from swine feces for feed additive composition. Arch. Microbiol. 2021, 204, 61. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.I.; Jung, M.Y.; Chang, Y.-H.; Kim, S.; Kim, S.-J.; Park, Y.-H. Probiotic properties of Lactobacillus and Bifidobacterium strains isolated from porcine gastrointestinal tract. Appl. Microbiol. Biotechnol. 2007, 74, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Isaacson, R.E. The pig gut microbial diversity: Understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet. Microbiol. 2015, 177, 242–251. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, H.P. The Application of Probiotic genomics in the Screening and Functional Evaluation of Lactic acid Bacteria. J. Chin. Inst. Food Sci. Technol. 2024, 24, 1. [Google Scholar]
- Ma, C.F.; Zhang, J.B.; Zhang, H.N.; Chen, H.Y.; Yang, Q.; Zhang, Q.E. Metabolomics technology and its application in animal production. Prog. Vet. Med. 2025, 46, 120–124. [Google Scholar]
- Zhang, W.; Gao, X.; Cui, Q.; Chen, C.; Liu, Y.; Peng, Y. Analyze the research progress of pig muscle growth and development based on omics technology. J. Chin. J. Anim. Sci. 2024, 60, 14–20. [Google Scholar]
- Holman, D.B.; Kommadath, A.; Tingley, J.P.; Abbott, D.W. Novel insights into the pig gut microbiome using metagenome-assembled genomes. Microbiol. Spectr. 2022, 10, e0238022. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Clarke, D.J.; Morris, J.G. Butyricin 7423: A Bacteriocin Produced by Clostridium butyricum NCIB7423. J. Gen. Microbiol. 1976, 95, 67–77. [Google Scholar] [CrossRef]
- Schilderink, R.; Verseijden, C.; Seppen, J.; Muncan, V.; van den Brink, G.R.; Lambers, T.T.; van Tol, E.A.; de Jonge, W.J. The SCFA butyrate stimulates the epithelial production of retinoic acid via inhibition of epithelial HDAC. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G1138–G1146. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.; Lim, S.D. The Inhibitory Effect of L. plantarum Q180 on Adipocyte Differentiation in 3T3-L1 and Reduction of Adipocyte Size in Mice Fed High-fat Diet. Korean J. Food Sci. Anim. Resour. 2018, 38, 99–109. [Google Scholar]
- Den Besten, G.; Bleeker, A.; Gerding, A.; Van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet–Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Diao, H.; Jiao, A.R.; Yu, B.; Mao, X.B.; Chen, D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr. 2019, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Laszczyńska, M.; Sipak, O.; Walczakiewicz, K.; Mizerski, A.; Rył, A.; Ratajczak, W. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Gao, W.; Fang, Z.; Lei, L.; Ju, L.; Jin, B.; Loor, J.J.; Liang, Y.; Shi, Z.; Shen, T.; Yu, H.; et al. Propionate alleviates palmitic acid–induced endoplasmic reticulum stress by enhancing autophagy in calf hepatic cells. J. Dairy Sci. 2021, 104, 9316–9326. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. short chain fatty acids and its producing organisms: An overlooked therapy for ibd? Ebiomedicine 2021, 66, 103293. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 2019, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Feng, N.; Xi, S.; Xu, J.; Su, Y. Genomics-based analysis of four swine-derived lactic acid bacteria strains and their evaluation as potential probiotics. Mol. Genet. Genom. 2024, 299, 24. [Google Scholar] [CrossRef]
- Gillor, O.; Etzion, A.; Riley, M.A. The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 2008, 81, 591–606. [Google Scholar] [CrossRef]
- Liang, J.F.; Ren, L.X.; Liu, P.; Tian, J.J.; Xiao, Y.B.; Dong, Z.L. The influence of porcine probiotics on the growth performance of fattening pigs. North. Anim. Husb. 2019, 10, 20. [Google Scholar]
- Dinata, R.; Baindara, P. Laterosporulin25: A probiotically produced, novel defensin-like bacteriocin and its immunogenic properties. Int. Immunopharmacol. 2023, 121, 110500. [Google Scholar] [CrossRef] [PubMed]
- Bosák, J.; Hrala, M.; Micenková, L.; Šmajs, D. Non-antibiotic antibacterial peptides and proteins of Escherichia coli: Efficacy and potency of bacteriocins. Expert. Rev. Anti. Infect. Ther. 2020, 19, 309–322. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Sugrue, I.; Ross, R.P.; Hill, C. Bacteriocin diversity, function, discovery and application as antimicrobials. Nat. Rev. Microbiol. 2024, 22, 556–571. [Google Scholar] [CrossRef]
- Du, K. Research Progress on the Antibacterial Characteristics of Lactic Acid Bacteria Bacteriocins and Their Applications in Food. China Brew. 2022, 41, 16–20. [Google Scholar]
- Baindara, P.; Mandal, S.M. Gut-Antimicrobial Peptides: Synergistic Co-Evolution with Antibiotics to Combat Multi-Antibiotic Resistance. Antibiotics 2023, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Ra, Y.E.; Bang, Y.-J. Balancing Act of the Intestinal Antimicrobial Proteins on Gut Microbiota and Health. J. Microbiol. 2024, 62, 167–179. [Google Scholar] [CrossRef]
- Grande Burgos, M.; Pulido, R.; Del Carmen López Aguayo, M.; Gálvez, A.; Lucas, R. The Cyclic Antibacterial Peptide Enterocin AS-48: Isolation, Mode of Action, and Possible Food Applications. Int. J. Mol. Sci. 2014, 5, 22706–22727. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Zhong, Z.; Zhang, W.-P.; Qian, P.-Y. Discovery of cationic nonribosomal peptides as Gram-negative antibiotics through global genome mining. Nat. Commun. 2018, 9, 3273. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Meneguetti, B.T.; Oliveira-Júnior, N.G.; Macedo, M.L.R.; Franco, O.L. Antimicrobial peptide production in response to gut microbiota imbalance. Peptides 2022, 157, 170865. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef] [PubMed]
- Madhu, M.; Kumar, D.; Sirohi, R.; Tarafdar, A.; Dhewa, T.; Aluko, R.E.; Badgujar, P.C.; Awasthi, M.K. Bioactive peptides from meat: Current status on production, biological activity, safety, and regulatory framework. Chemosphere 2022, 307 Pt 1, 135650. [Google Scholar] [CrossRef]
- Hynönen, U.; Palva, A. Lactobacillus surface layer proteins: Structure, function and applications. Appl. Microbiol. Biotechnol. 2013, 97, 5225–5243. [Google Scholar] [CrossRef]
- Salazar, N.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P. Exopolysaccharides Produced by Lactic Acid Bacteria and Bifidobacteria as Fermentable Substrates by the Intestinal Microbiota. Crit. Rev. Food Sci. Nutr. 2015, 56, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Dey, P. Bacterial exopolysaccharides as emerging bioactive macromolecules: From fundamentals to applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, I.; Ktari, N.; Ben Slima, S.; Triki, M.; Bardaa, S.; Mnif, H.; Ben Salah, R. Evaluation of dermal wound healing activity and in vitro antibacterial and antioxidant activities of a new exopolysaccharide produced by Lactobacillus sp. Ca 6. Int. J. Biol. Macromol. 2017, 103, 194–201. [Google Scholar] [CrossRef]
- Bhagat, N.; Raghav, M.; Dubey, S.; Bedi, N. Bacterial Exopolysaccharides: Insight into Their Role in Plant Abiotic Stress Tolerance. J. Microbiol. Biotechnol. 2021, 31, 1045–1059. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, G.; Tian, Y. Biological activities and applications of exopolysaccharides produced by lactic acid bacteria: A mini-review. World J. Microbiol. Biotechnol. 2023, 39, 155. [Google Scholar] [CrossRef]
- Rahbar Saadat, Y.; Yari Khosroushahi, A.; Pourghassem Gargari, B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Laiño, J.; Villena, J.; Kanmani, P.; Kitazawa, H. Immunoregulatory Effects Triggered by Lactic Acid Bacteria Exopolysaccharides: New Insights into Molecular Interactions with Host Cells. Microorganisms 2016, 4, 27. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef]
- Liang, S.; Wang, X.; Li, C.; Liu, L. Biological Activity of Lactic Acid Bacteria Exopolysaccharides and Their Applications in the Food and Pharmaceutical Industries. Foods 2024, 13, 1621. [Google Scholar] [CrossRef]
- Patten, D.A.; Laws, A.P. Lactobacillus-produced exopolysaccharides and their potential health benefits: A review. Benef. Microbes 2015, 6, 457–471. [Google Scholar] [CrossRef]
- Simpson, A.; Martinez, F.D. The role of lipopolysaccharide in the development of atopy in humans. Clin. Exp. Allergy 2010, 40, 209–223. [Google Scholar] [CrossRef]
- Eribo, O.A.; du Plessis, N.; Chegou, N.N. The Intestinal Commensal, Bacteroides fragilis, Modulates Host Responses to Viral Infection and Therapy: Lessons for Exploration during Mycobacterium tuberculosis Infection. Infect. Immun. 2022, 90, e0032121. [Google Scholar] [CrossRef] [PubMed]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of Surface Exopolysaccharides From Bifidobacterium and Lactobacillus Within the Intestinal Environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef] [PubMed]
- Olesen, S.V.; Rajabi, N.; Svensson, B.; Olsen, C.A.; Madsen, A.S. An NAD+-Dependent Sirtuin Depropionylase and Deacetylase (Sir2La) from the Probiotic Bacterium Lactobacillus acidophilus NCFM. Biochemistry 2018, 57, 3903–3915. [Google Scholar] [CrossRef]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef]
- Powers, H.J.; Hill, M.H.; Mushtaq, S.; Dainty, J.R.; Majsak-Newman, G.; Williams, E.A. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). Am. J. Clin. Nutr. 2011, 93, 1274–1284. [Google Scholar] [CrossRef]
- Kennedy, D. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Fusaro, M.; Cosmai, L.; Evenepoel, P.; Nickolas, T.L.; Cheung, A.M.; Aghi, A.; Tripepi, G.; Plebani, M.; Iervasi, G.; Vettor, R.; et al. Vitamin K and Kidney Transplantation. Nutrients 2020, 12, 2717. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.; Meinitzer, A.; Fritz-Petrin, E.; Enko, D.; Herrmann, M. Role of Vitamin K in Bone and Muscle Metabolism. Calcif. Tissue Int. 2022, 112, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Kirschning, A. On the evolution of coenzyme biosynthesis. Nat. Prod. Rep. 2022, 39, 2175–2199. [Google Scholar] [CrossRef] [PubMed]
- Pereira, W.A.; Franco, S.M.; Reis, I.L.; Mendonça, C.M.N.; Piazentin, A.C.; Azevedo, P.O.S.; Tse, M.L.P.; De Martinis, E.C.P.; Gierus, M.; Oliveira, R.P.S. Beneficial effects of probiotics on the pig production cycle: An overview of clinical impacts and performance. Vet. Microbiol. 2022, 269, 109431. [Google Scholar] [CrossRef]
- Liu, G.; Gu, K.; Liu, X.; Jia, G.; Zhao, H.; Chen, X.; Wang, J. Dietary glutamate enhances intestinal immunity by modulating microbiota and Th17/Treg balance-related immune signaling in piglets after lipopolysaccharide challenge. Food Res. Int. 2023, 166, 112597. [Google Scholar] [CrossRef]
- Wang, W.; Dang, G.; Hao, W.; Li, A.; Zhang, H.; Guan, S.; Ma, T. Dietary Supplementation of Compound Probiotics Improves Intestinal Health by Modulated Microbiota and Its SCFA Products as Alternatives to In-Feed Antibiotics. Probiotics Antimicrob. Proteins 2024, 17, 1969–1984. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, X.; Azad, M.A.K.; Ma, C.; Gao, Q.; He, J.; Kong, X. Dietary supplementation with Bacillus subtilis and xylo-oligosaccharides improves growth performance and intestinal morphology and alters intestinal microbiota and metabolites in weaned piglets. Food Funct. 2021, 12, 5837–5849. [Google Scholar] [CrossRef]
- Ma, S.; Yeom, J.; Lim, Y.-H. Specific activation of hypoxia-inducible factor-2α by propionate metabolism via a β-oxidation-like pathway stimulates MUC2 production in intestinal goblet cells. Biomed. Pharmacother. 2022, 155, 113672. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef]
- Chakravortty, D.; Mao, X.; Gu, C.; Hu, H.; Tang, J.; Chen, D.; Yu, B.; He, J.; Yu, J.; Luo, J.; et al. Dietary Lactobacillus rhamnosus GG Supplementation Improves the Mucosal Barrier Function in the Intestine of Weaned Piglets Challenged by Porcine Rotavirus. PLoS ONE 2016, 11, e0146312. [Google Scholar]
- Yue, Y.X.; Wang, Y.Q.; Yan, F.F.; Li, N.; Li, B.L.; Huo, J.C. Research progress on the Production Methods of Butyric acid and its physiological functions in the Intestinal Tract. Sci. Technol. Food Ind. 2019, 40, 339–344. [Google Scholar]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mikami, D.; Kimura, H.; Kamiyama, K.; Morikawa, Y.; Yokoi, S.; Kasuno, K.; Takahashi, N.; Taniguchi, T.; Iwano, M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem. Biophys. Res. Commun. 2017, 486, 499–505. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, L.; Li, H.; Li, J.; Zhang, Z.; Tan, B.; Wang, J. Ningxiang Pig-Derived Parabacteroides distasonis HNAU0205 Alleviates ETEC-Induced Intestinal Apoptosis, Oxidative Damage, and Inflammation in Piglets. Animals 2024, 14, 2156. [Google Scholar] [CrossRef]
- Luu, M.; Visekruna, A. Short-chain fatty acids: Bacterial messengers modulating the immunometabolism of T cells. Eur. J. Immunol. 2019, 49, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Rodríguez, J.M.; Bongaerts, R.J.; Gasson, M.J.; Horn, N. Nisin-Controlled Extracellular Production of Interleukin-2 inLactococcus lactisStrains, without the Requirement for a Signal Peptide Sequence. Appl. Environ. Microbiol. 2007, 73, 7781–7784. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Liu, Y.; Zhang, T.; Liu, Y.; Zhang, Z.; Yi, H. Bacteriocin-Producing Lactiplantibacillus plantarum YRL45 Enhances Intestinal Immunity and Regulates Gut Microbiota in Mice. Nutrients 2023, 15, 3437. [Google Scholar] [CrossRef]
- Wang, K.; Cao, G.; Zhang, H.; Li, Q.; Yang, C. Effects of Clostridium butyricum and Enterococcus faecalison growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019, 10, 7844–7854. [Google Scholar] [CrossRef]
- Mizumachi, K.; Aoki, R.; Ohmori, H.; Saeki, M.; Kawashima, T. Effect of fermented liquid diet prepared with Lactobacillus plantarum LQ80 on the immune response in weaning pigs. Animal 2009, 3, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Han, J.; Zhu, S.; Wang, Y.; Zhang, W.; Wu, Z. Structural elucidation of the exopolysaccharide from Streptococcus thermophilus XJ53 and the effect of its molecular weight on immune activity. Int. J. Biol. Macromol. 2023, 230, 123177. [Google Scholar] [CrossRef]
- Kavitake, D.; Devi, P.B.; Delattre, C.; Reddy, G.B.; Shetty, P.H. Exopolysaccharides produced by Enterococcus genus—An overview. Int. J. Biol. Macromol. 2023, 226, 111–120. [Google Scholar] [CrossRef]
- Kaushik, A.; Kest, H.; Sood, M.; Steussy, B.; Thieman, C.; Gupta, S. Biofilm Producing Methicillin-Resistant Staphylococcus aureus (MRSA) Infections in Humans: Clinical Implications and Management. Pathogens 2024, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Woodward, A.; Zijlstra, R.T.; Gänzle, M.G.; Griffiths, M.W. Exopolysaccharides Synthesized by Lactobacillus reuteri Protect against Enterotoxigenic Escherichia coli in Piglets. Appl. Environ. Microbiol. 2014, 80, 5752–5760. [Google Scholar] [CrossRef] [PubMed]
- Maalej, H.; Boisset, C.; Hmidet, N.; Colin-Morel, P.; Buon, L.; Nasri, M. Depolymerization of Pseudomonas stutzeri exopolysaccharide upon fermentation as a promising production process of antibacterial compounds. Food Chem. 2017, 227, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Y.-J.; Yang, X.-Y.; Liu, Y.; Wang, J.-Y.; Man, C.-X. Immunoregulatory effects on Caco-2 cells and mice of exopolysaccharides isolated from Lactobacillus acidophilus NCFM. Food Funct. 2014, 5, 3261–3268. [Google Scholar] [CrossRef]
- Engevik, M.A.; Versalovic, J.; Britton, R.A.; Cani, P.D. Biochemical Features of Beneficial Microbes: Foundations for Therapeutic Microbiology. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Järbrink-Sehgal, E.; Andreasson, A. The gut microbiota and mental health in adults. Curr. Opin. Neurobiol. 2020, 62, 102–114. [Google Scholar] [CrossRef]
- Knaus, U.G.; Hertzberger, R.; Pircalabioru, G.G.; Yousefi, S.P.M.; Branco dos Santos, F. Pathogen control at the intestinal mucosa–H2O2to the rescue. Gut microbes 2017, 8, 67–74. [Google Scholar] [CrossRef]
- Xi, M.; Zhao, P.; Li, F.; Bao, H.; Ding, S.; Ji, L.; Yan, J. MicroRNA-16 inhibits the TLR4/NF-kappaB pathway and maintains tight junction integrity in irritable bowel syndrome with diarrhea. J. Biol. Chem. 2022, 298, 102461. [Google Scholar] [CrossRef]
- Yin, H.; Wang, C.; Shuai, Y.; Xie, Z.; Liu, J. Pig-Derived Probiotic Bacillus tequilensis YB-2 Alleviates Intestinal Inflammation and Intestinal Barrier Damage in Colitis Mice by Suppressing the TLR4/NF-kappaB Signaling Pathway. Animals 2024, 14, 1989. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, S.; Yang, C.; Niu, Y.; Feng, J. The protective effects of Bacillus licheniformis against inflammatory responses and intestinal barrier damage in broilers with necrotic enteritis induced by Clostridium perfringens. J. Sci. Food Agric. 2023, 103, 6958–6965. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Yu, F. The functions of probiotics and their application in the pig breeding industry. China Anim. Health 2025, 27, 127–128. [Google Scholar]
- Zhou, Y.L.; Yao, M.X.; Yang, J.J.; Yin, X.H.; Zhang, Y.J.; Zhai, S.Q.; Wang, J. The effects of dietary supplementation with different levels of porcine Lactobacillus plantarum on the digestive function of weaned piglets. Feed Ind. 2025, 46, 51–57. [Google Scholar]
- Chen, W.J.; Luo, W.T.; Huang, J.M.; Chen, C.; Jiang, Y.J.; Wang, Z.D.; Zhao, L.M.; Zhang, H.T. The application of Clostridium butyricum in livestock, poultry and aquaculture. Guangdong J. Anim. Vet. Sci. 2025, 3, 82–89. [Google Scholar]
- Merlo, G.; Bachtel, G.; Sugden, S.G. Gut microbiota, nutrition, and mental health. Front. Nutr. 2024, 11, 1337889. [Google Scholar] [CrossRef] [PubMed]
- Tang, X. Probiotic Roles of Clostridium butyricum in Piglets: Considering Aspects of Intestinal Barrier Function. Animals 2024, 14, 1069. [Google Scholar] [CrossRef]
- Hou, C.; Zeng, X.; Yang, F.; Liu, H.; Qiao, S. Study and use of the probiotic Lactobacillus reuteri in pigs: A review. JASB 2015, 6, 14. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Jiang, Z. Dietary Additive Probiotics Modulation of the Intestinal Microbiota. Protein Pept. Lett. 2017, 24, 382–387. [Google Scholar] [CrossRef]
- Sen, U.; Lützhøft, D.O.; Bækgård, C.; Wimborne, E.; Straarup, E.M.; Pedersen, K.-M.; Swann, J.R.; Pedersen, H.D.; Kristensen, K.; Morgills, L.; et al. High fat diet is associated with gut microbiota dysbiosis and decreased gut microbial derived metabolites related to metabolic health in young Göttingen Minipigs. PLoS ONE 2024, 19, e0298602. [Google Scholar]
- Yun, Y.; Ji, S.; Yu, G.; Jia, P.; Niu, Y.; Zhang, H.; Zhang, X.; Wang, T.; Zhang, L. Effects of Bacillus subtilis on jejunal integrity, redox status, and microbial composition of intrauterine growth restriction suckling piglets. J. Anim. Sci. 2021, 99, skab255. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2016, 61, 1600240. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhao, Y.; Chen, W.; Li, Y.; Han, Y.; Zhang, B.; Pineda, L.; Li, X.; Jiang, X. Effect of an organic acid blend as an antibiotic alternative on growth performance, antioxidant capacity, intestinal barrier function, and fecal microbiota in weaned piglets. J. Anim. Sci. 2024, 102, skae149. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Z.; Azad, M.A.K.; Zhang, W.; Blachier, F.; Wang, Z.; Kong, X. Dietary supplementation with Bacillus mixture modifies the intestinal ecosystem of weaned piglets in an overall beneficial way. J. Appl. Microbiol. 2020, 130, 233–246. [Google Scholar] [CrossRef]
- Bai, P.T.; Kong, J.M.; Pei, T.; Cheng, Z.X.; Ren, Y.H. The effects of compound microecological preparations on the growth performance, immune function and cecal flora structure of weaned piglets. China Anim. Husb. Vet. Med. 2022, 49, 942–952. [Google Scholar]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A Novel Postbiotic From Lactobacillus rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wu, H.; Wang, W.S.; Chen, Z.X.; Xu, E.R.; Chen, L.; Zheng, R.T. The effects of compound microecological preparations on the production performance and nutrient digestion and metabolism of ruminants. Feed Ind. 2025, 46, 37. [Google Scholar]
- Zhao, C.; Li, Y.; Wang, H.; Solomon, A.I.; Wang, S.; Dong, X.; Song, B.; Ren, Z. Dietary supplementation with compound microecological preparations: Effects on the production performance and gut microbiota of lactating female rabbits and their litters. Microbiol. Spectr. 2025, 13, e0006724. [Google Scholar] [CrossRef]
- Tang, J.; Li, W.; Zhou, Q.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Zhuo, Y.; Jiang, X.; Zhao, H.; et al. Effect of heating, microbial fermentation, and enzymatic hydrolysis of soybean meal on growth performance, nutrient digestibility, and intestinal microbiota of weaned piglets. J. Anim. Sci. 2023, 101, skad384. [Google Scholar] [CrossRef]
- Xu, X.; Duarte, M.E.; Kim, S.W. Postbiotic effects of Lactobacillus fermentate on intestinal health, mucosa-associated microbiota, and growth efficiency of nursery pigs challenged with F18+Escherichia coli. J. Anim. Sci. 2022, 100, skac210. [Google Scholar] [CrossRef]
- Yu, J.; Zuo, B.; Li, Q.; Zhao, F.; Wang, J.; Huang, W.; Sun, Z.; Chen, Y. Dietary supplementation with Lactiplantibacillus plantarum P-8 improves the growth performance and gut microbiota of weaned piglets. Microbiol. Spectr. 2024, 12, e0234522. [Google Scholar] [CrossRef]
- Kiernan, D.P.; O’Doherty, J.V.; Sweeney, T. The effect of maternal probiotic or synbiotic supplementation on sow and offspring gastrointestinal microbiota, health, and performance. Animals 2023, 13, 2996. [Google Scholar] [CrossRef]
- Wang, T.; Zhong, Y.Q. Research Progress on the Application of Microbial Fermented Feed in Pig Breeding. China Anim. Ind. 2024, 12, 50–51. [Google Scholar]
- Ma, N.N.; Zhang, X.Q.; Yang, C.L.; Yang, D.D.; Li, J.L.; Wang, X.Z.; Cao, Q.J. Optimization and application evaluation of mixed bacteria compounding in Fermented Feed. Shandong Agric. Sci. 2020, 52, 112–117+125. [Google Scholar]
- Zhang, G.F.; Zeng, W.L.; Zheng, M.L.; Chen, Q.H. Research Progress on the Characteristics of Microbial Fermented Feed and Its Application in Animal Production. Feed Ind. 2023, 44, 79–85. [Google Scholar]
- Kenny, M.; Smidt, H.; Mengheri, E.; Miller, B. Probiotics–do they have a role in the pig industry? Animal 2011, 5, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, X.; Chang, J.; Jiang, X.; Che, L.; Lin, Y.; Zhuo, Y.; Feng, B.; Fang, Z.; Li, J.; et al. Effect of yeast culture supplementation in sows during late gestation and lactation on growth performance, antioxidant properties, and intestinal microorganisms of offspring weaned piglets. Front. Microbiol. 2023, 13, 1105888. [Google Scholar] [CrossRef]
- Vasquez, R.; Oh, J.K.; Song, J.H.; Kang, D.-K. Gut microbiome-produced metabolites in pigs: A review on their biological functions and the influence of probiotics. J. Anim. Sci. Technol. 2022, 64, 671–695. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Wang, Y.J.; Zheng, T.L.; Wang, S.B.; Wang, L.N.; Zheng, C.J.; Wu, R.F.; Jiang, Q.Y.; Shu, G. Screening and identification of endogenous Lactobacillus in porcine intestines and its application in liquid fermented feed. Acta Agric. Univ. Jiangxiensis 2025, 47, 152–166. [Google Scholar]
- Liu, M.; Luan, H.; Qiu, W.; Zhang, Y.; Feng, W.; Xu, W.; Wang, F.; Xuan, H.; Song, P. Antibiotic alternatives in livestock feeding. Sci. Total Environ. 2025, 989, 179867. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.F.; de Glória, R.A.; Americo, M.F.; Freitas, A.D.S.; de Jesus, L.C.L.; Barroso, F.A.L.; Laguna, J.G.; Coelho-Rocha, N.D.; Tavares, L.M.; le Loir, Y. Unlocking the potential of probiotics: A comprehensive review on research, production, and regulation of probiotics. Probiotics Antimicrob. Prot. 2024, 16, 1687–1723. [Google Scholar] [CrossRef]
| Genus | Species | Effect on Animal Health | |
|---|---|---|---|
| Bacteria | Lactobacillus | L. acidophilus | A core probiotic species primarily residing in the small intestine; supports digestive health [11]. |
| L. casei | Widely used in fermented foods; supports gut microbiota balance [12]. | ||
| L. paracasei | Commonly researched for immune support [13]. | ||
| L. rhamnosus | One of the most clinically documented strains; effective against antibiotic-associated diarrhea [14]. | ||
| L. plantarum | Highly adaptable; often studied for managing IBS symptoms [15]. | ||
| Bifidobacterium | B. animalis subsp. lactis | Extremely common in dairy products; supports immune and digestive health [16]. | |
| B. longum subsp. longum | A dominant inhabitant of the adult gut; researched for IBS and anti-inflammatory effects [17]. | ||
| B. breve | Prevalent in infants; studied for the prevention of necrotizing enterocolitis (NEC) [18]. | ||
| Bacillus | B. coagulans | Spore-forming bacterium; highly stable and resistant to heat and acid [19]. | |
| B. subtilis | Spore-forming; used for its enzymatic activity and support for protein digestion and gut health [20]. | ||
| B. clausii | Spore-forming; widely used, especially in Europe, for preventing antibiotic-associated diarrhea [21]. | ||
| Enterococcus | E. faecium | Used for management of diarrhea. Note: Some strains are vancomycin-resistant (VRE), so careful selection is critical [22]. | |
| fungus | Saccharomyces | S. boulardii | The most well-known probiotic yeast; effective against antibiotics [23]. |
| Kluyveromyces | K. marxianus | Used in dairy fermentations; also researched for its potential probiotic properties in gut health [24]. |
| Disease/Disorder | Target Population/Species | Intervention (Dosage/Duration) | Key Efficacy Outcomes & Mechanisms |
|---|---|---|---|
| Necrotic Enteritis | Livestock: Broiler chickens (Poultry). | Antibiotic: Ionophores (Salinomycin) or therapeutic antibiotics (Bacitracin). Probiotic: Bacillus subtilis or Clostridium butyricum spores. ~1–5 × 105 CFU/g of feed. Duration: Throughout the grow-out period. | Antibiotic: Effective for prevention and treatment but faces resistance and withdrawal issues. Probiotic: Reduces mortality and intestinal lesions caused by Clostridium perfringens. Mechanism: Competitive exclusion, production of antimicrobial compounds (bacteriocins), stimulation of immune responses [137]. |
| Acute Infectious Diarrhea | Humans: Infants & Children. Livestock: Neonatal piglets. | Antibiotic: Only for bacterial causes (e.g., E. coli scours). Probiotic: L. rhamnosus GG or Bacillus clausii. ~1–10 Billion CFU/day for 5–7 days. | Antibiotic: Targets specific pathogens but may disrupt microbiota. Probiotic: Shortens duration of diarrhea. Mechanism: Inhibition of pathogen adhesion, secretion of antibacterial substances, support of mucosal immunity [59]. |
| Post-Weaning Diarrhea | Livestock: Weaned piglets. | Antibiotic: ZnO (pharmacological dose) is widely used, but being phased out in the EU. Therapeutic antibiotics (Colistin). Probiotic: Enterococcus faecium or Bacillus licheniformis. ~1–5 × 109 CFU/kg feed. Duration: 2–4 weeks post-weaning. | Antibiotic: Effective but contributes to antimicrobial resistance (AMR). Probiotic: Improves growth performance and reduces diarrhea incidence. Mechanism: Stabilizes gut microbiota during stress, enhances digestive enzyme activity, improves gut barrier integrity [101]. |
| Helicobacter pylori Infection | Humans: Infected adults. | Antibiotic: Standard triple/quadruple therapy (PPI + Amoxicillin + Clarithromycin). Probiotic: As adjunct: Specific strains of Lactobacillus and Bifidobacterium. ~10–20 Billion CFU/day. | Antibiotic: Eradicates pathogen but has high side effect rate. Probiotic: Increases eradication rates and reduces antibiotic side effects (especially diarrhea). Mechanism: May inhibit Hp adhesion, modulate local immune response [138]. |
| Mastitis | Livestock: Dairy cows. | Antibiotic: Intramammary infusions (Cephapirin) during the dry period or for treatment. Probiotic: Intramammary infusions of lactic acid bacteria (e.g., Lactococcus lactis) or oral administration. | Antibiotic: Standard treatment but leads to milk discard periods. Probiotic: Promising for prevention and treatment, reducing somatic cell count (SCC). Mechanism: Competitive exclusion of pathogens like S. aureus, modulation of the local immune response in the mammary gland [137]. |
| General Growth Promotion/Health Maintenance | Livestock: Poultry and Swine. | Antibiotic: Growth-Promoting Antibiotics (AGPs)—now banned/restricted in many regions. Probiotic: Various (Bacillus, Lactobacillus, Enterococcus strains). ~0.5–2 × 109 CFU/kg feed. Duration: Continuous. | Antibiotic: Historically improved growth rate and feed efficiency. Probiotic: Modestly improves Average Daily Gain (ADG) and Feed Conversion Ratio (FCR). Mechanism: Competitive exclusion of pathogens, improved nutrient digestibility, enhanced intestinal health and morphology [126]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Chen, B.; Peng, S.; Mei, G.; Li, M.; Lin, F.; Sun, T.; Li, Z. Swine-Derived Probiotics and Their Metabolites as an Alternative to Veterinary Antibiotics. Vet. Sci. 2025, 12, 1100. https://doi.org/10.3390/vetsci12111100
Zhao M, Chen B, Peng S, Mei G, Li M, Lin F, Sun T, Li Z. Swine-Derived Probiotics and Their Metabolites as an Alternative to Veterinary Antibiotics. Veterinary Sciences. 2025; 12(11):1100. https://doi.org/10.3390/vetsci12111100
Chicago/Turabian StyleZhao, Mengshi, Bihong Chen, Song Peng, Guiheng Mei, Meiqin Li, Fengqiang Lin, Tiecheng Sun, and Zhaolong Li. 2025. "Swine-Derived Probiotics and Their Metabolites as an Alternative to Veterinary Antibiotics" Veterinary Sciences 12, no. 11: 1100. https://doi.org/10.3390/vetsci12111100
APA StyleZhao, M., Chen, B., Peng, S., Mei, G., Li, M., Lin, F., Sun, T., & Li, Z. (2025). Swine-Derived Probiotics and Their Metabolites as an Alternative to Veterinary Antibiotics. Veterinary Sciences, 12(11), 1100. https://doi.org/10.3390/vetsci12111100





