1. Introduction
Small ruminants play a critical role in sustainable livestock production systems globally due to their capacity to thrive in marginal environments and provide essential resources [
1]. Goat products, including high-quality protein, milk, and fiber, contribute significantly to the livelihoods of millions of households, particularly in developing regions where other livestock may struggle to survive [
2]. However, despite these advantages, small ruminants face numerous health challenges that impede their productivity, such as parasitism, metabolic stress, and subclinical diseases. These health issues lead to considerable economic losses and jeopardize the sustainability of production systems that depend on these animals [
3].
The economic impact of health challenges, particularly due to parasitic infections, is significant [
4]. For instance, gastrointestinal nematodes, predominantly
Haemonchus contortus, have been identified as a leading cause of morbidity and mortality in sheep and goats. Effective management of these infections is further complicated by the growing resistance to commonly used anthelmintics, which has been thoroughly documented in numerous studies [
5,
6]. This situation underscores the necessity for continuous monitoring and effective control strategies to alleviate the burden of parasitism in small ruminants.
Traditional monitoring practices, such as body condition scoring, FAMACHA© charting, and periodic Packed Cell Volume (PCV) hematological testing, have served as essential tools for assessing the health status of these animals [
7]. Yet, they also present several shortcomings such as primarily being labor-intensive, subjective for addressing health issues.
The FAMACHA© system has been recognized for its ability to identify anemia due to
H. contortus infections with high sensitivity as to
H. contortus infection [
8]. However, its performance can vary significantly depending on the training of the personnel conducting the assessments, which can lead to discrepancies and render results somewhat subjective [
9]. Additionally, reliance on visual assessments can introduce inconsistencies in health monitoring, making it imperative to seek more robust and precise alternatives. The FAMACHA© chart remains a practical tool for detecting anemia; however, its accuracy depends on the user’s training, experience, and ambient lighting conditions. In this study, a radio-frequency (RF) sensing approach was introduced to generate numerical dielectric measurements reflective of tissue composition and hydration status. This method enables objective, reproducible, and user-independent evaluation of physiological condition. By transitioning from observer-based scoring to data-driven sensing, the system directly mitigates the subjectivity inherent in manual assessment.
As the demand for efficient, welfare-oriented, and climate-resilient livestock production systems increases, there is an emergent need for innovative approaches to animal health monitoring. For example, more sophisticated analytics such as optimizing deductions via Artificial Intelligence for optimal early detection of especially relatively slowly declining animal health conditions, including hemonchosis, under less-than-optimal epidemiological conditions. One promising direction lies in the integration of non-invasive and scalable technologies that can provide real-time health assessments. For example, advances in machine learning applications for predicting anthelmintic resistance and managing gastrointestinal nematode infections show potential for transforming traditional practices into more automated and precise methodologies [
10]. The use of technology, such as digital applications that can analyze data collected from various health indicators (e.g., blood packed cell volume (PCV), fecal egg counts or physical health assessments), including such as the following, which could streamline health monitoring, allowing for timely interventions when health declines are detected [
11].
Radio-Wave Frequency
Radio-frequency (RF) wave sensing represents a promising technology in this regard. The RF waves, occupying the electromagnetic spectrum between 2 GHz and 18 GHz, have unique penetration and interaction properties with biological tissues [
12]. When RF waves pass through or reflect from biological materials, they generate distinctive signatures determined by tissue composition, density, hydration status, and structural properties [
13]. These dielectric properties make RF waves particularly attractive for non-destructive and non-invasive sensing applications. Unlike optical approaches, which are limited to surface-level features, RF signals can penetrate tissues, enabling the detection of subsurface physiological changes [
14,
15]. Moreover, RF systems can operate with minimal sample preparation, offer rapid measurement times, and are adaptable to field conditions, making them well-suited for livestock applications.
The concept of RF-based monitoring has been explored in several agricultural contexts. In cereals and grains, RF spectroscopy has been used to estimate moisture content and bulk density with high accuracy, supporting postharvest management [
16]. Similarly, in poultry science, RF and microwave sensors have been evaluated as alternatives to near-infrared and hyperspectral imaging for detecting muscle myopathies, such as woody breast, due to their ability to capture tissue-level differences in water content and structure [
15]. Translating this principle to small ruminants, RF sensing has the potential to detect early physiological alterations associated with health and welfare conditions such as anemia, hydration deficits, and changes in muscle or fat composition before they are clinically observable [
17]. Thus, the application of RF-based methods could provide producers with a rapid, non-invasive, and scalable system for monitoring animal health in real time.
The rationale for pursuing alternative non-destructive RF (RF-NDT) wave sensing in small ruminant health monitoring is threefold. First, RF technology offers objectivity and repeatability, overcoming the biases and inconsistencies of manual scoring systems. Second, it provides a non-invasive and rapid diagnostic approach, reducing the need for invasive sampling or repeated handling that stresses animals and requires skilled labor. Third, RF sensing aligns with sustainability and welfare goals, as it enables early detection and targeted interventions, thereby reducing mortality, lowering drug use, and improving feed efficiency.
Despite these advantages, gaps in knowledge remain. The application of RF wave sensing in live small ruminants has not been extensively studied, and its ability to discriminate specific health-related physiological changes requires empirical validation. Biological variability across breeds, ages, and production environments introduces challenges for developing robust predictive models [
18,
19,
20]. Furthermore, while RF waves are theoretically well-suited for tissue characterization, their utility in differentiating subtle variations associated with parasitism or metabolic status in live animals has yet to be systematically investigated. These uncertainties highlight the need for proof-of-concept studies to establish baseline evidence for feasibility and to guide future development of integrated animal health monitoring systems.
The present study addresses this gap by evaluating the feasibility of using a RF wave device operating between 2 and 18 GHz as a non-destructive proof-of-concept tool for small ruminant health monitoring. Specifically, this study investigates whether RF wave interactions with biological tissues can provide measurable signatures that may serve as proxies for physiological status. By generating and analyzing RF-based datasets, the goal is to assess the potential of this approach as a foundation for future multimodal sensing platforms. While the present work is exploratory, it is envisioned as a steppingstone toward the development of comprehensive animal health monitoring systems that integrate RF sensing with complementary modalities and AI-driven analytics to enhance precision, sustainability, and resilience in small ruminant production.
2. Materials and Methods
2.1. Animals and Housing
A total of eight intact male Spanish goats (Capra hircus) were used in this proof-of-concept study. The animals were 24 months of age and weighed between 36 and 50 kg at the time of data collection. All goats were maintained at the Fort Valley State University (FVSU) Agriculture Technology Center farm in Fort Valley, Georgia, USA. The animals were raised under standard husbandry conditions, with unrestricted access to fresh water and grazing pastures. Goats were managed on a mixed-grass pasture system that permitted natural exposure to gastrointestinal nematodes during the grazing season (March–September 2023). This natural grazing regime ensured that the animals developed physiological variability in parasitic status and hematological indicators (e.g., PCV), under field conditions while allowing for the evaluation of RF sensing under realistic management scenarios.
2.2. Experimental Design
The proof-of-concept study employed a longitudinal, non-invasive design in which RF measurements were repeatedly collected from each goat at multiple body locations. Although only eight goats were used, the design leveraged repeated within-animal sampling to capture intra- and inter-individual variation in RF signatures over time. Four anatomical sides per goat (left lateral, right lateral, dorsal, and ventral regions) were selected for RF data acquisition, thereby maximizing spatial coverage of body tissues and physiological states. This approach yielded a robust dataset while minimizing animal numbers, in line with the 3Rs principle (Replacement, Reduction, and Refinement) of ethical animal use.
2.3. Device Safety and Certification
The RF sensor unit functions at low power and possesses C1D2 (Class 1 Division 2) certification, guaranteeing safe operation in environments potentially containing combustible gasses or vapors under abnormal conditions. The certification verifies that the device design inhibits sparking, overheating, or igniting, in accordance with safety requirements. This study utilized equipment in conventional animal housing and laboratory environments, devoid of such dangers. The C1D2 certification offers enhanced assurance of electrical safety and non-incendive functionality, hence facilitating its applicability in many agricultural and healthcare fields.
2.4. Radio-Frequency Wave Data Collection
To confirm the physiological validity of the FAMACHA© scoring system, packed cell volume (PCV) was measured using the standard microhematocrit centrifugation method. The mean ± standard deviation of PCV for each FAMACHA© category was 30.74 ± 2.23% (Score 1), 24.99 ± 1.48% (Score 2), and 20.43 ± 1.32% (Score 3) [
21,
22]. The progressive decline in PCV with increasing FAMACHA© scores demonstrated that the field-based classifications accurately reflected the severity of hematological anemia. In this proof-of-concept, FAMACHA© served only as a pragmatic field label; RF is intended as a non-invasive screening adjunct to prioritize animals for confirmatory PCV and/or fecal egg counts, not a replacement.
Radio-frequency wave signals were collected using a custom-designed RF device (Compass technology group, LLC, Alpharetta, GA, USA) consisting of a broadband transmitting–receiving antenna pair and an integrated vector network analyzer module, enabling dielectric reflectometry configured to emit and record signals across a frequency range of 2 to 18 GHz. The device performed a continuous frequency sweep across the 2–18 GHz spectrum, consisting of 1601 evenly spaced frequency steps per scan (
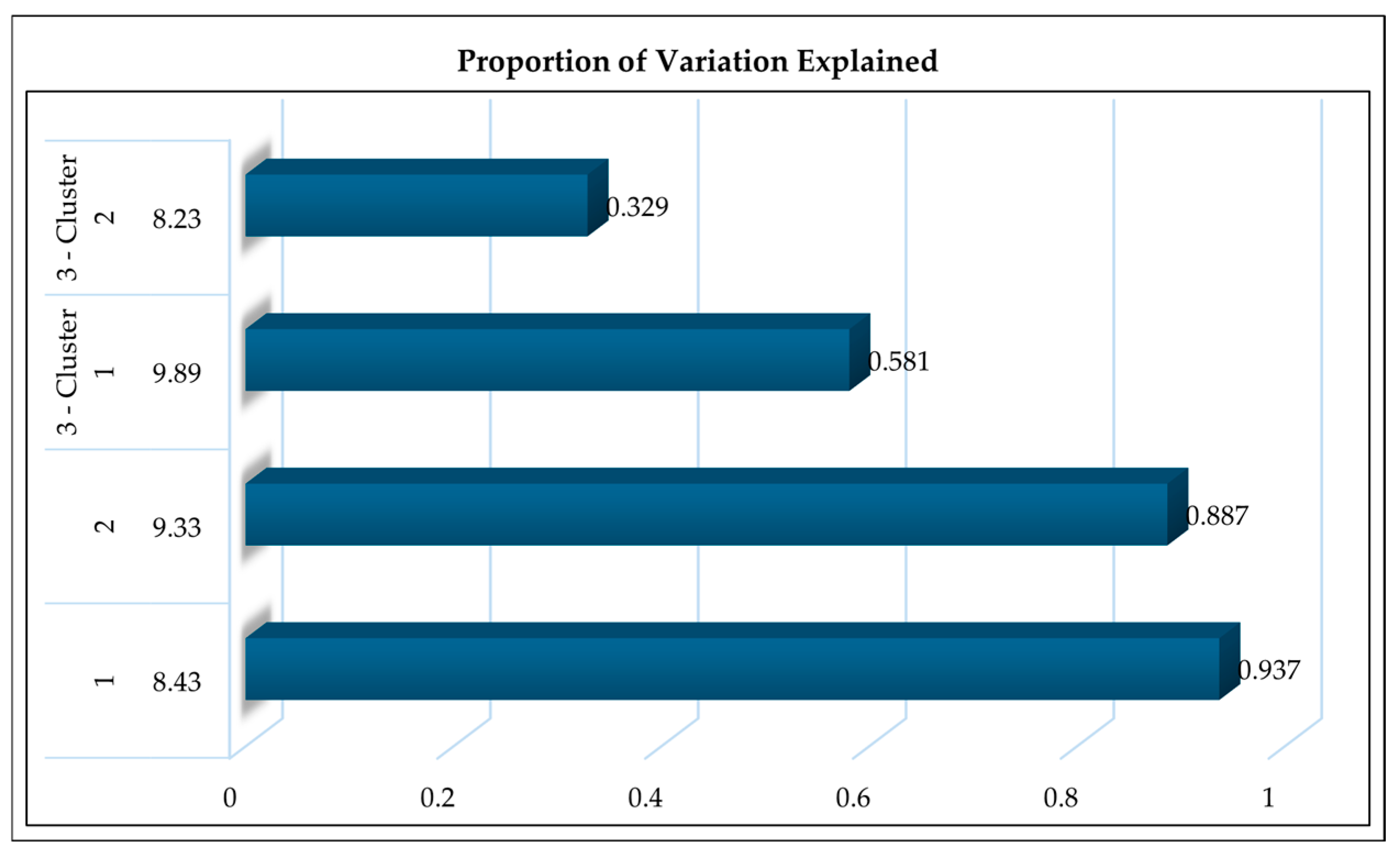
Supplementary File, Figure S1). This configuration enabled high-resolution spectral acquisition suitable for identifying narrowband dielectric changes in biological tissue. From these spectra, the top ten discriminative frequencies (8.23, 8.43, 8.67, 8.89, 9.11, 9.33, 9.56, 9.78, 9.89, and 10.12 GHz) were selected based on false discovery rate (FDR) filtering and Bootstrap Forest screening for downstream modeling.
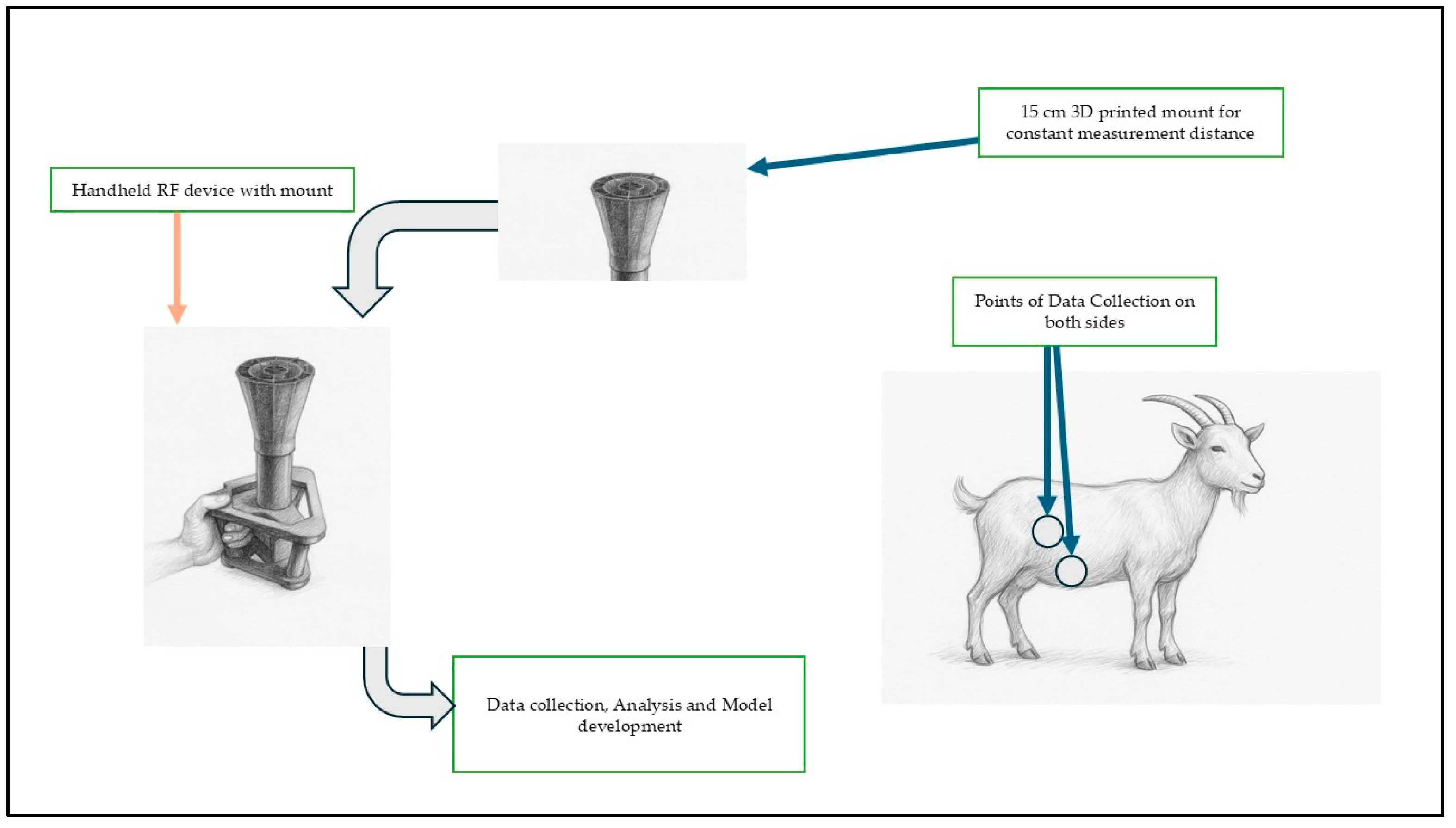
Animals were restrained in a standing posture, and data were collected from left and right ventral regions, and under the stomach area on both sides. Each scan lasted 5–10 s and produced 1601 discrete frequencies on each of the readings across the band, resulting in 6404 data points per animal per session. This spectrum was selected because it spans both ultra-high frequency (UHF) and super-high frequency (SHF) bands, enabling sufficient penetration and interaction with soft tissues, while preserving sensitivity to dielectric properties of biological material. For each anatomical side, a total of 1601 discrete frequencies were scanned. Across all eight goats, the experiment produced a cumulative dataset of 51,232 raw RF observations. Measurements were conducted with the animals in a standing, restrained position to minimize movement noises, and the device was held at a standardized distance (15 cm) and orientation relative to the body surface to ensure measurement consistency (
Figure 1).
2.5. Data Preprocessing
After acquisition, RF data were exported for preprocessing and modeling. Raw frequency-domain readings were cleaned to remove noise from the environment, and prevent motion, or instrument drift, before being filtered, normalized, and smoothed in the frequency domain. The cleaned data were prepared for machine learning, in order to allow frequency signatures to be assessing as predictors of physiological or health states. Although this is a proof-of-concept study, the strict adherence to best practices for RF signal analysis reassures the reliability of our results and supports reproducibility and future integration into predictive analytics pipelines, consistent with Siddique et al. [
15].
2.6. Ethical Considerations
All animal use protocols were reviewed and approved by the Fort Valley State University Agricultural and Laboratory Animal Care and Use Committee (ALACUC approval number F-T-01-2022). The study was designed to comply fully with institutional and national regulations governing animal welfare. In particular, care was taken to reduce conditions that could lead to mortality or distress in the experimental goats, especially those associated with low PCV values caused by parasitic infection. No animals were sacrificed for this study, and all goats were monitored daily by trained personnel for health and welfare throughout the experimental period. The study adhered to the guiding principles of minimizing animal numbers, refining procedures to reduce stress, and replacing invasive techniques with non-invasive monitoring approaches whenever feasible.
3. Data Analysis
The raw RF data collected from the device, consisting of both amplitude and phase components across the 2–18 GHz frequency range, were exported for computational processing. Data preprocessing and statistical analyses were conducted using Python (Ver. 3.10) and JMP Pro 16.0 (SAS Institute Inc., Cary, NC, USA). The analytical workflow was designed to systematically clean, screen, and extract meaningful frequency-domain features associated with physiological variation, while minimizing the risk of spurious findings inherent with high-dimensional datasets [
15].
For consistency across models and a common plotting scale, we treated the ordinal labels (1–3) as evenly spaced and applied a min-max mapping to preserve order and spacing while standardizing the target range for regression and visualization: 1 → 0.0, 2 → 0.5, 3 → 1.0 [
15]. Initial data screening was performed to identify and remove noise that could arise from environmental interference, device variability, or motion during measurement. To reduce false positives and control for Type I error rates, a false discovery rate (FDR) analysis was applied to the complete RF spectrum [
15]. This approach allowed us to filter out spurious associations and retain only frequencies with a high likelihood of biological relevance. Subsequently, the Predictor Screening (PS) module in JMP Pro was employed, using a Bootstrap Forest algorithm, to isolate frequency ranges most strongly associated with classification outcomes [
23]. The combination of FDR and Bootstrap Forest screening ensured that the retained predictors were statistically robust and biologically interpretable.
From this two-step screening process, the top ten discriminative frequencies were selected for further analysis. These frequencies were then subjected to variable clustering, which enabled dimensionality reduction by grouping correlated frequencies into clusters. Each cluster represented a set of RF “signature frequencies” potentially linked to distinct physiological states. This variable clustering step [
15] allowed for the identification of frequency subsets that were most informative for health status classification while reducing redundancy in the predictor space. The outcome variable was defined by FAMACHA© scoring, a widely used field proxy for anemia in small ruminants [
19]. In the dataset, goats were classified into three ordinal categories: FAMACHA© 1 (healthy), FAMACHA© 2 (healthy/mild risk), and FAMACHA© 3 (borderline anemia) [
19]. However, the class distribution was imbalanced, with relatively fewer observations in the healthy category. To mitigate the risk of biased model performance caused by class imbalance, the Synthetic Minority Over-sampling Technique (SMOTE) was employed. SMOTE generated synthetic instances of the minority class, thereby balancing the dataset and enhancing the model’s ability to discriminate across all health categories [
24,
25,
26,
27,
28].
For predictive modeling, the cleaned and balanced dataset was used to train multi-class classification models in Python. A nested 10-fold cross-validation framework was adopted to provide an unbiased estimate of model performance and to minimize overfitting [
15,
29,
30,
31]. The outer loop of the nested design was used to evaluate generalization performance, while the inner loop optimized model hyperparameters [
32,
33,
34]. Performance metrics included accuracy, precision, recall, and area under the receiver operating characteristic curve (AUROC), which were calculated for each class. This combined workflow comprising FDR-based screening, frequency clustering, data balancing via SMOTE, and rigorous nested cross-validation, which provided a robust and reproducible pipeline for evaluating the diagnostic potential of RF wave signatures. By integrating statistical rigor with machine learning approaches, this study established a foundational methodology for linking RF signal variation with physiological markers of health in small ruminants.
5. Discussion
The observed spectrum homogeneity in non-anemic animals indicates stable physiological conditions where tissue hydration, blood composition, and cellular architecture sustain consistent dielectric properties. This stability corresponds with earlier research by Ley et al. [
33], which demonstrated that low-GHz frequencies consistently characterized healthy tissue properties. In this range, water molecules and membrane polarization mostly influence the dielectric response, facilitating effective differentiation of healthy physiological conditions. The 8.43 GHz signal presumably relates to extracellular water and membrane capacitance, both of which remain stable in animals exhibiting normal hemoglobin and circulation.
As anemia advances, tissue dielectric responses exhibit greater heterogeneity. The delineation of clusters at 9.89 GHz and 8.23 GHz in borderline-anemic subjects signifies varying penetration depths and modified blood volume distribution. These changes align with previous studies associating dehydration or hemoconcentration with frequency-dependent dielectric variability [
35,
36]. Consequently, RF signal dispersion reflects physiological anomalies associated with decreasing hematocrit levels.
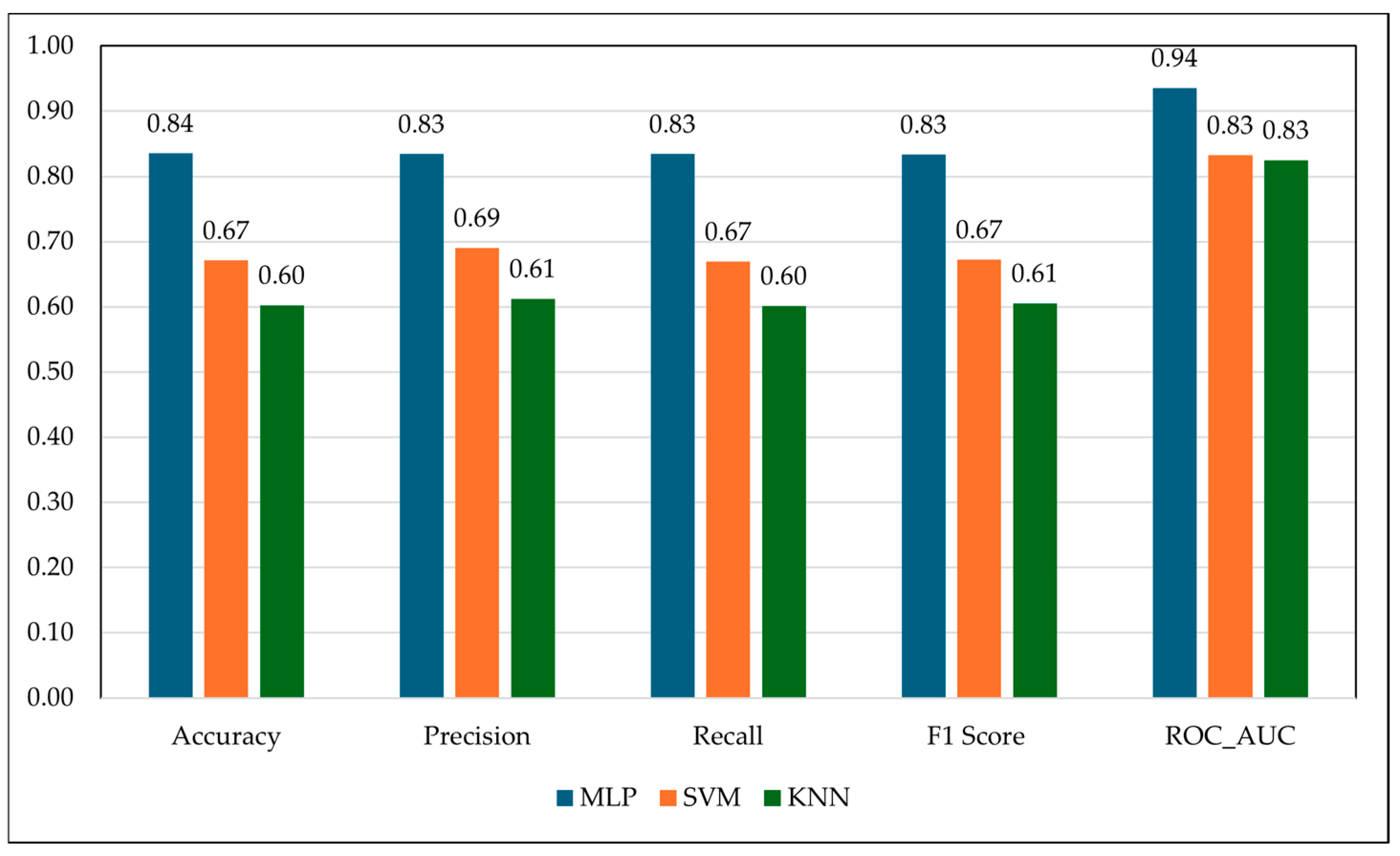
Among the evaluated models, the multilayer perceptron (MLP) showed a greater capacity to capture non-linear spectral interactions than SVM and KNN [
31,
32]. This result corresponds with previous research in biological sensing, wherein neural networks proficiently managed overlapping spectral characteristics of tissues and blood constituents [
33,
34]. Instead of highlighting numerical dominance, our findings indicate that deep designs may more effectively generalize the intricate dielectric interactions in biological systems. The Random Forest algorithm demonstrated consistent performance, highlighting that ensemble learning effectively addresses the noise and variability prevalent in field data. The nearly flawless performance of the linear regression model is approached with caution, since it may suggest residual overfitting or unintentional data leaking, despite the implementation of cross-validation measures. Subsequent research will use independent validation datasets to verify the generalizability of the concept.
Comparisons with prior radio-frequency (RF) investigations in livestock and biomedical fields underscore both the potential and the constraints of this methodology. RF-based rumen health monitoring in dairy cattle and non-invasive blood-glucose estimates in humans have shown that GHz-range dielectric shifts coincide with internal physiological changes, hence affirming the significance of the frequencies identified herein. Nonetheless, those investigations underscore the necessity for comprehensive calibration across breeds, ages, and tissue compositions prior to diagnostic use. This study’s sample size was restricted and done under controlled conditions; larger trials are necessary prior to field implementation.
Consequently, the current findings offer a proof of concept rather than clinical validation. Although RF sensing integrated with machine learning demonstrates significant promise for large-scale anemia screening in herds, its application in commercial or agricultural settings necessitates extensive, multi-site investigations to address environmental interference, coat variability, and equipment discrepancies. The incorporation of supplementary biomarkers, such temperature, heart rate, or fecal egg counts, may augment diagnostic confidence.
As a quick, field-deployable screening tool, radio-frequency (RF) sensing has great potential, but this proof-of-concept study does not recommend replacing hematological or parasitological diagnostics. Anemia-related tissue dielectric changes can be detected in a 5–10 s non-invasive scan with the RF system, providing a feasible triage step before PCV or FEC testing. In resource-limited or extensive grazing systems, the approach can detect early physiological aberrations connected to anemia in real time, reducing animal handling and labor expenses. In quantitative terms, frequency clustering accounted nearly 90% of physiological variation across health states (8.43 GHz = 93.7% for healthy, 9.33 GHz = 88.7% for moderate, and 9.89 GHz/8.23 GHz = 91.0% for borderline). The regression study validated the biological relevance of these spectral properties (Random Forest R2 = 0.79; RMSE = 0.07), with the Multilayer Perceptron classifier achieving 84% accuracy and 94% ROC-AUC values. These findings show a meaningful and reproducible relationship between RF dielectric signatures and anemia severity, demonstrating the method’s diagnostic value as a decision-support tool. This integration of RF detecting and AI improves small ruminant health monitoring on farms in terms of accessibility, speed, and welfare compliance.
This study illustrates that GHz-range RF waves provide physiologically significant information regarding tissue condition in goats. The machine-learning models demonstrate quantifiable patterns associated with early stages of anemia, validating the feasibility of non-invasive spectrum monitoring. However, its practical application will rely on validation across various field settings and comparability with existing hematological and parasitological diagnoses.
This approach is potentially transformative in pastoral and resource-limited farming systems, where labor, equipment, and veterinary access are scarce. A lightweight RF sensing device coupled with a MLP classifier could provide farmers with a rapid, non-invasive, and repeatable diagnostic tool. Such technology would reduce reliance on manual FAMACHA© scoring, minimize observer bias, and enable more timely interventions against parasitic infections, ultimately supporting improved animal health, productivity, and sustainability.
6. Conclusions and Future Research
The present study intentionally focused on developing a non-invasive diagnostic framework. Direct hematological measurements such as PCV or hemoglobin were not obtained, as the goal was to determine whether radio-frequency responses alone could infer anemia-related physiological differences. Although FAMACHA© provides a convenient visual reference, it does not fully quantify hematological status. Therefore, future validation experiments will integrate direct blood parameters (e.g., PCV, hemoglobin, total protein) alongside dielectric data to establish robust physiological baselines and strengthen the biological interpretability of the RF-anemia relationship. This work demonstrates the utility of radio-frequency (RF) wave characteristics as a non-invasive analytical tool for identifying physiologically significant patterns associated with anemia in small ruminants. The findings indicate that GHz-range dielectric responses systematically correlate with FAMACHA© scores, and that machine learning methods, especially multilayer perceptrons and ensemble models, may accurately assess health status based on these spectral attributes. This novel methodology underscores the capabilities of RF-based sensing in veterinary diagnostics.
This work is inherently exploratory. The sample size was limited, and testing was conducted in controlled settings. Although the results are encouraging, they should not be construed as definitive proof of diagnostic readiness at the field level. Further validation utilizing larger, independent datasets and multi-location trials is essential to verify the model’s generalizability and robustness. The incorporation of additional biomarkers, such as temperature, heart rate, or parasitological data, may enhance predictive accuracy and clinical reliability. This study facilitates further research on the application of RF sensing and artificial intelligence in animal health diagnosis. The potential of this research is extensive, and further refinement, calibration, and validation will be critical steps toward its eventual implementation in on-farm decision support systems.