A Hydroxynaphthol Blue-Based Loop-Mediated Isothermal Amplification Assay for Closed-Tube Detection of the Streptomycin Resistance Gene aadA1 in Salmonella
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Salmonella Strains and Antimicrobial Susceptibility Testing
2.2. DNA Extraction
2.3. Design of the LAMP Primers
2.4. Optimized LAMP Assay
2.5. Specificity and Sensitivity of the LAMP Assay
2.6. Parallel Detection of Clinical Isolates by LAMP and PCR Assays
2.7. Statistical Analysis
3. Results
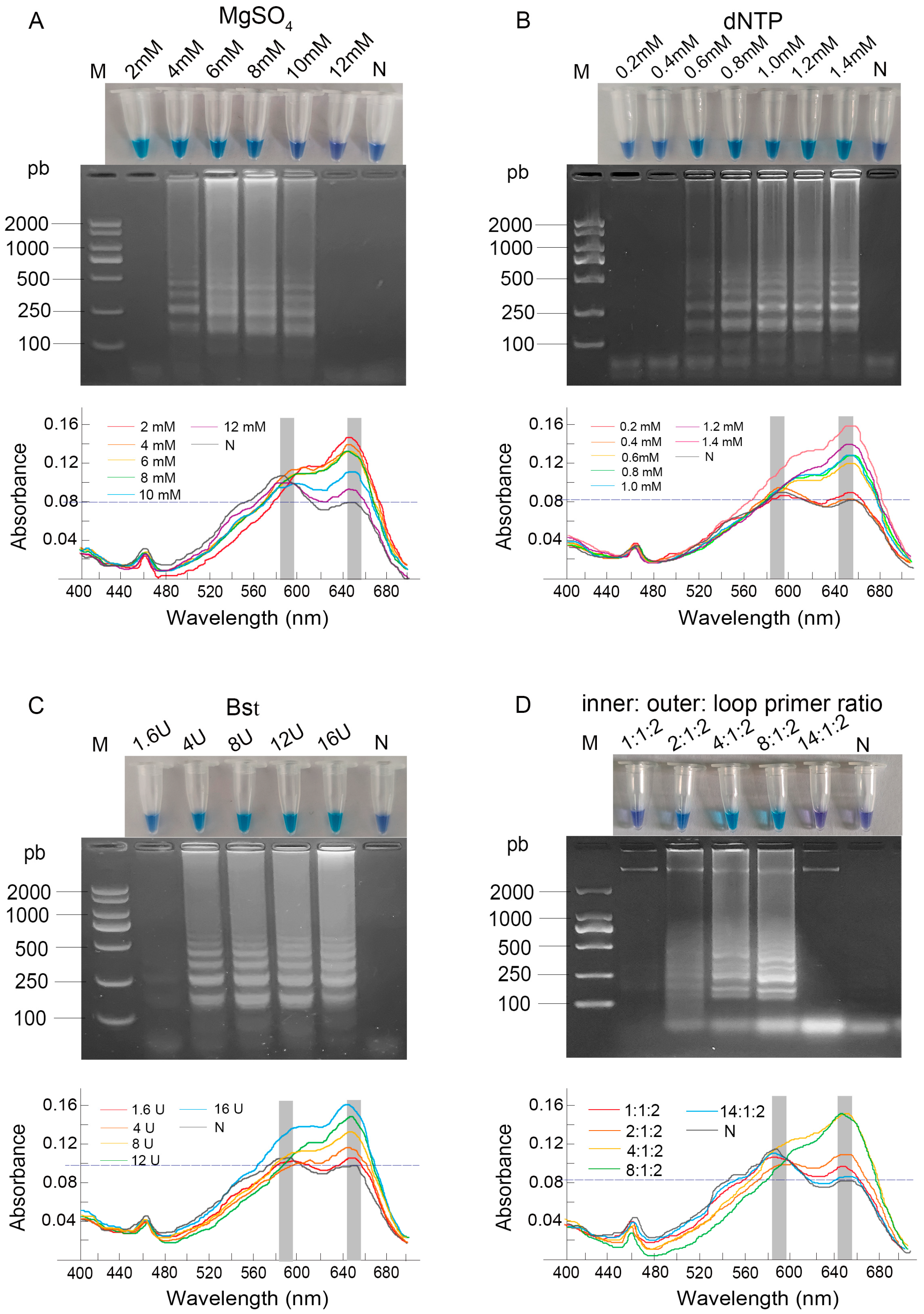
3.1. Optimization of LAMP Reaction Component Concentrations
3.2. Optimization of LAMP Conditions
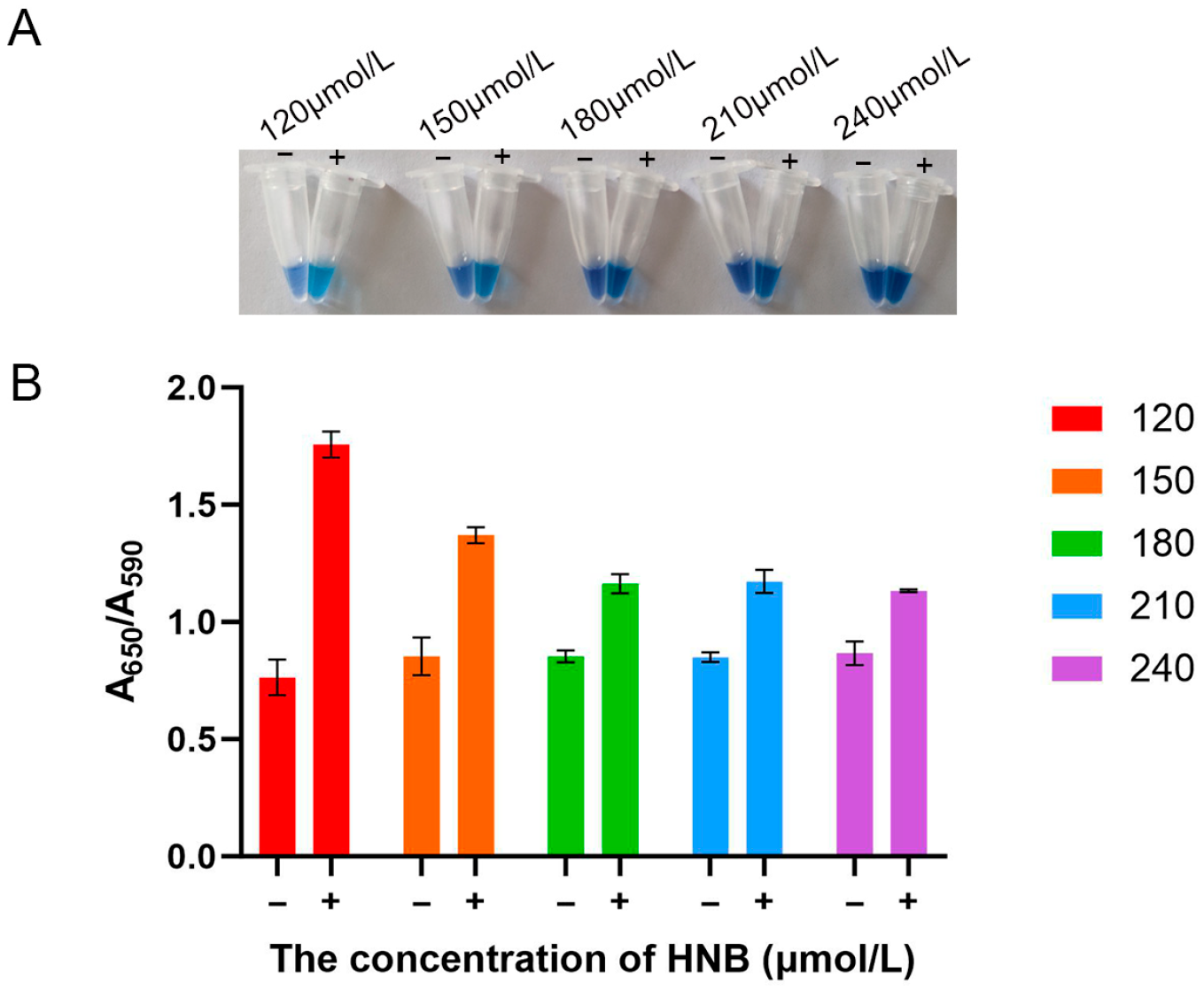
3.3. Optimization of HNB Concentration for the LAMP Assay
3.4. Specificity of the Optimized LAMP Assay for aadA1 Gene
3.5. Sensitivity of the Optimized LAMP Assay for aadA1 Gene Against PCR
3.6. Clinical Application of a Visual LAMP Assay for the aadA1 Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, A.; Zhang, T.; Cheng, F.; Yang, H.; Guo, Z.; Zhao, S.; Zhang, Y.N.; Qu, J. Comprehensive analysis and risk assessment of Antibiotic contaminants, antibiotic-resistant bacteria, and resistance genes: Patterns, drivers, and implications in the Songliao Basin. Environ. Pollut. 2024, 361, 124852. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial Resistance in Bacteria: Mechanisms, Evolution, and Persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef]
- Daubin, V.; Szollosi, G.J. Horizontal Gene Transfer and the History of Life. Cold Spring Harb. Perspect. Biol. 2016, 8, a018036. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updates 2010, 13, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Lertwatcharasarakul, P.; Phatthanakunanan, S.; Tulayakul, P. Retrospective analysis of antimicrobial resistance of Salmonella spp. isolated from livestock and its environment in Thailand. Front. Vet. Sci. 2025, 12, 1584940. [Google Scholar] [CrossRef]
- Misiak, M.; Kurpas, D. The only constant in life is change: Summary of the last 4 years of Advances in Clinical and Experimental Medicine. Adv. Clin. Exp. Med. 2024, 33, 1311–1315. [Google Scholar] [CrossRef]
- Habib, I.; Mohamed, M.I.; Lakshmi, G.B.; Al Marzooqi, H.M.; Afifi, H.S.; Shehata, M.G.; Elbediwi, M. First detection and genomic analysis of mcr-1-positive Salmonella Infantis isolated from a broiler production system in the United Arab Emirates. Front. Vet. Sci. 2025, 12, 1592955. [Google Scholar] [CrossRef]
- Hembach, N.; Bierbaum, G.; Schreiber, C.; Schwartz, T. Facultative pathogenic bacteria and antibiotic resistance genes in swine livestock manure and clinical wastewater: A molecular biology comparison. Environ. Pollut. 2022, 313, 120128. [Google Scholar] [CrossRef]
- Ruuskanen, M.; Muurinen, J.; Meierjohan, A.; Parnanen, K.; Tamminen, M.; Lyra, C.; Kronberg, L.; Virta, M. Fertilizing with Animal Manure Disseminates Antibiotic Resistance Genes to the Farm Environment. J. Environ. Qual. 2016, 45, 488–493. [Google Scholar] [CrossRef]
- Hussain, E.A.; Qasim Hameed, H.; Mujahid Al-Shuwaikh, A.; Mujahid Abdullah, R. Detection of the aadA1 and aac (3)-1V resistance genes in Acinetobacter baumannii. Arch. Razi Inst. 2022, 77, 959–966. [Google Scholar] [CrossRef]
- Ferreira, C.; Otani, S.; Aarestrup, F.M.; Manaia, C.M. Quantitative PCR versus metagenomics for monitoring antibiotic resistance genes: Balancing high sensitivity and broad coverage. FEMS Microbes 2023, 4, xtad008. [Google Scholar] [CrossRef]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Wang, Y.; Gao, J.; Bai, J.; Zhang, Q.; Shi, L.; Wang, X.; Wang, C. Rapid detection of Salmonella based on loop-mediated isothermal amplification. Ann. Palliat. Med. 2021, 10, 6850–6858. [Google Scholar] [CrossRef] [PubMed]
- Pavon, R.D.N.; Rivera, W.L. Loop-Mediated Isothermal Amplification Assay for Visual Detection of Salmonella enterica Serovar Typhimurium in Food Animal Meat Products. Foods 2025, 14, 1731. [Google Scholar] [CrossRef]
- Schafer, L.; Volker, E.; Eule, M.; Ahrens, B.; Beyer, K.; Holzhauser, T. Development and Validation of a Loop-Mediated Isothermal Amplification (LAMP) Method for the Rapid, Sensitive, and Specific Detection of Hazelnut as an Allergen in Food Matrix. J. Agric. Food Chem. 2024, 72, 24093–24100. [Google Scholar] [CrossRef]
- Sadeghi, Y.; Kananizadeh, P.; Moghadam, S.O.; Alizadeh, A.; Pourmand, M.R.; Mohammadi, N.; Afshar, D.; Ranjbar, R. The Sensitivity and Specificity of Loop-Mediated Isothermal Amplification and PCR Methods in Detection of Foodborne Microorganisms: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2021, 50, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhuang, L.; Zhang, D.; Zhang, P.; Dou, X.; Wang, C. Establishment of a Multiplex Loop-Mediated Isothermal Amplification Method for Rapid Detection of Sulfonamide Resistance Genes (sul1, sul2, sul3) in Clinical Enterobacteriaceae Isolates from Poultry. Foodborne Pathog. Dis. 2018, 15, 413–419. [Google Scholar] [CrossRef]
- Prakash, S.; Priyatma; Aasarey, R.; Pandey, P.K.; Mathur, P.; Arulselvi, S. An inexpensive and rapid diagnostic method for detection of SARS-CoV-2 RNA by loop-mediated isothermal amplification (LAMP). MethodsX 2023, 10, 102011. [Google Scholar] [CrossRef]
- Qi, J.; Du, Y.; Zhu, R.; Zhu, X.; Bai, H.; Hu, M.; Luo, Y.; Hu, X.; Wu, C.; Shen, J.; et al. A loop-mediated isothermal amplification method for rapid detection of the multidrug-resistance gene cfr. Gene 2012, 504, 140–143. [Google Scholar] [CrossRef]
- Esmaeeli, A.; Ravan, H.; Hassanshahian, M.; Khaleghi, M. Rapid LAMP-based detection of A. baumannii and aminoglycoside resistance genes in ESKAPE pathogens. Microb. Pathog. 2025, 202, 107436. [Google Scholar] [CrossRef]
- Askari, N.; Momtaz, H.; Tajbakhsh, E. Acinetobacter baumannii in sheep, goat, and camel raw meat: Virulence and antibiotic resistance pattern. AIMS Microbiol. 2019, 5, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, I.H.; Beshiru, A.; Ikediashi, S.C.; Igbinosa, E.O. Identification and Characterization of Salmonella Serovars Isolated from Pig Farms in Benin City, Edo State, Nigeria: One Health Perspective. Microb. Drug Resist. 2021, 27, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Carriere, M.; Vijayabaskar, V.; Applefield, D.; Harvey, I.; Garneau, P.; Lorsch, J.; Lapidot, A.; Pelletier, J. Inhibition of protein synthesis by aminoglycoside-arginine conjugates. RNA 2002, 8, 1267–1279. [Google Scholar] [CrossRef]
- Nastri, H.G.; Algranati, I.D. Inhibition of protein synthesis by aminoglycoside antibiotics in polyamine-requiring bacteria. Biochem. Biophys. Res. Commun. 1988, 150, 947–954. [Google Scholar] [CrossRef]
- Pagkalis, S.; Mantadakis, E.; Mavros, M.N.; Ammari, C.; Falagas, M.E. Pharmacological considerations for the proper clinical use of aminoglycosides. Drugs 2011, 71, 2277–2294. [Google Scholar] [CrossRef]
- Choopara, I.; Teethaisong, Y.; Arunrut, N.; Thunyaharn, S.; Kiatpathomchai, W.; Somboonna, N. Specific and sensitive, ready-to-use universal fungi detection by visual color using ITS1 loop-mediated isothermal amplification combined hydroxynaphthol blue. PeerJ 2021, 9, e11082. [Google Scholar] [CrossRef]
- Mollasalehi, H.; Esmaili, F.; Minai-Tehrani, D. Development and evaluation of a colorimetric LAMP-based biosensor for rapid detection of a nosocomial infection agent, Citrobacter freundii. Sci. Rep. 2023, 13, 21896. [Google Scholar] [CrossRef]
- Balaga, K.B.; Pavon, R.D.N.; Calayag, A.M.B.; Justo, C.A.C.; Adao, D.E.V.; Rivera, W.L. Development of a closed-tube, calcein-based loop-mediated isothermal amplification assay to detect Salmonella spp. in raw meat samples. J. Microbiol. Methods 2024, 220, 106922. [Google Scholar] [CrossRef]
- Chen, N.; Li, G.; Si, Y.; Zhang, W.; Ye, Y.; Wang, Y.; Wang, K.; Zong, M.; Fan, L. Evaluation of LAMP assay using phenotypic tests and PCR for detection of blaKPC gene among clinical samples. J. Clin. Lab. Anal. 2022, 36, e24310. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.Q.; Liu, B.B.; Hui, E.; Huang, W.; Yao, L.C.; Duo, L.B.; Sun, W.Y.; Li, G.Q.; Wang, F.X.; Liu, S.L. A rapid loop-mediated isothermal amplification (LAMP) method for detection of the macrolide-streptogramin type B resistance gene msrA in Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2016, 7, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; El-Tarabili, R.M.; Abd El-Ghany, W.A.; Almanzalawi, E.A.; Alqahtani, T.M.; Ghabban, H.; Al-Otaibi, A.S.; Alatfeehy, N.M.; Abosleima, N.M.; Hetta, H.F.; et al. Resistance profiles, virulence and antimicrobial resistance genes of XDR S. Enteritidis and S. Typhimurium. AMB Express 2023, 13, 110. [Google Scholar] [CrossRef]
- Panno, S.; Matic, S.; Tiberini, A.; Caruso, A.G.; Bella, P.; Torta, L.; Stassi, R.; Davino, A.S. Loop Mediated Isothermal Amplification: Principles and Applications in Plant Virology. Plants 2020, 9, 461. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Sethy, K.; Mohapatra, S.; Panda, D. Loop mediated isothermal amplification: An innovative gene amplification technique for animal diseases. Vet. World 2016, 9, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wen, J.; Liao, X.; Lin, Q.; Zhang, J.; Chen, K.; Wang, S.; Zhang, J. A Sensitive, Highly Specific Novel Isothermal Amplification Method Based on Single-Nucleotide Polymorphism for the Rapid Detection of Salmonella Pullorum. Front. Microbiol. 2020, 11, 560791. [Google Scholar] [CrossRef]
- Muhummed, A.; Alemu, A.; Hosch, S.; Osman, Y.; Tschopp, R.; Yersin, S.; Schindler, T.; Hattendorf, J.; Zinsstag, J.; Cisse, G.; et al. Fecal carriage of ESBL-producing E. coli and genetic characterization in rural children and livestock in the Somali region, Ethiopia: A one health approach. Antimicrob. Resist. Infect. Control. 2024, 13, 148. [Google Scholar] [CrossRef]
- Yang, B.; Xin, X.; Cao, X.; Nasifu, L.; Nie, Z.; He, B. Phenotypic and genotypic perspectives on detection methods for bacterial antimicrobial resistance in a One Health context: Research progress and prospects. Arch. Microbiol. 2024, 206, 409. [Google Scholar] [CrossRef]
- Enne, V.I.; Delsol, A.A.; Roe, J.M.; Bennett, P.M. Evidence of antibiotic resistance gene silencing in Escherichia coli. Antimicrob. Agents Chemother. 2006, 50, 3003–3010. [Google Scholar] [CrossRef]
- Jarmula, A.; Oblak, E.; Wawrzycka, D.; Gutowicz, J. Efflux-mediated antimicrobial multidrug resistance. Postep. Hig. Med. Dosw. 2011, 65, 216–227. [Google Scholar] [CrossRef]
- Li, W.; Atkinson, G.C.; Thakor, N.S.; Allas, U.; Lu, C.C.; Chan, K.Y.; Tenson, T.; Schulten, K.; Wilson, K.S.; Hauryliuk, V.; et al. Mechanism of tetracycline resistance by ribosomal protection protein Tet(O). Nat. Commun. 2013, 4, 1477. [Google Scholar] [CrossRef] [PubMed]

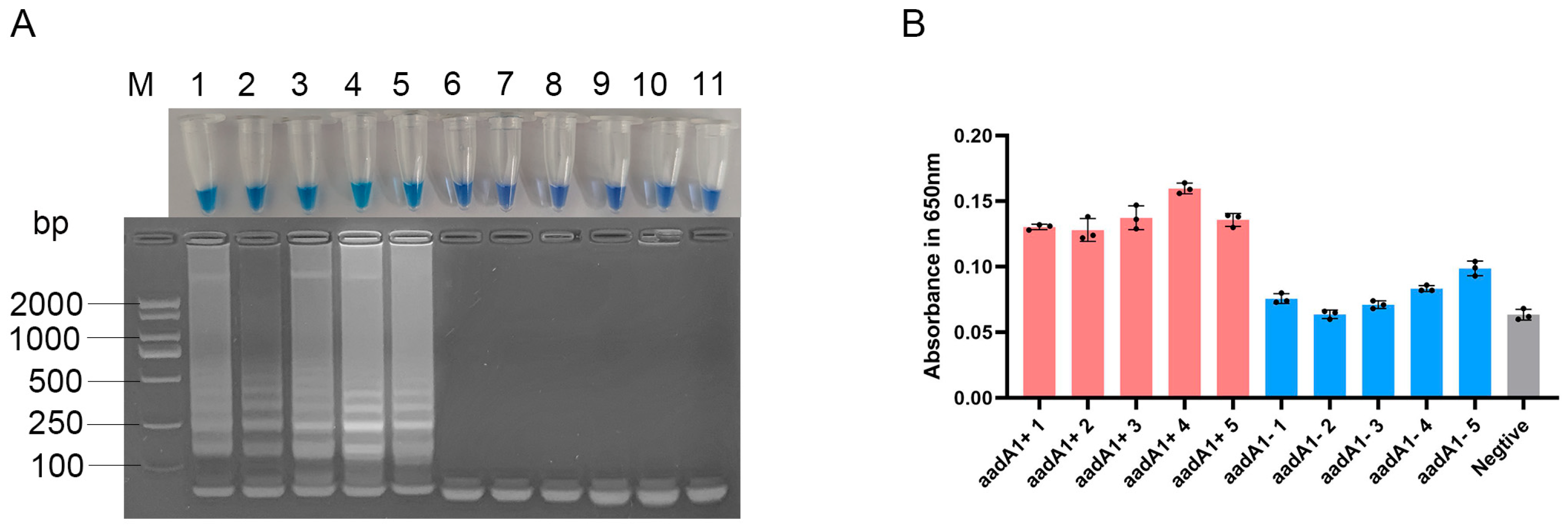

| NO. | Strains | Serotype | aadA1 |
|---|---|---|---|
| 1 | LLC-35 | S. pullorum | + |
| 2 | LLC-41 | S. pullorum | + |
| 3 | LLC-51 | S. pullorum | + |
| 4 | HO-43 | S. Newport | + |
| 5 | HO-96 | S. pullorum | + |
| 6 | HO-78 | S. pullorum | − |
| 7 | LLC-17 | S. enteritidis | − |
| 8 | LLC-19 | S. pullorum | − |
| 9 | LLC-8 | S. Newport | − |
| 10 | BSC-16 | S. enteritidis | − |
| Method | Primers | Sequence (5′–3′) | References |
|---|---|---|---|
| LAMP | F3 | GTTGTGCACGACGACATCA | This study |
| B3 | GGATCAAAGAGTTCCTCCGC | ||
| FIP | TGCAAGAATGTCATTGCGCTGCGTGGCGTTATCCAGCTAAGC | ||
| BIP | GGTATCTTCGAGCCAGCCACGCCAAGGCAACGCTATGTTCT | ||
| LB | TCTGGCTATCTTGCTGACAAAAGC | ||
| PCR | aadA1-F | TATCCAGCTAAGCGCGAACT | [1] |
| aadA1-R | ATTTGCCGACTACCTTGGTG |
| Parameters | Assay Range | Optimized |
|---|---|---|
| Isothermal Amplification Buffer | 1× | 1× |
| MgSO4 | 2–12 mM | 6 mM |
| dNTPs | 0.2–1.4 mM | 1.4 mM |
| Bst Polymerase | 1.6–16 U | 8 U |
| FIP/BIP Primers | 0.2–2.8 µM | 1.6 µM |
| F3/B3 Primers | 0.2 µM | 0.2 µM |
| Loop F Primers | 0.4 µM | 0.4 µM |
| Nuclease-Free H2O | Make up to 25 µL | Make up to 25 µL |
| Betaine | 1 M | 1 M |
| DNA Template | 1 µL | 1 µL |
| Hydroxynaphthol blue | 120–240 μmol/L | 120 μmol/L |
| Temperature | 58–66 °C | 64 °C |
| Time | 25–55 min | 35 min |
| Phenotypic Tests | LAMP | PCR | Total | ||
|---|---|---|---|---|---|
| + | − | + | − | ||
| Streptomycin-resistant | 27 (100.0%) | 0 | 26 (96.3%) | 1 (3.7%) | 27 |
| Streptomycin-susceptible | 5 (38.5%) | 8 (61.5%) | 3 (23.1%) | 10 (76.9%) | 13 |
| Total | 32 (75.0%) | 8 (25.0%) | 29 (72.5%) | 11 (27.5%) | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Zheng, Y.; Li, M.; Du, Y.; Yang, H.; Li, F.; Wang, B.; Wang, X. A Hydroxynaphthol Blue-Based Loop-Mediated Isothermal Amplification Assay for Closed-Tube Detection of the Streptomycin Resistance Gene aadA1 in Salmonella. Vet. Sci. 2025, 12, 1094. https://doi.org/10.3390/vetsci12111094
Shen Y, Zheng Y, Li M, Du Y, Yang H, Li F, Wang B, Wang X. A Hydroxynaphthol Blue-Based Loop-Mediated Isothermal Amplification Assay for Closed-Tube Detection of the Streptomycin Resistance Gene aadA1 in Salmonella. Veterinary Sciences. 2025; 12(11):1094. https://doi.org/10.3390/vetsci12111094
Chicago/Turabian StyleShen, Yuxiang, Yeqing Zheng, Meiquan Li, Yanli Du, Heng Yang, Fangjie Li, Bin Wang, and Xiao Wang. 2025. "A Hydroxynaphthol Blue-Based Loop-Mediated Isothermal Amplification Assay for Closed-Tube Detection of the Streptomycin Resistance Gene aadA1 in Salmonella" Veterinary Sciences 12, no. 11: 1094. https://doi.org/10.3390/vetsci12111094
APA StyleShen, Y., Zheng, Y., Li, M., Du, Y., Yang, H., Li, F., Wang, B., & Wang, X. (2025). A Hydroxynaphthol Blue-Based Loop-Mediated Isothermal Amplification Assay for Closed-Tube Detection of the Streptomycin Resistance Gene aadA1 in Salmonella. Veterinary Sciences, 12(11), 1094. https://doi.org/10.3390/vetsci12111094





