Integrated Metabolomic and Transcriptomic Analysis Decodes Heat Stress-Induced Metabolic Shifts in Gilt Granulosa Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Evaluation of Cumulus Expansion and Cell Viability
2.3. Non-Targeted Metabolomics Analysis
2.4. RNA Sequencing (RNA-seq) Analysis
2.5. Integrated Transcriptome and Metabolome Analysis
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
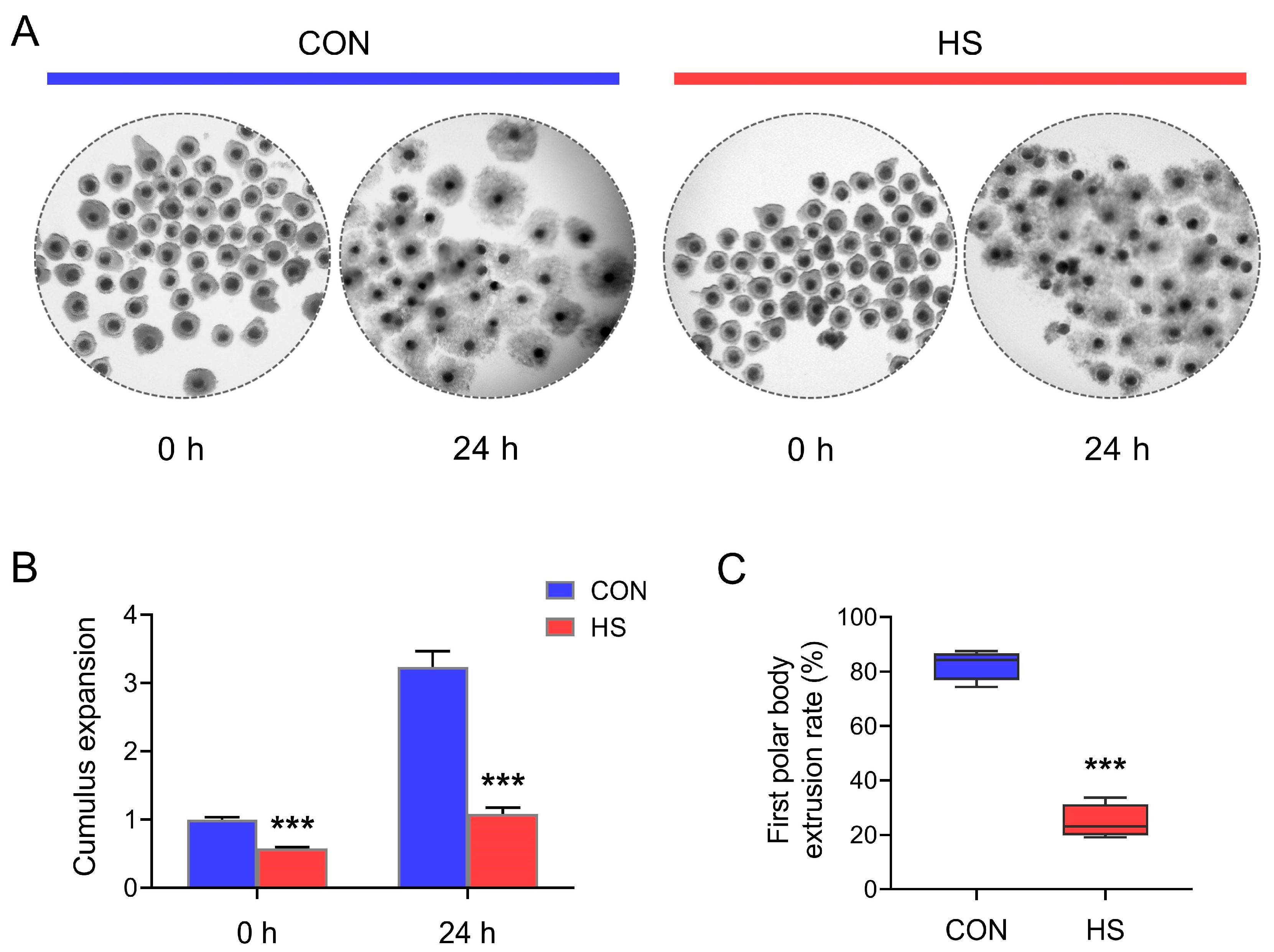
3.1. HS Reduced the Cumulus Expansion and Oocyte Maturation
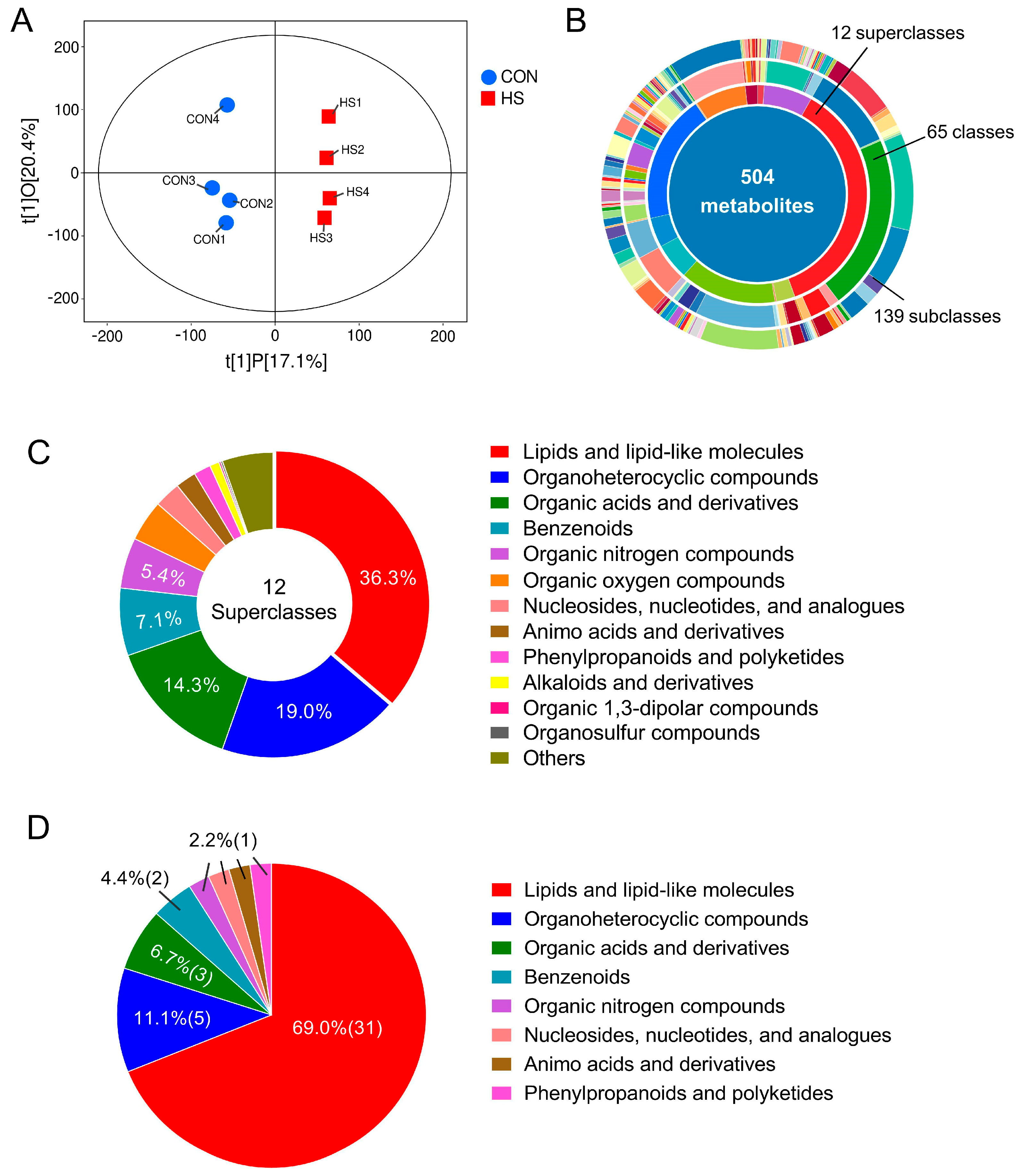
3.2. HS Altered the Metabolic Profiles of Porcine Follicular Granulosa Cell
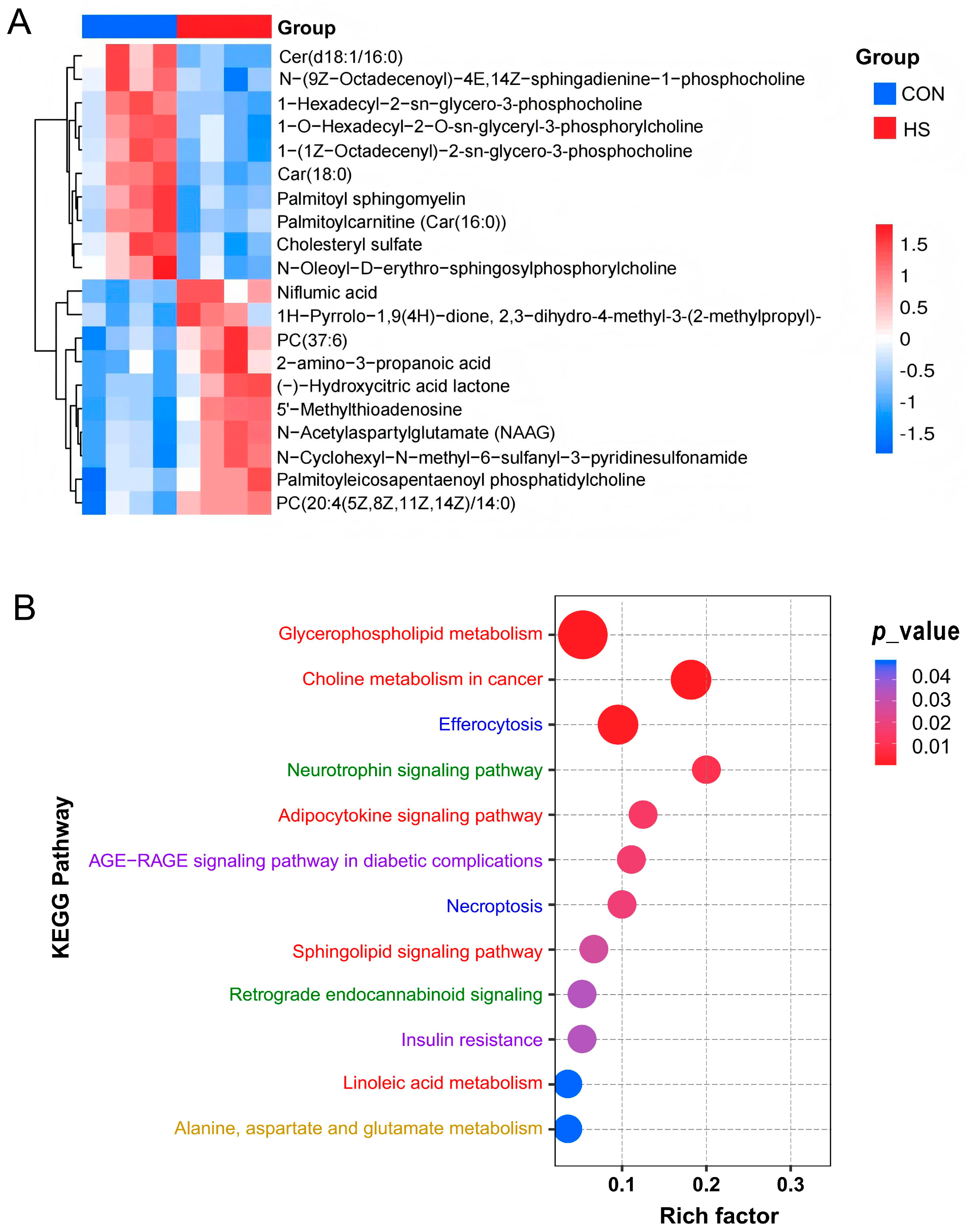
3.3. Enrichment and Functional Annotation of DAMs in Response to HS
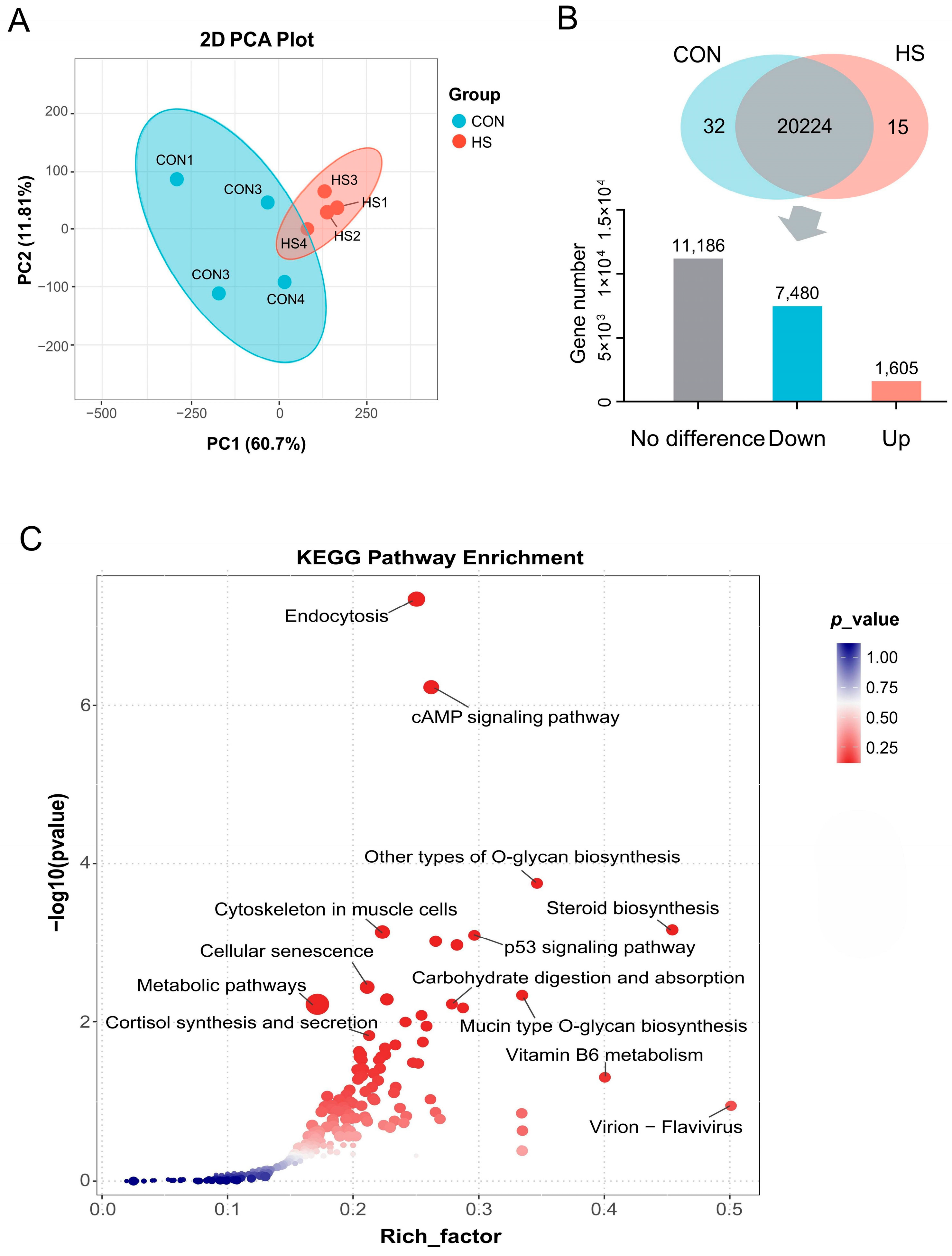
3.4. Differential Expression and Enrichment Analysis of Genes in GCs
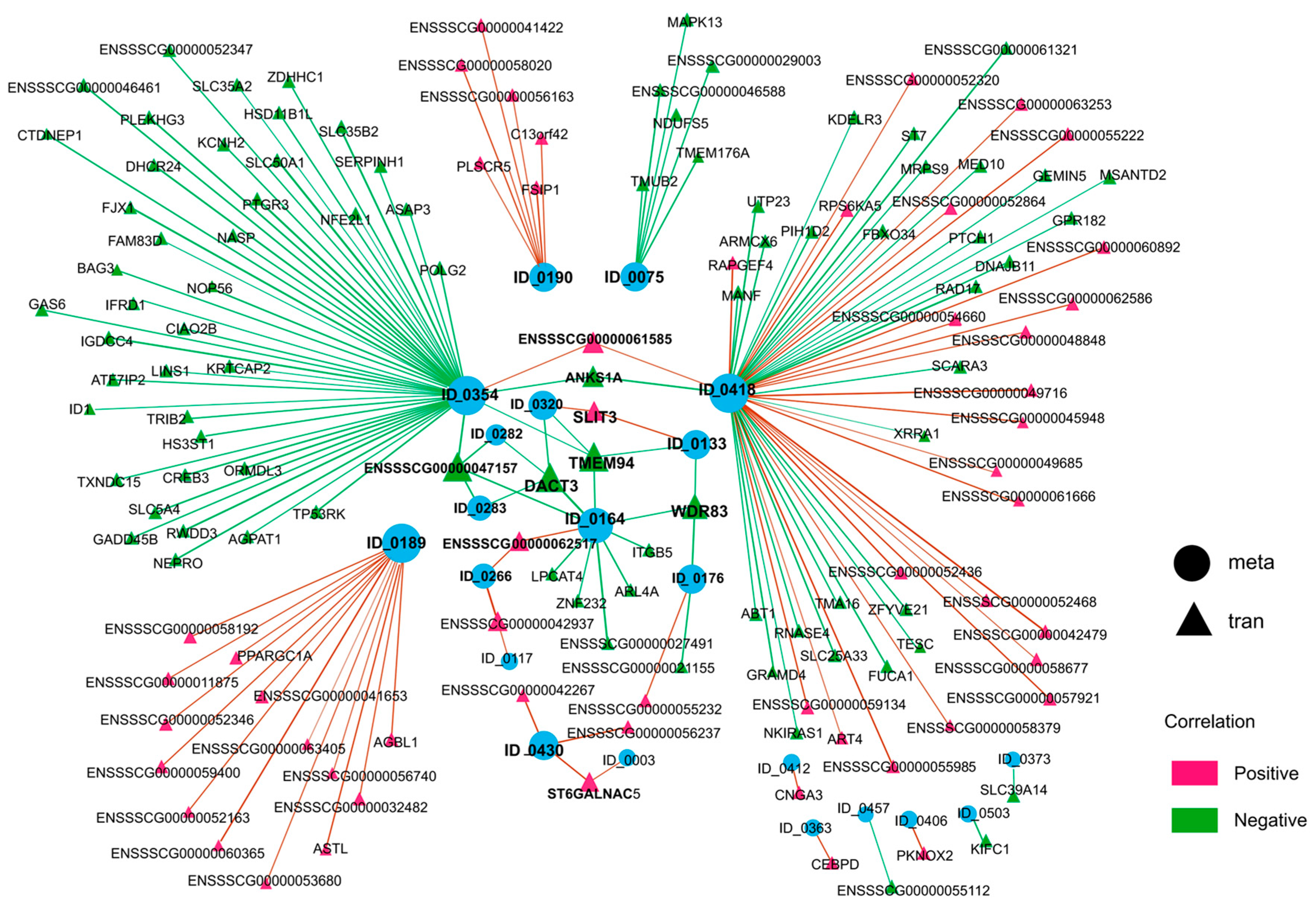
3.5. Integrated Analysis of Differentially Expressed mRNAs and DAMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, F.; Zhao, W.; Le, H.H.; Cottrell, J.J.; Green, M.P.; Leury, B.J.; Dunshea, F.R.; Bell, A.W. Review: What have we learned about the effects of heat stress on the pig industry? Animal 2022, 16 (Suppl. 2), 100349. [Google Scholar] [CrossRef]
- Knox, R.V. Swine fertility in a changing climate. Anim. Reprod. Sci. 2024, 269, 107537. [Google Scholar] [CrossRef]
- Hunter, M.G. Oocyte maturation and ovum quality in pigs. Rev. Reprod. 2000, 5, 122–130. [Google Scholar] [CrossRef]
- Cecconi, S.; Ciccarelli, C.; Barberi, M.; Macchiarelli, G.; Canipari, R. Granulosa cell-oocyte interactions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115 (Suppl. 1), S19–S22. [Google Scholar] [CrossRef]
- De Rensis, F.; Ziecik, A.J.; Kirkwood, R.N. Seasonal infertility in gilts and sows: Aetiology, clinical implications and treatments. Theriogenology 2017, 96, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.A.; Rizos, D.; Rodriguez-Martinez, H.; Funahashi, H. Oocyte-cumulus cells crosstalk: New comparative insights. Theriogenology 2023, 205, 87–93. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Wang, L.; Li, J.; Li, E.; Luo, Y. Multi-omics technology and its applications to life sciences: A review. Sheng Wu Gong Cheng Xue Bao 2022, 38, 3581–3593. [Google Scholar] [PubMed]
- Karahalil, B. Overview of systems biology and omics technologies. Curr. Med. Chem. 2016, 23, 4221–4230. [Google Scholar] [CrossRef]
- Pan, B.; Chai, J.; Fei, K.; Zheng, T.; Jiang, Y. Dynamic changes in the transcriptome and metabolome of pig ovaries across developmental stages and gestation. BMC Genom. 2024, 25, 1193. [Google Scholar] [CrossRef]
- Yin, C.; Liu, J.; Chang, Z.; He, B.; Yang, Y.; Zhao, R. Heat exposure impairs porcine oocyte quality with suppressed actin expression in cumulus cells and disrupted f-actin formation in transzonal projections. J. Anim. Sci. Biotechnol. 2020, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yong, Y.; Ju, X. Effect of heat stress on growth and production performance of livestock and poultry: Mechanism to prevention. J. Therm. Biol. 2021, 99, 103019. [Google Scholar] [CrossRef]
- Turner, A.I.; Hemsworth, P.H.; Tilbrooka, A.J. Susceptibility of reproduction in female pigs to impairment by stress and the role of the hypothalamo-pituitary-adrenal axis. Reprod. Fertil. Dev. 2002, 14, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Canipari, R. Oocyte-granulosa cell interactions. Hum. Reprod. Update 2000, 6, 279–289. [Google Scholar] [CrossRef]
- Zhou, L.T.; Gokyer, D.; Madkins, K.; Beestrum, M.; Horton, D.E.; Duncan, F.E.; Babayev, E. The effects of heat stress on the ovary, follicles and oocytes: A systematic review. Biol. Reprod. 2025, ioaf150. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.I.; Wang, Z.Z.; Liu, B.; Shen, C.-Q.; Tu, J.-Y.; Wang, S.-C.; Lei, R.-L.; Peng, S.-Q.; Xiao, X.; Zhao, Y.-J.; et al. The effects and mechanisms of heat stress on mammalian oocyte and embryo development. J. Therm. Biol. 2024, 124, 103927. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yin, Q.; Jin, E.; Chen, H.; He, S. Selenium attenuates chronic heat stress-induced apoptosis via the inhibition of endoplasmic reticulum stress in mouse granulosa cells. Molecules 2020, 25, 557. [Google Scholar] [CrossRef]
- Alemu, T.W.; Pandey, H.O.; Salilew, W.D.; Gebremedhn, S.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to heat stress. Theriogenology 2018, 110, 130–141. [Google Scholar] [CrossRef]
- Li, X.; Wu, Q.; Gao, H.; Cheng, J.; Chen, H.; Duan, J.; Sun, W.; Zhang, Z.; Yang, L.; Hua, R.; et al. Isorhamnetin protects porcine oocytes from heat stress by maintaining cumulus cells-oocyte communications. Free. Radic. Biol. Med. 2025, 237, 239–250. [Google Scholar] [CrossRef]
- Yin, C.; Liu, J.; He, B.; Jia, L.; Gong, Y.; Guo, H.; Zhao, R. Heat stress induces distinct responses in porcine cumulus cells and oocytes associated with disrupted gap junction and trans-zonal projection colocalization. J. Cell. Physiol. 2019, 234, 4787–4798. [Google Scholar] [CrossRef]
- Sammad, A.; Ahmed, T.; Ullah, K.; Hu, L.; Luo, H.; Alphayo, K.P.; Faisal, S.; Zhu, H.; Li, Y.; Wang, Y. Vitamin C alleviates the negative effects of heat stress on reproductive processes by regulating amino acid metabolism in granulosa cells. Antioxidants 2024, 13, 653. [Google Scholar] [CrossRef]
- Sammad, A.; Hu, L.; Luo, H.; Abbas, Z.; Umer, S.; Zhao, S.; Xu, Q.; Khan, A.; Wang, Y.; Zhu, H.; et al. Investigation of metabolome underlying the biological mechanisms of acute heat stressed granulosa cells. Int. J. Mol. Sci. 2022, 23, 2146. [Google Scholar] [CrossRef]
- Sammad, A.; Luo, H.; Hu, L.; Zhao, S.; Gong, J.; Umer, S.; Khan, A.; Zhu, H.; Wang, Y. Joint transcriptome and metabolome analysis prevails the biological mechanisms underlying the pro-survival fight in in vitro heat-stressed granulosa cells. Biology 2022, 11, 839. [Google Scholar] [CrossRef]
- Liu, J.; He, B.; Yin, C.; Chang, Z.; Zhao, R. Transcriptomic responses of porcine cumulus cells to heat exposure during oocytes in vitro maturation. Mol. Reprod. Dev. 2021, 88, 43–54. [Google Scholar] [CrossRef]
- Gebremedhn, S.; Gad, A.; Aglan, H.S.; Laurincik, J.; Prochazka, R.; Salilew-Wondim, D.; Hoelker, M.; Schellander, K.; Tesfaye, D. Extracellular vesicles shuttle protective messages against heat stress in bovine granulosa cells. Sci. Rep. 2020, 10, 15824. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Swelum, A.A.; Elsafadi, M.; Mahmood, A.; Osama, A.; Shikshaky, H.; Alfayez, M.; Alowaimer, A.N.; Magdeldin, S. Thermotolerance and plasticity of camel somatic cells exposed to acute and chronic heat stress. J. Adv. Res. 2020, 22, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Zhang, M.; Yu, H.; Mustafa, S.; Shafiq, M.; Wei, Q.; Wang, W.; Jan, M.; Mao, D. Heat exposure affected the reproductive performance of pregnant mice: Enhancement of autophagy and alteration of subcellular structure in the corpus luteum. Reprod. Biol. 2019, 19, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Bharati, J.; Kumar, S.; Devi, S.J.; Mohan, N.H.; Gupta, V.K. Transcriptional dynamics of porcine granulosa cells during cellular acclimation to thermal challenge. J. Therm. Biol. 2025, 127, 104064. [Google Scholar] [CrossRef]
- Richani, D.; Dunning, K.R.; Thompson, J.G.; Gilchrist, R.B. Metabolic co-dependence of the oocyte and cumulus cells: Essential role in determining oocyte developmental competence. Hum. Reprod. Update. 2021, 27, 27–47. [Google Scholar] [CrossRef]
- Zhang, C.H.; Liu, X.Y.; Wang, J. Essential role of granulosa cell glucose and lipid metabolism on oocytes and the potential metabolic imbalance in polycystic ovary syndrome. Int. J. Mol. Sci. 2023, 24, 16247. [Google Scholar] [CrossRef]
- Auclair, S.; Uzbekov, R.; Elis, S.; Sanchez, L.; Kireev, I.; Lardic, L.; Dalbies-Tran, R.; Uzbekova, S. Absence of cumulus cells during in vitro maturation affects lipid metabolism in bovine oocytes. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E599–E613. [Google Scholar] [CrossRef]
- Liu, T.; Qu, J.; Tian, M.; Yang, R.; Song, X.; Li, R.; Yan, J.; Qiao, J. Lipid metabolic process involved in oocyte maturation during folliculogenesis. Front. Cell Dev. Biol. 2022, 10, 806890. [Google Scholar] [CrossRef]
- Dickinson, R.E.; Hryhorskyj, L.; Tremewan, H.; Hogg, K.; Thomson, A.A.; McNeilly, A.S.; Duncan, W.C. Involvement of the SLIT/ROBO pathway in follicle development in the fetal ovary. Reproduction 2010, 139, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.E.; Myers, M.; Duncan, W.C. Novel regulated expression of the SLIT/ROBO pathway in the ovary: Possible role during luteolysis in women. Endocrinology 2008, 149, 5024–5034. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, R.; Qin, N.; Xu, X.; Sun, X.; Chen, X.; Zhao, J. Implication of SLIT3-ROBO1/ROBO2 in granulosa cell proliferation, differentiation and follicle selection in the prehierarchical follicles of hen ovary. Cell Biol. Int. 2018, 42, 1643–1657. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Fan, X.C.; Zhang, Y.Y.; Xu, X.X.; Tyasi, T.L.; Jing, Y.; Mu, F.; Wei, M.L.; Xu, R.F. New insights into implication of the SLIT/ROBO pathway in the prehierarchical follicle development of hen ovary. Poult. Sci. 2015, 94, 2235–2246. [Google Scholar] [CrossRef]
- Thangaraju, M.; Rudelius, M.; Bierie, B.; Raffeld, M.; Sharan, S.; Hennighausen, L.; Huang, A.-M.; Sterneck, E. C/EBPdelta is a crucial regulator of pro-apoptotic gene expression during mammary gland involution. Development 2005, 132, 4675–4685. [Google Scholar] [CrossRef]
- Huang, A.M.; Rudelius, M.; Sharan, S.; McAllister, J.M.; Raffeld, M.; Christenson, L.K.; Sterneck, E. The CEBPD (C/EBPdelta) gene is induced by luteinizing hormones in ovarian theca and interstitial cells but is not essential for mouse ovary function. PLoS ONE 2007, 2, e1334. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, P.; Si, X.; Xie, X.; Liu, X.; Huang, J.; Shi, Y.; Yin, C. Integrated Metabolomic and Transcriptomic Analysis Decodes Heat Stress-Induced Metabolic Shifts in Gilt Granulosa Cells. Vet. Sci. 2025, 12, 1087. https://doi.org/10.3390/vetsci12111087
Tang P, Si X, Xie X, Liu X, Huang J, Shi Y, Yin C. Integrated Metabolomic and Transcriptomic Analysis Decodes Heat Stress-Induced Metabolic Shifts in Gilt Granulosa Cells. Veterinary Sciences. 2025; 12(11):1087. https://doi.org/10.3390/vetsci12111087
Chicago/Turabian StyleTang, Peng, Xiangyu Si, Xun Xie, Xiaomei Liu, Jianzhen Huang, Yun Shi, and Chao Yin. 2025. "Integrated Metabolomic and Transcriptomic Analysis Decodes Heat Stress-Induced Metabolic Shifts in Gilt Granulosa Cells" Veterinary Sciences 12, no. 11: 1087. https://doi.org/10.3390/vetsci12111087
APA StyleTang, P., Si, X., Xie, X., Liu, X., Huang, J., Shi, Y., & Yin, C. (2025). Integrated Metabolomic and Transcriptomic Analysis Decodes Heat Stress-Induced Metabolic Shifts in Gilt Granulosa Cells. Veterinary Sciences, 12(11), 1087. https://doi.org/10.3390/vetsci12111087






