Probiotics, Prebiotics, and Synbiotics in Pigs and Poultry: A Review of Gut Health, Performance, and Environmental Outcomes
Simple Summary
Abstract
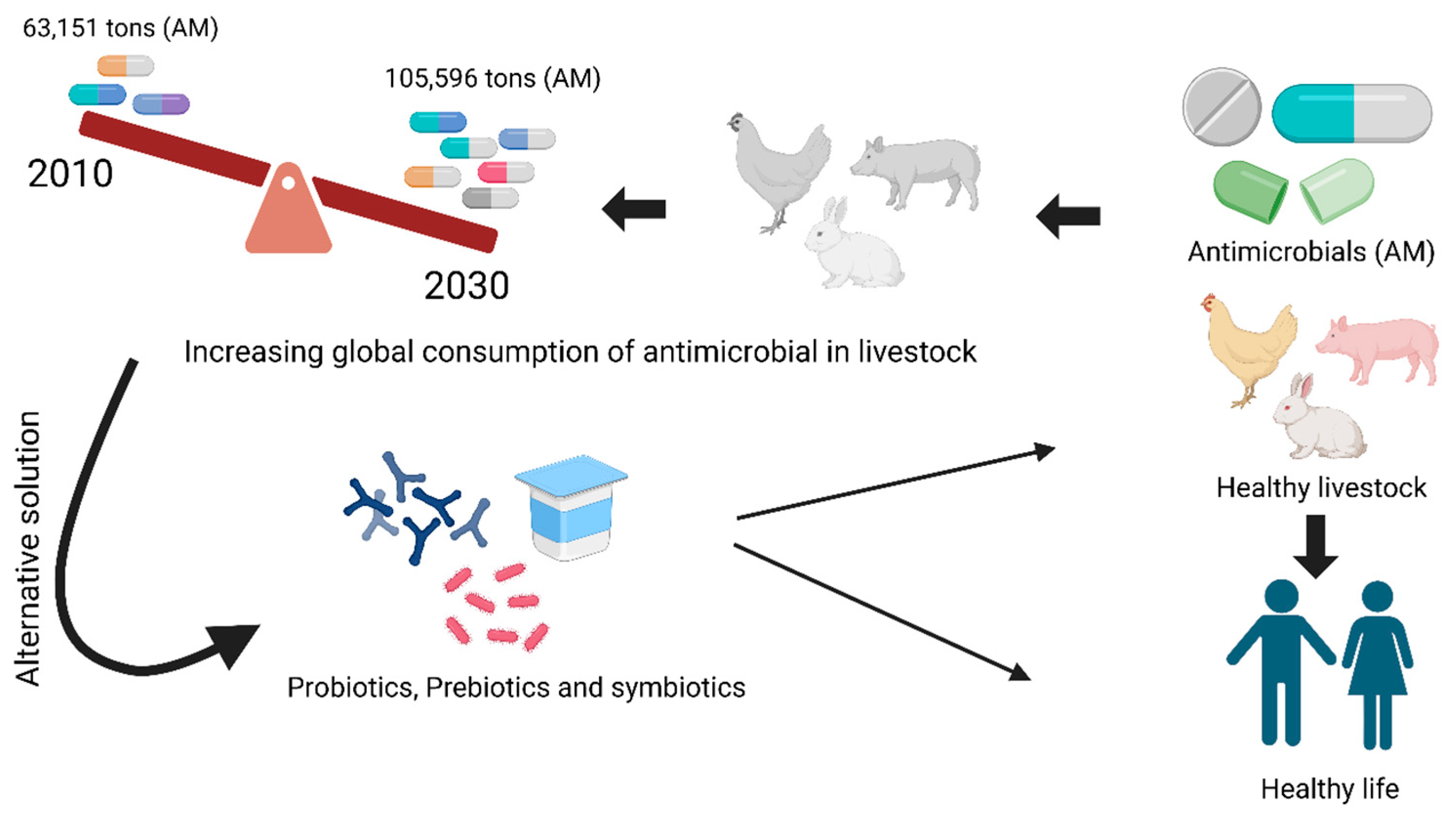
1. Introduction
2. Literature Search & Evidence Grading
2.1. Search Strategy
2.2. Eligibility Criteria (PICOS)
- Population: Monogastric food-producing species limited to pigs (suckling, weaned, grower–finisher, and sows) and poultry (broiler and layer chickens). Studies focusing exclusively on ruminants or non-target species were excluded from the review.
- Intervention: Dietary administration (feed or water) of probiotics (live microorganisms), prebiotics (selectively utilized substrates), or synbiotics (combined probiotic–prebiotic). Postbiotic studies were included only when the mechanistic endpoints were directly linked to in vivo outcomes in pigs or poultry.
- Comparator: Placebo/basal diet, standard management practices, or active controls (e.g., antibiotic growth promoters, alternative feed additives). Challenge models with pathogen exposure were eligible if a concurrent control group was present.
- Outcomes: At least one primary in vivo outcome relevant to health or productivity was required, including one or more of the following: average daily gain (ADG), body weight (BW), feed intake, FCR, morbidity (e.g., diarrhea incidence) or mortality, nutrient digestibility/retention, nitrogen or phosphorus excretion, and environmental emission proxies (e.g., ammonia). Mechanistic and physiological endpoints were included when measured alongside in vivo performance or health outcomes in pigs or poultry. These endpoints included gut barrier integrity, tight junction expression, mucin, immune markers such as IgA and cytokines, SCFA, microbiota composition and diversity, and pathogen load. Endpoints were also included when they were clearly linked to in vivo performance or health outcomes in pigs or poultry.
- Study design: In vivo randomized controlled trials (RCTs), controlled field trials, challenge trials with controls, and meta-analyses/systematic reviews. Relevant mechanistic studies were included only if they were linked to in vivo outcomes in the target species.
2.3. Additional Inclusion Parameters
3. Nutritional Strategies for Optimizing Gut Health
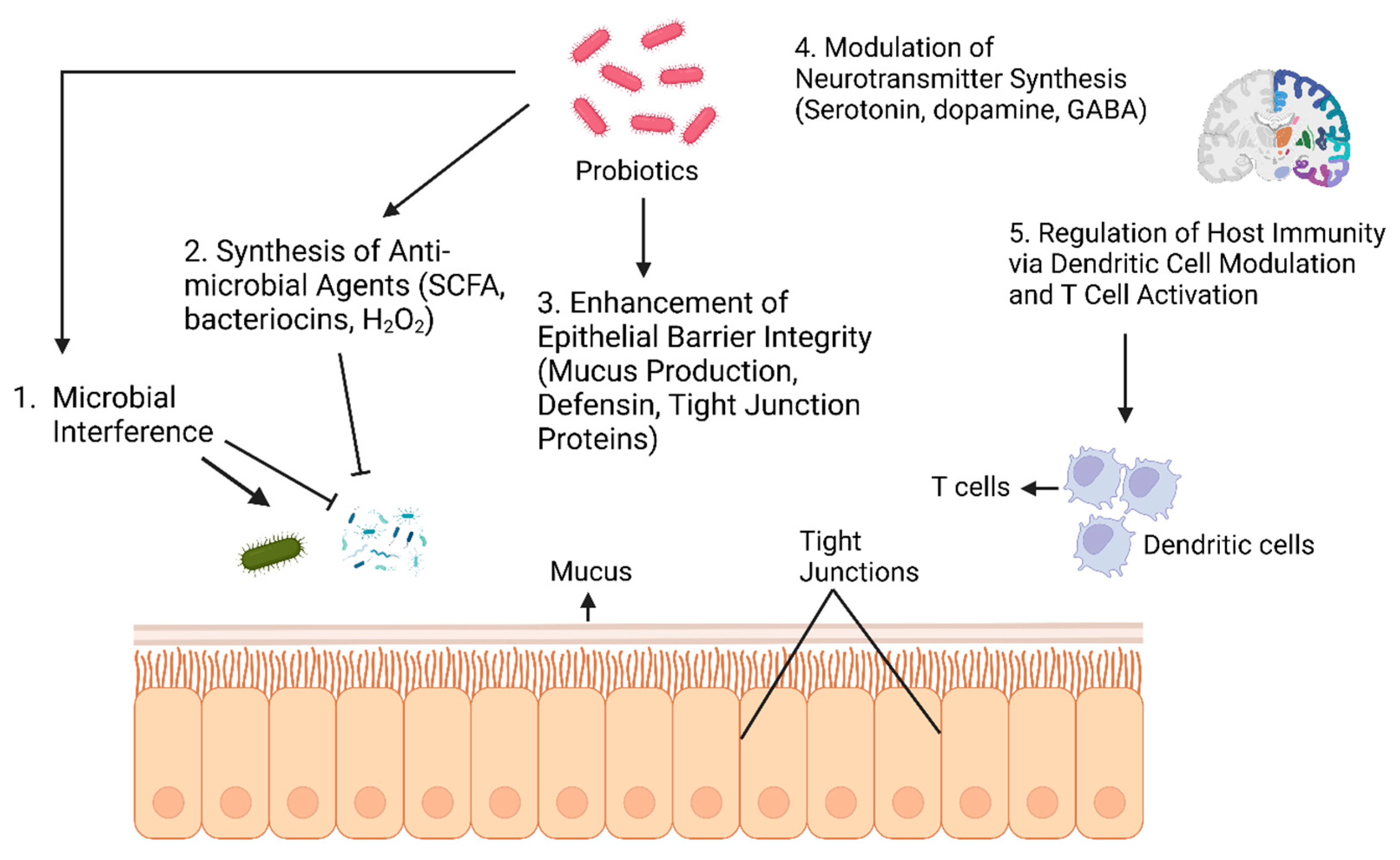
4. Probiotics and Prebiotics: Mechanisms of Action
Prebiotic Regulation Mechanisms
5. Synergistic Effects of Probiotics and Prebiotics
6. Impact on Nutrient Absorption and Utilization
7. Environmental Implications of Gut Health and Nutritional Efficiency
8. Challenges and Considerations
9. Future Directions and Research Gaps
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.W.; Gormley, A.; Jang, K.B.; Duarte, M.E. Current Status of Global Pig Production: An Overview and Research Trends. Anim. Biosci. 2024, 37, 719–729. [Google Scholar] [CrossRef]
- Galli, C. Current Techniques of Gene Editing in Pigs for Xenotransplantation. Transpl. Int. 2025, 38, 13807. [Google Scholar] [CrossRef]
- Shirini, K.; Ladowski, J.M.; Meier, R.P.H. Xenotransplantation Literature Update July–December 2024. Xenotransplantation 2025, 32, e70027. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Pan, D.; Guo, H.; Jiang, H.; Wang, J.; Feng, H.; He, S.; Du, J.; Zhang, M.; et al. Pig-to-Human Kidney Xenotransplants Using Genetically Modified Minipigs. Cell Rep. Med. 2024, 5, 101744. [Google Scholar] [CrossRef]
- Leone, F.; Ferrante, V. Effects of Prebiotics and Precision Biotics on Performance, Animal Welfare and Environmental Impact. A Review. Sci. Total Environ. 2023, 901, 165951. [Google Scholar] [CrossRef]
- Wu, S.-B. Advancements in Animal Nutrition: The Interplay of Feed Enzymes, Gut Health, and Nutrient Supply in Poultry and Pig Production—A Tribute to Professor Mingan Choct’s 30-Year Scientific Legacy. Anim. Nutr. 2024, 17, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Bhogoju, S.; Nahashon, S. Recent Advances in Probiotic Application in Animal Health and Nutrition: A Review. Agriculture 2022, 12, 304. [Google Scholar] [CrossRef]
- Rawal, S.; Kaur, H.; Bhathan, S.; Mittal, D.; Kaur, G.; Ali, S.A. Ruminant Gut Microbiota: Interplay, Implications, and Innovations for Sustainable Livestock Production. In Sustainable Agriculture Reviews; Kumar Yata, V., Mohanty, A.K., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer Nature: Cham, Switzerland, 2024; Volume 62, pp. 205–228. ISBN 978-3-031-54371-5. [Google Scholar]
- Giorgi, S. Nutritional Strategies for Improving the Gut Health of Monogastric Animals. Ph.D. Thesis, University of Milan, Milan, Italy, 2021. Available online: https://hdl.handle.net/20.500.14242/170068 (accessed on 17 September 2025).
- Kumar, P.; Abubakar, A.A.; Verma, A.K.; Umaraw, P.; Adewale Ahmed, M.; Mehta, N.; Nizam Hayat, M.; Kaka, U.; Sazili, A.Q. New Insights in Improving Sustainability in Meat Production: Opportunities and Challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 11830–11858. [Google Scholar] [CrossRef]
- Capper, J.L. Healthy Livestock Produce Sustainable Food–The Impacts of Livestock Health and the Performance-Enhancing Technologies on Environmental and Economic Sustainability. Merck Anim. Health 2021. [Google Scholar]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.-M.; Verlhac, V. Gastrointestinal Functionality in Animal Nutrition and Health: New Opportunities for Sustainable Animal Production. Anim. Feed. Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Asml, A.; Invernizzi, G.; Bontempo, V.; Savoini, G. The Beneficial Role of Probiotics in Monogastric Animal Nutrition and Health. J. Dairy Vet. Anim. Res. 2015, 2, 00041. [Google Scholar]
- Richards, J.D.; Gong, J.; De Lange, C.F.M. The Gastrointestinal Microbiota and Its Role in Monogastric Nutrition and Health with an Emphasis on Pigs: Current Understanding, Possible Modulations, and New Technologies for Ecological Studies. Can. J. Anim. Sci. 2005, 85, 421–435. [Google Scholar] [CrossRef]
- Bakke, A.M.; Glover, C.; Krogdahl, Å. Feeding, Digestion and Absorption of Nutrients. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 30, pp. 57–110. [Google Scholar]
- Clements, K.D.; Raubenheimer, D. Feeding and Nutrition. Physiol. Fishes 2006, 47, 82. [Google Scholar]
- Saha, S.K.; Pathak, N.N. Digestion, Absorption and Metabolism of Nutrients. In Fundamentals of Animal Nutrition; Springer: Singapore, 2021; pp. 219–246. ISBN 978-981-15-9124-2. [Google Scholar]
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in Pig and Poultry Nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Dalby, F.R.; Hansen, M.J.; Guldberg, L.B.; Hafner, S.D.; Feilberg, A. Simple Management Changes Drastically Reduce Pig House Methane Emission in Combined Experimental and Modeling Study. Environ. Sci. Technol. 2023, 57, 3990–4002. [Google Scholar] [CrossRef]
- Seradj, A.R.; Balcells, J.; Sarri, L.; Fraile, L.J.; de la Fuente Oliver, G. The Impact of Producing Type and Dietary Crude Protein on Animal Performances and Microbiota Together with Greenhouse Gases Emissions in Growing Pigs. Animals 2020, 10, 1742. [Google Scholar] [CrossRef]
- Jayaraman, B.; Nyachoti, C.M. Husbandry Practices and Gut Health Outcomes in Weaned Piglets: A Review. Anim. Nutr. 2017, 3, 205–211. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Z.; Wang, Y.; Li, L.; Jia, H.; Zhang, L. Compound Probiotics Can Improve Intestinal Health by Affecting the Gut Microbiota of Broilers. J. Anim. Sci. 2023, 101, skad388. [Google Scholar] [CrossRef]
- Sun, W.; Chen, W.; Meng, K.; Cai, L.; Li, G.; Li, X.; Jiang, X. Dietary Supplementation with Probiotic Bacillus Licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets. Biology 2023, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, R.; Kim, S.H.; Oh, J.K.; Song, J.H.; Hwang, I.-C.; Kim, I.H.; Kang, D.-K. Multispecies Probiotic Supplementation in Diet with Reduced Crude Protein Levels Altered the Composition and Function of Gut Microbiome and Restored Microbiome-Derived Metabolites in Growing Pigs. Front. Microbiol. 2023, 14, 1192249. [Google Scholar] [CrossRef]
- Kumar, R.; Sood, U.; Gupta, V.; Singh, M.; Scaria, J.; Lal, R. Recent Advancements in the Development of Modern Probiotics for Restoring Human Gut Microbiome Dysbiosis. Indian J. Microbiol. 2020, 60, 12–25. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Probert, H.M.; Smejkal, C.W.; Gibson, G.R. Using Probiotics and Prebiotics to Improve Gut Health. Drug Discov. Today 2003, 8, 692–700. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Saxena, M.J. Feed Additives in Animal Health. In Nutraceuticals in Veterinary Medicine; Gupta, R.C., Srivastava, A., Lall, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 345–362. ISBN 978-3-030-04623-1. [Google Scholar]
- Qi, X.; Tester, R.F. Utilisation of Dietary Fibre (Non-Starch Polysaccharide and Resistant Starch) Molecules for Diarrhoea Therapy: A Mini-Review. Int. J. Biol. Macromol. 2019, 122, 572–577. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Wenk, C. Recent Advances in Animal Feed Additives Such as Metabolic Modifiers, Antimicrobial Agents, Probiotics, Enzymes and Highly Available Minerals—Review. Asian-Australas. J. Anim. Sci. 2000, 13, 86–95. [Google Scholar] [CrossRef]
- Franz, C.; Baser, K.; Windisch, W. Essential Oils and Aromatic Plants in Animal Feeding—A European Perspective. A Review. Flavour Fragr. J. 2010, 25, 327–340. [Google Scholar] [CrossRef]
- Swanson, K.S.; Allenspach, K.; Amos, G.; Auchtung, T.A.; Bassett, S.A.; Bjørnvad, C.R.; Everaert, N.; Martín-Orúe, S.M.; Ricke, S.C.; Ryan, E.P.; et al. Use of Biotics in Animals: Impact on Nutrition, Health, and Food Production. J. Anim. Sci. 2025, 103, skaf061. [Google Scholar] [CrossRef]
- Kiczorowska, B.; Samolińska, W.; Al-Yasiry, A.R.M.; Kiczorowski, P.; Winiarska-Mieczan, A. The Natural Feed Additives as Immunostimulants in Monogastric Animal Nutrition—A Review. Ann. Anim. Sci. 2017, 17, 605–625. [Google Scholar] [CrossRef]
- Vanderaar, P.J.; Molist, F.; van der Klis, J.D. The Central Role of Intestinal Health on the Effect of Feed Additives on Feed Intake in Swine and Poultry. Anim. Feed. Sci. Technol. 2016, 233, 64–75. [Google Scholar] [CrossRef]
- Adedokun, S.A.; Olojede, O.C. Optimizing Gastrointestinal Integrity in Poultry: The Role of Nutrients and Feed Additives. Front. Vet. Sci. 2019, 5, 348. [Google Scholar] [CrossRef]
- Das, M.; Fox, C.F. Molecular Mechanism of Mitogen Action: Processing of Receptor Induced by Epidermal Growth Factor. Proc. Natl. Acad. Sci. USA 1978, 75, 2644–2648. [Google Scholar] [CrossRef]
- Malijan, G.M.; Howteerakul, N.; Ali, N.; Siri, S.; Kengganpanich, M.; Nascimento, R.; Booton, R.D.; Turner, K.M.E.; Cooper, B.S.; Meeyai, A. A Scoping Review of Antibiotic Use Practices and Drivers of Inappropriate Antibiotic Use in Animal Farms in WHO Southeast Asia Region. One Health 2022, 15, 100412. [Google Scholar] [CrossRef] [PubMed]
- Price, L.B.; Koch, B.J.; Hungate, B.A. Ominous Projections for Global Antibiotic Use in Food-Animal Production. Proc. Natl. Acad. Sci. USA 2015, 112, 5554–5555. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N. Mitigation of Antibiotic Resistance Using Probiotics, Prebiotics and Synbiotics. A Review. Environ. Chem. Lett. 2022, 20, 1295–1308. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Badawy, N.K.; Ashraf, Y.; Zatioun, A.A.; Masriya, H.H.; Ammar, M.M.; Mohamed, N.A.; Mourad, S.; Assy, A.M. Combating Antibiotic Resistance: Mechanisms, Multidrug-Resistant Pathogens, and Novel Therapeutic Approaches: An Updated Review. Pharmaceuticals 2025, 18, 402. [Google Scholar] [CrossRef]
- Kober, A.K.M.H.; Riaz Rajoka, M.S.; Mehwish, H.M.; Villena, J.; Kitazawa, H. Immunomodulation Potential of Probiotics: A Novel Strategy for Improving Livestock Health, Immunity, and Productivity. Microorganisms 2022, 10, 388. [Google Scholar] [CrossRef]
- Pokharel, S.; Shrestha, P.; Adhikari, B. Antimicrobial Use in Food Animals and Human Health: Time to Implement ‘One Health’ Approach. Antimicrob. Resist. Infect Control. 2020, 9, 181. [Google Scholar] [CrossRef]
- Sucena Afonso, J.; El Tholth, M.; Mcintyre, K.M.; Carmo, L.P.; Coyne, L.; Manriquez, D.; Raboisson, D.; Lhermie, G.; Rushton, J. Strategies to Reduce Antimicrobials in Livestock and Aquaculture, and Their Impact under Field Conditions: A Structured Scoping Literature Review. J. Antimicrob. Chemother. 2024, 79, 11–26. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A.O. Fermentation of Prebiotics by Human Colonic Microbiota in Vitro and Short-Chain Fatty Acids Production: A Critical Review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Chen, J.; Guo, X.; Yan, L.; Guo, Y.; Wang, B.; Yuan, J. The Duration of Food Withdrawal Affects the Intestinal Structure, Nutrients Absorption, and Utilization in Broiler Chicken. FASEB J. 2021, 35, e21178. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Habte-Tsion, H.-M.; Kumar, V. Chapter 9—Nonstarch Polysaccharide Enzymes—General Aspects. In Enzymes in Human and Animal Nutrition; Nunes, C.S., Kumar, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 183–209. ISBN 978-0-12-805419-2. [Google Scholar]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, W.; Wu, G. Composition of Amino Acids in Foodstuffs for Humans and Animals. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1332, pp. 189–210. ISBN 978-3-030-74179-2. [Google Scholar]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative Stress, Hormones, and Effects of Natural Antioxidants on Intestinal Inflammation in Inflammatory Bowel Disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Rahaman, M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural Antioxidants from Some Fruits, Seeds, Foods, Natural Products, and Associated Health Benefits: An Update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-Martins, N.; Kuča, K.; Chopra, C.; Singh, R.; Kumar, H.; Șen, F.; et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules 2021, 11, 440. [Google Scholar] [CrossRef]
- Dai, Z.; Cui, L.; Li, J.; Wang, B.; Guo, L.; Wu, Z.; Zhu, W.; Wu, G. Chapter 24—Fermentation Techniques in Feed Production. In Animal Agriculture; Bazer, F.W., Lamb, G.C., Wu, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 407–429. ISBN 978-0-12-817052-6. [Google Scholar]
- Vandenberghe, L.P.S.; Pandey, A.; Carvalho, J.C.; Letti, L.A.J.; Woiciechowski, A.L.; Karp, S.G.; Thomaz-Soccol, V.; Martínez-Burgos, W.J.; Penha, R.O.; Herrmann, L.W.; et al. Solid-State Fermentation Technology and Innovation for the Production of Agricultural and Animal Feed Bioproducts. Syst. Microbiol. Biomanuf. 2021, 1, 142–165. [Google Scholar] [CrossRef]
- Makkar, H. Smart Livestock Feeding Strategies for Harvesting Triple Gain—The Desired Outcomes in Planet, People and Profit Dimensions: A Developing Country Perspective. Anim. Prod. Sci. 2016, 56, 519–534. [Google Scholar] [CrossRef]
- Bocquier, F.; González-García, E. Sustainability of Ruminant Agriculture in the New Context: Feeding Strategies and Features of Animal Adaptability into the Necessary Holistic Approach. Animal 2010, 4, 1258–1273. [Google Scholar] [CrossRef]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The Promotion Mechanism of Prebiotics for Probiotics: A Review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Cereals & Grains Association. Available online: https://www.cerealsgrains.org/Pages/default.aspx (accessed on 1 September 2025).
- Ng, S.C.; Hart, A.L.; Kamm, M.A.; Stagg, A.J.; Knight, S.C. Mechanisms of Action of Probiotics: Recent Advances. Inflamm. Bowel Dis. 2009, 15, 300–310. [Google Scholar] [CrossRef]
- Khare, A.; Thorat, G.; Bhimte, A.; Yadav, V. Mechanism of Action of Prebiotic and Probiotic. Immunity 2018, 3, 27. [Google Scholar]
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I.S. Mechanism of Action of Probiotics. Braz. Arch. Biol. Technol. 2013, 56, 113–119. [Google Scholar] [CrossRef]
- Gogineni, V.K.; Morrow, L.E.; Malesker, M.A. Probiotics: Mechanisms of Action and Clinical Applications. J. Prob. Health 2013, 1, 1–11. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Yue, T.; Lu, Y.; Ding, W.; Xu, B.; Zhang, C.; Li, L.; Jian, F.; Huang, S. The Role of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Livestock and Poultry Gut Health: A Review. Metabolites 2025, 15, 478. [Google Scholar] [CrossRef]
- Liu, S.; Cai, P.; You, W.; Yang, M.; Tu, Y.; Zhou, Y.; Valencak, T.G.; Xiao, Y.; Wang, Y.; Shan, T. Enhancement of Gut Barrier Integrity by a Bacillus Subtilis Secreted Metabolite through the GADD45A-Wnt/Β-catenin Pathway. Imeta 2025, 4, e70005. [Google Scholar] [CrossRef]
- Dhama, K.; Mahendran, M.; Tomar, S.; Chauhan, R.S. Beneficial Effects of Probiotics and Prebiotics in Livestock and Poultry: The Current Perspectives. Intas Polivet 2008, 9, 1–12. [Google Scholar]
- Markowiak, P.; Śliżewska, K. The Role of Probiotics, Prebiotics and Synbiotics in Animal Nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Samanta, A.K.; Jayapal, N.; Senani, S.; Kolte, A.P.; Sridhar, M. Prebiotic Inulin: Useful Dietary Adjuncts to Manipulate the Livestock Gut Microflora. Braz. J. Microbiol. 2013, 44, 1–14. [Google Scholar] [CrossRef]
- Uyeno, Y.; Shigemori, S.; Shimosato, T. Effect of Probiotics/Prebiotics on Cattle Health and Productivity. Microbes Environ. 2015, 30, 126–132. [Google Scholar] [CrossRef]
- Saghir, S.A.M.; Al Suede, F.S. Synergistic Efficacy and Mechanism of Probiotics and Prebiotics in Enhancing Health Impact. Microb. Bioact. 2024, 7, 1–11. [Google Scholar] [CrossRef]
- Ren, H.; Vahjen, W.; Dadi, T.; Saliu, E.-M.; Boroojeni, F.G.; Zentek, J. Synergistic Effects of Probiotics and Phytobiotics on the Intestinal Microbiota in Young Broiler Chicken. Microorganisms 2019, 7, 684. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Tiihonen, K.; Mäkivuokko, H.; Rautonen, N. Synbiotics: Combining the Benefits of Pre-and Probiotics. In Functional Dairy Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 195–213. [Google Scholar]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Ohshima, T.; Seneviratne, C.J.; Maeda, N. Combining Prebiotics and Probiotics to Develop Novel Synbiotics That Suppress Oral Pathogens. J. Oral Biosci. 2016, 58, 27–32. [Google Scholar] [CrossRef]
- Femia, A.P.; Luceri, C.; Dolara, P.; Giannini, A.; Biggeri, A.; Salvadori, M.; Clune, Y.; Collins, K.J.; Paglierani, M.; Caderni, G. Antitumorigenic Activity of the Prebiotic Inulin Enriched with Oligofructose in Combination with the Probiotics Lactobacillus Rhamnosus and Bifidobacterium Lactis on Azoxymethane-Induced Colon Carcinogenesis in Rats. Carcinogenesis 2002, 23, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Fayed, B.; El-Sayed, H.; Ismail, S. Novel Assessment of Synergistic Stimulatory Effect of Prebiotic Chitooligosaccharide and Some Commercial Prebiotics on the Probiotic Growth: A Preliminary Study. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e3341. [Google Scholar] [CrossRef]
- de Souza Oliveira, R.P.; Perego, P.; de Oliveira, M.N.; Converti, A. Effect of Inulin as Prebiotic and Synbiotic Interactions between Probiotics to Improve Fermented Milk Firmness. J. Food Eng. 2011, 107, 36–40. [Google Scholar] [CrossRef]
- Adamberg, S.; Sumeri, I.; Uusna, R.; Ambalam, P.; Kondepudi, K.K.; Adamberg, K.; Wadström, T.; Ljungh, Å. Survival and Synergistic Growth of Mixed Cultures of Bifidobacteria and Lactobacilli Combined with Prebiotic Oligosaccharides in a Gastrointestinal Tract Simulator. Microb. Ecol. Heal. Dis. 2014, 25, 23062. [Google Scholar] [CrossRef]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef]
- Seo, K.-H.; Jeong, J.; Kim, H. Synergistic Effects of Heat-Killed Kefir Paraprobiotics and Flavonoid-Rich Prebiotics on Western Diet-Induced Obesity. Nutrients 2020, 12, 2465. [Google Scholar] [CrossRef]
- Sekhon, B.S.; Jairath, S. Prebiotics, Probiotics and Synbiotics: An Overview. J. Pharm. Educ. Res. 2010, 1, 13–36. [Google Scholar]
- Roberfroid, M.B. Prebiotics and Probiotics: Are They Functional Foods? Am. J. Clin. Nutr. 2000, 71, 1682S–1687S. [Google Scholar] [CrossRef]
- Manigandan, T.; Mangaiyarkarasi, S.P.; Hemalatha, R.; Hemalatha, V.T.; Murali, N.P. Probiotics, Prebiotics and Synbiotics-a Review. Biomed. Pharmacol. J. 2012, 5, 295. [Google Scholar] [CrossRef]
- Legette, L.L.; Lee, W.-H.; Martin, B.R.; Story, J.A.; Arabshahi, A.; Barnes, S.; Weaver, C.M. Genistein, a Phytoestrogen, Improves Total Cholesterol, and Synergy, a Prebiotic, Improves Calcium Utilization, but There Were No Synergistic Effects. Menopause 2011, 18, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium Strains and Galactooligosaccharides Improve Intestinal Barrier Function in Obese Adults but Show No Synergism When Used Together as Synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.A.; Marette, A. Potential Health Benefits of Combining Yogurt and Fruits Based on Their Probiotic and Prebiotic Properties. Adv. Nutr. 2017, 8, 155S–164S. [Google Scholar] [CrossRef]
- Choi, S.M.; Lin, H.; Xie, W.; Chu, I.K. Study of Potential Synergistic Effect of Probiotic Formulas on Acrylamide Reduction. Int. J. Mol. Sci. 2023, 24, 4693. [Google Scholar] [CrossRef]
- Shini, S.; Bryden, W.L. Probiotics and Gut Health: Linking Gut Homeostasis and Poultry Productivity. Anim. Prod. Sci. 2021, 62, 1090–1112. [Google Scholar] [CrossRef]
- Mucci, R. Effects of Different Probiotics and Synbiotics and Mode of Their Administration on Productive Performance, Carcass Traits and Meat Quality in Broiler Chickens. Ph.D. Thesis, University of Molise, Campobasso, Italy, 2017. [Google Scholar]
- Mounir, M.; Ibijbijen, A.; Farih, K.; Rabetafika, H.N.; Razafindralambo, H.L. Synbiotics and Their Antioxidant Properties, Mechanisms, and Benefits on Human and Animal Health: A Narrative Review. Biomolecules 2022, 12, 1443. [Google Scholar] [CrossRef]
- Lambo, M.T.; Chang, X.; Liu, D. The Recent Trend in the Use of Multistrain Probiotics in Livestock Production: An Overview. Animals 2021, 11, 2805. [Google Scholar] [CrossRef]
- Malik, J.K.; Prakash, A.; Srivastava, A.K.; Gupta, R.C. Synbiotics in Animal Health and Production. In Nutraceuticals in Veterinary Medicine; Gupta, R.C., Srivastava, A., Lall, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 287–301. ISBN 978-3-030-04623-1. [Google Scholar]
- Goh, J.X.H.; Tan, L.T.; Law, J.W.; Ser, H.; Khaw, K.; Letchumanan, V.; Lee, L.; Goh, B. Harnessing the Potentialities of Probiotics, Prebiotics, Synbiotics, Paraprobiotics, and Postbiotics for Shrimp Farming. Rev. Aquac. 2022, 14, 1478–1557. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, T.; Wang, Y.; Li, T.; Chi, Q. Multifaceted Impacts of Nanoparticles on Plant Nutrient Absorption and Soil Microbial Communities. Front. Plant Sci. 2024, 15, 1497006. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Jha, R. Strategies to Modulate the Intestinal Microbiota and Their Effects on Nutrient Utilization, Performance, and Health of Poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Xu, P.; Gao, Y.; Cui, Z.; Wu, B.; Yan, B.; Wang, Y.; Zaitongguli, K.; Wen, M.; Wang, H.; Jing, N. Research Progress on Effects of Biochar on Soil Environment and Crop Nutrient Absorption and Utilization. Sustainability 2023, 15, 4861. [Google Scholar] [CrossRef]
- Singh, A.K.; Kim, W.K. Effects of Dietary Fiber on Nutrients Utilization and Gut Health of Poultry: A Review of Challenges and Opportunities. Animals 2021, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, V.; Kornegay, E.T.; Webb Jr, K.E. Effects of Fiber and Virginiamycin on Nutrient Absorption, Nutrient Retention and Rate of Passage in Growing Swine. J. Anim. Sci. 1984, 59, 400–408. [Google Scholar] [CrossRef]
- Rani, R.P.; Nishant, A.K.; Kumar, G. Nutrient utilization and crop metabolism. Crop Physiol. A Collab. Insights 2023, 86. [Google Scholar]
- Gilbert, E.R.; Wong, E.A.; Webb Jr, K.E. Board-Invited Review: Peptide Absorption and Utilization: Implications for Animal Nutrition and Health. J. Anim. Sci. 2008, 86, 2135–2155. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light Regulation of Horticultural Crop Nutrient Uptake and Utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Trovato, A.; Nuhlicek, D.N.; Midtling, J.E. Drug-Nutrient Interactions. Am. Fam. Physician 1991, 44, 1651–1658. [Google Scholar]
- Selim, S.; Abdel-Megeid, N.S.; Khalifa, H.K.; Fakiha, K.G.; Majrashi, K.A.; Hussein, E. Efficacy of Various Feed Additives on Performance, Nutrient Digestibility, Bone Quality, Blood Constituents, and Phosphorus Absorption and Utilization of Broiler Chickens Fed Low Phosphorus Diet. Animals 2022, 12, 1742. [Google Scholar] [CrossRef]
- Ren, W.; Li, X.; Liu, T.; Chen, N.; Xin, M.; Liu, B.; Qi, Q.; Li, G. Impact of Fertilization Depth on Sunflower Yield and Nitrogen Utilization: A Perspective on Soil Nutrient and Root System Compatibility. Front. Plant Sci. 2024, 15, 1440859. [Google Scholar] [CrossRef] [PubMed]
- Piantoni, P.; VandeHaar, M.J. Symposium Review: The Impact of Absorbed Nutrients on Energy Partitioning throughout Lactation. J. Dairy Sci. 2023, 106, 2167–2180. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Adavi, S.B.; Kumar, A.; Bamatov, I.; Ivanova, E.; Behera, L.; Jena, R.; Kumar, R. Deciphering Phenomics Approaches for Understanding Plant–Microbe Interactions in Nutrient Absorption and Utilization. Plant Physiol. Rep. 2024, 29, 769–785. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Farmer, S.; Cash, H.A.; Keller, D. Probiotic Bacillus Coagulans GBI-30, 6086 Improves Protein Absorption and Utilization. Probiotics Antimicrob. Proteins 2018, 10, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liao, S.F. Physiological Effects of Dietary Amino Acids on Gut Health and Functions of Swine. Front. Vet. Sci. 2019, 6, 169. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Lin, Z.; Liu, C.; Zhang, Y.; Zhang, S.; Zhou, M.; Zhao, J.; Liu, H.; Ma, X. Clostridium Butyricum Alleviates Weaned Stress of Piglets by Improving Intestinal Immune Function and Gut Microbiota. Food Chem. 2023, 405, 135014. [Google Scholar] [CrossRef]
- Kim, K.; Song, M.; Liu, Y.; Ji, P. Enterotoxigenic Escherichia coli Infection of Weaned Pigs: Intestinal Challenges and Nutritional Intervention to Enhance Disease Resistance. Front. Immunol. 2022, 13, 885253. [Google Scholar] [CrossRef]
- Kobek-Kjeldager, C.; Schönherz, A.A.; Canibe, N.; Pedersen, L.J. Diet and Microbiota-Gut-Brain Axis in Relation to Tail Biting in Pigs: A Review. Appl. Anim. Behav. Sci. 2022, 246, 105514. [Google Scholar] [CrossRef]
- Lauridsen, C. Effects of Dietary Fatty Acids on Gut Health and Function of Pigs Pre-and Post-Weaning. J. Anim. Sci. 2020, 98, skaa086. [Google Scholar] [CrossRef] [PubMed]
- Katouli, M.; Melin, L.; Jensen-Waern, M.; Wallgren, P.; Möllby, R. The Effect of Zinc Oxide Supplementation on the Stability of the Intestinal Flora with Special Reference to Composition of Coliforms in Weaned Pigs. J. Appl. Microbiol. 1999, 87, 564–573. [Google Scholar] [CrossRef]
- Jia, R.; Sadiq, F.A.; Liu, W.; Cao, L.; Shen, Z. Protective Effects of Bacillus Subtilis ASAG 216 on Growth Performance, Antioxidant Capacity, Gut Microbiota and Tissues Residues of Weaned Piglets Fed Deoxynivalenol Contaminated Diets. Food Chem. Toxicol. 2021, 148, 111962. [Google Scholar] [CrossRef] [PubMed]
- Fouhse, J.M.; Zijlstra, R.T.; Willing, B.P. The Role of Gut Microbiota in the Health and Disease of Pigs. Anim. Front. 2016, 6, 30–36. [Google Scholar] [CrossRef]
- Wahlqvist, P.; Reilly, M.C.; Barkun, A. Systematic Review: The Impact of Gastro-oesophageal Reflux Disease on Work Productivity. Aliment. Pharmacol. Ther. 2006, 24, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Voordt, T.V.D.; Jensen, P.A. The Impact of Healthy Workplaces on Employee Satisfaction, Productivity and Costs. J. Corp. Real Estate 2023, 25, 29–49. [Google Scholar] [CrossRef]
- Ford, M.T.; Cerasoli, C.P.; Higgins, J.A.; Decesare, A.L. Relationships between Psychological, Physical, and Behavioural Health and Work Performance: A Review and Meta-Analysis. Work. Stress 2011, 25, 185–204. [Google Scholar] [CrossRef]
- Diet, I.B.S.; Foods-Why, I.D.T.; List-Stop, I.T.F.; Can’t Eat Anything, W.Y.; Deprivation, S.N.; Diet, F.; Teas, T.; Books, I.B.S.; Books, A.; Hypnotherapy, G.D. Impairment in Work Productivity and Health-Related Quality of Life in Patients with IBS. Am. J. Manag. Care 2005, 11, S17–S26. [Google Scholar]
- Frändemark, Å.; Törnblom, H.; Hreinsson, J.P.; Andresen, V.; Benninga, M.A.; Corazziari, E.S.; Fukudo, S.; Mulak, A.; Santos, J.; Sperber, A.D.; et al. Work Productivity and Activity Impairment in Disorders of Gut-brain Interaction: Data from the Rome Foundation Global Epidemiology Study. UEG J. 2023, 11, 503–513. [Google Scholar] [CrossRef]
- Frändemark, Å.; Törnblom, H.; Jakobsson, S.; Simrén, M. Work Productivity and Activity Impairment in Irritable Bowel Syndrome (IBS): A Multifaceted Problem. Off. J. Am. Coll. Gastroenterol. ACG 2018, 113, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- van Gennep, S.; Gielen, M.E.; Rietdijk, S.T.; de Boer, N.K.; Duijvestein, M.; Gecse, K.B.; Ponsioen, C.Y.; D’Haens, G.R.; de Boer, A.G.; Löwenberg, M. Work Productivity Loss Is Determined by Fatigue and Reduced Quality of Life in Employed Inflammatory Bowel Disease Patients: A Prospective Multicentre Cohort Study. Eur. J. Gastroenterol. Hepatol. 2021, 33, e594–e602. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.X.; DiBonaventura, M.; Purayidathil, F.W.; Wagner, J.-S.; Dabbous, O.; Mody, R. Impact of Chronic Constipation on Health-Related Quality of Life, Work Productivity, and Healthcare Resource Use: An Analysis of the National Health and Wellness Survey. Dig. Dis. Sci. 2011, 56, 2688–2695. [Google Scholar] [CrossRef]
- Loeppke, R.; Taitel, M.; Haufle, V.; Parry, T.; Kessler, R.C.; Jinnett, K. Health and Productivity as a Business Strategy: A Multiemployer Study. J. Occup. Environ. Med. 2009, 51, 411–428. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Mondragon, A.d.C.; Cardelle-Cobas, A.; Santos, E.M.; Porto-Arias, J.J.; Cepeda, A.; Miranda, J.M. Effects of Unconventional Work and Shift Work on the Human Gut Microbiota and the Potential of Probiotics to Restore Dysbiosis. Nutrients 2023, 15, 3070. [Google Scholar] [CrossRef]
- Santin, E. Gut Health: Environment, Climate Change and Agriculture Sustainability. In Environmental Effects on Gut Health in Production Animals; Wageningen Academic: Wageningen, The Netherlands, 2024; pp. 129–138. [Google Scholar]
- Raiten, D.J.; Aimone, A.M. The Intersection of Climate/Environment, Food, Nutrition and Health: Crisis and Opportunity. Curr. Opin. Biotechnol. 2017, 44, 52–62. [Google Scholar] [CrossRef]
- Lindberg, J.E. Nutrient and Energy Supply in Monogastric Food Producing Animals with Reduced Environmental and Climatic Footprint and Improved Gut Health. Animal 2023, 17, 100832. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The Interplay between Gut Microbiota, Short-Chain Fatty Acids, and Implications for Host Health and Disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Huang, S.-C.; He, Y.-F.; Chen, P.; Liu, K.-L.; Shaukat, A. Gut Microbiota as a Target in the Bone Health of Livestock and Poultry: Roles of Short-Chain Fatty Acids. Anim. Dis. 2023, 3, 23. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary Gut Microbial Metabolites, Short-Chain Fatty Acids, and Host Metabolic Regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Jha, R.; Mishra, P. Dietary Fiber in Poultry Nutrition and Their Effects on Nutrient Utilization, Performance, Gut Health, and on the Environment: A Review. J. Anim. Sci. Biotechnol. 2021, 12, 51. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Moradi, S.; Abdollahi, M.R.; Moradi, A.; Jamshidi, L. Effect of Bacterial Phytase on Growth Performance, Nutrient Utilization, and Bone Mineralization in Broilers Fed Pelleted Diets. Animals 2023, 13, 1450. [Google Scholar] [CrossRef] [PubMed]
- Nuamah, E.; Okon, U.M.; Jeong, E.; Mun, Y.; Cheon, I.; Chae, B.; Odoi, F.N.A.; Kim, D.; Choi, N.-J. Unlocking Phytate with Phytase: A Meta-Analytic View of Meat-Type Chicken Muscle Growth and Bone Mineralization Potential. Animals 2024, 14, 2090. [Google Scholar] [CrossRef] [PubMed]
- Pirzado, S.A.; Liu, G.; Purba, M.A.; Cai, H. Enhancing the Production Performance and Nutrient Utilization of Laying Hens by Augmenting Energy, Phosphorous and Calcium Deficient Diets with Fungal Phytase (Trichoderma reesei) Supplementation. Animals 2024, 14, 376. [Google Scholar] [CrossRef]
- Selle, P.H.; Macelline, S.P.; Chrystal, P.V.; Liu, S.Y. The Contribution of Phytate-Degrading Enzymes to Chicken-Meat Production. Animals 2023, 13, 603. [Google Scholar] [CrossRef]
- Valente Junior, D.T.; Genova, J.L.; Kim, S.W.; Saraiva, A.; Rocha, G.C. Carbohydrases and Phytase in Poultry and Pig Nutrition: A Review beyond the Nutrients and Energy Matrix. Animals 2024, 14, 226. [Google Scholar] [CrossRef]
- Bindelle, J.; Leterme, P.; Buldgen, A. Nutritional and Environmental Consequences of Dietary Fibre in Pig Nutrition: A Review. Biotechnol. Agron. Société Et Environ. 2008, 12, 69–80. [Google Scholar]
- Chang, S.Y.; Song, M.H.; Lee, J.H.; Oh, H.J.; Kim, Y.J.; An, J.W.; Go, Y.B.; Song, D.C.; Cho, H.A.; Cho, S.Y.; et al. Phytogenic Feed Additives Alleviate Pathogenic Escherichia coli-Induced Intestinal Damage through Improving Barrier Integrity and Inhibiting Inflammation in Weaned Pigs. J. Anim. Sci. Biotechnol. 2022, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jinno, C.; Li, C.; Johnston, S.L.; Xue, H.; Liu, Y.; Ji, P. Effects of a Blend of Essential Oils, Medium-Chain Fatty Acids, and a Toxin-Adsorbing Mineral on Diarrhea and Gut Microbiome of Weanling Pigs Experimentally Infected with a Pathogenic Escherichia coli. J. Anim. Sci. 2021, 100, skab365. [Google Scholar] [CrossRef] [PubMed]
- Nathan, V.B.; Eckrote, S.; Li, S.; Reddivari, L. Crude Blueberry Phenolic Extracts Improve Gut Barrier Integrity and Exert Anti-Inflammatory and Antimicrobial Activity in an In Vitro Weaning Stress Model. Antioxidants 2024, 13, 1044. [Google Scholar] [CrossRef]
- Yang, T.; Liu, B.; Wang, Y.; Huang, X.; Yan, Z.; Jiang, Q.; Chen, Q. Ellagic Acid Improves Antioxidant Capacity and Intestinal Barrier Function of Heat-Stressed Broilers via Regulating Gut Microbiota. Animals 2022, 12, 1180. [Google Scholar] [CrossRef]
- Bialkowski, S.; Toschi, A.; Yu, L.; Schlitzkus, L.; Mann, P.; Grilli, E.; Li, Y. Effects of Microencapsulated Blend of Organic Acids and Botanicals on Growth Performance, Intestinal Barrier Function, Inflammatory Cytokines, and Endocannabinoid System Gene Expression in Broiler Chickens. Poult. Sci. 2022, 102, 102460. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Ashouri, G.; Marisaldi, L.; Candelma, M.; Basili, D.; Zimbelli, A.; Notarstefano, V.; Salvini, L.; Randazzo, B.; Zarantoniello, M.; et al. Reducing the Use of Antibiotics in European Aquaculture with Vaccines, Functional Feed Additives and Optimization of the Gut Microbiota. J. Mar. Sci. Eng. 2024, 12, 204. [Google Scholar] [CrossRef]
- Mârza, S.M.; Munteanu, C.; Papuc, I.; Radu, L.; Purdoiu, R.C. The Role of Probiotics in Enhancing Animal Health: Mechanisms, Benefits, and Applications in Livestock and Companion Animals. Animals 2025, 15, 2986. [Google Scholar] [CrossRef]
- Upadhaya, S.-D.; Kim, I.-H. The Impact of Weaning Stress on Gut Health and the Mechanistic Aspects of Several Feed Additives Contributing to Improved Gut Health Function in Weanling Piglets—A Review. Animals 2021, 11, 2418. [Google Scholar] [CrossRef]
- Ashworth, C.J.; Toma, L.M.; Hunter, M.G. Nutritional Effects on Oocyte and Embryo Development in Mammals: Implications for Reproductive Efficiency and Environmental Sustainability. Phil. Trans. R. Soc. B 2009, 364, 3351–3361. [Google Scholar] [CrossRef] [PubMed]
- Aruwa, C.E.; Pillay, C.; Nyaga, M.M.; Sabiu, S. Poultry Gut Health—Microbiome Functions, Environmental Impacts, Microbiome Engineering and Advancements in Characterization Technologies. J. Anim. Sci. Biotechnol. 2021, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Gickel, J.; Hartung, C.B.; Abd El-Wahab, A.; Hankel, J.; Visscher, C. Influence of Vaccination against Infectious Diseases on the Carbon Footprint of Fattening Pigs: A Systematic Review. Front. Vet. Sci. 2024, 11, 1487742. [Google Scholar] [CrossRef]
- Kyriazakis, I.; Arndt, C.; Aubry, A.; Charlier, J.; Ezenwa, V.O.; Godber, O.F.; Krogh, M.; Mostert, P.F.; Orsel, K.; Robinson, M.W.; et al. Improve Animal Health to Reduce Livestock Emissions: Quantifying an Open Goal. Proc. R. Soc. B Biol. Sci. 2024, 291, 20240675. [Google Scholar] [CrossRef]
- Pelton, R.E.O.; Kazanski, C.E.; Keerthi, S.; Racette, K.A.; Gennet, S.; Springer, N.; Yacobson, E.; Wironen, M.; Ray, D.; Johnson, K.; et al. Greenhouse Gas Emissions in US Beef Production Can Be Reduced by up to 30% with the Adoption of Selected Mitigation Measures. Nat. Food 2024, 5, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Pereyra-Goday, F.; Jebari, A.; Takahashi, T.; Rovira, P.; Ayala, W.; Lee, M.R.F.; Rivero, M.J.; McAuliffe, G.A. Carbon Footprint of Mixed Farming Crop-Livestock Rotational-Based Grazing Beef Systems Using Long Term Experimental Data. Agron. Sustain. Dev. 2024, 44, 1–14. [Google Scholar] [CrossRef]
- Tamilselvan, G.; Tyagi, N. Life Cycle Assessment as an Evaluation Tool- A Critical Review on Carbon Footprint in Dairy Sector: Carbon Footprint of Milk. Lett. Anim. Biol. 2024, 4, 10–16. [Google Scholar] [CrossRef]
- Carter, L.J.; Dennis, S.; Allen, K.; McKenna, P.; Chen, X.; Daniell, T.J.; Evans, B.; Guest, J.S.; Guo, H.; Kirk, S.; et al. Mitigating Contaminant-Driven Risks for the Safe Expansion of the Agricultural─Sanitation Circular Economy in an Urbanizing World. ACS EST Water 2024, 4, 1166–1176. [Google Scholar] [CrossRef]
- Dou, Z. Leveraging livestock to promote a circular food system. Front. Agric. Sci. Eng. 2021, 8, 188–192. [Google Scholar] [CrossRef]
- Pinotti, L. 447 Ex-Food4feed: Keeping Nutrients in the Food Chain—Sponsored by EAAP. J. Anim. Sci. 2024, 102, 252–253. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Afshar, R.K. Livestock Manure: From Waste to Resource in a Circular Economy. J. Agric. Food Res. 2024, 17, 101255. [Google Scholar] [CrossRef]
- Simon, W.J.; Hijbeek, R.; Frehner, A.; Cardinaals, R.; Talsma, E.F.; van Zanten, H.H.E. Circular Food System Approaches Can Support Current European Protein Intake Levels While Reducing Land Use and Greenhouse Gas Emissions. Nat. Food 2024, 5, 402–412. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, G.; Gupta, T.; Mittal, D.; Ali, S.A. Integrating Omics Technologies for a Comprehensive Understanding of the Microbiome and Its Impact on Cattle Production. Biology 2023, 12, 1200. [Google Scholar] [CrossRef]
- Kazemi, M. Biochar in Animal Agriculture: Enhancing Health, Efficiency and Environmental Sustainability. Vet. Med. Sci. 2025, 11, e70629. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Gras, M.A.; Untea, A.E.; Lefter, N.A.; Rotar, M.C. Advancing Livestock Technology: Intelligent Systemization for Enhanced Productivity, Welfare, and Sustainability. AgriEngineering 2024, 6, 1479–1496. [Google Scholar] [CrossRef]
- Harrison, M.T.; Cullen, B.R.; Mayberry, D.E.; Cowie, A.L.; Bilotto, F.; Badgery, W.B.; Liu, K.; Davison, T.; Christie, K.M.; Muleke, A.; et al. Carbon Myopia: The Urgent Need for Integrated Social, Economic and Environmental Action in the Livestock Sector. Glob. Change Biol. 2021, 27, 5726–5761. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.A.; Penner, G.B.; Chaucheyras-Durand, F. Development and Physiology of the Rumen and the Lower Gut: Targets for Improving Gut Health. J. Dairy Sci. 2016, 99, 4955–4966. [Google Scholar] [CrossRef] [PubMed]
- Osofsky, S.A. Conservation and Development Interventions at the Wildlife/Livestock Interface: Implications for Wildlife, Livestock and Human Health: Proceedings of the Southern and East African Experts Panel on Designing Successful Conserva-tion and Development Interventions at the Wildlife/Livestock Interface: Implications for Wildlife, Livestock and Human Health, AHEAD (Animal Health for the Environment And Development) Forum, IUCN Vth World Parks Congress, Durban, South Africa, 14th and 15th September 2003; IUCN: Gland, Switzerland, 2005. [Google Scholar]
- Hafez, H.M.; Attia, Y.A. Challenges to the Poultry Industry: Current Perspectives and Strategic Future after the COVID-19 Outbreak. Front. Vet. Sci. 2020, 7, 516. [Google Scholar] [CrossRef]
- Doyle, M.P.; Erickson, M.C. Opportunities for Mitigating Pathogen Contamination during On-Farm Food Production. Int. J. Food Microbiol. 2012, 152, 54–74. [Google Scholar] [CrossRef]
- Adewole, D.I.; Kim, I.H.; Nyachoti, C.M. Gut Health of Pigs: Challenge Models and Response Criteria with a Critical Analysis of the Effectiveness of Selected Feed Additives—A Review. Asian-Australas. J. Anim. Sci. 2016, 29, 909–924. [Google Scholar] [CrossRef]
- Lobo, E.; Bajagai, Y.S.; Kayal, A.; Ramirez, S.; Nikolić, A.; Valientes, R.; Stanley, D. Precision Glycan Supplementation Improves Gut Microbiota Diversity, Performance, and Disease Outbreak Resistance in Broiler Chickens. Animals 2023, 14, 32. [Google Scholar] [CrossRef]
- Gupta, S.; Shrivastava, D.N. Brachyspira-Induced Gut Microbiome Disruption in Livestock: Mechanisms, Consequences, and Interventions. J. Sci. Res. Technol. 2025, 3, 9–20. [Google Scholar] [CrossRef]
- Pangga, G.M.; Star-Shirko, B.; Psifidi, A.; Xia, D.; Corcionivoschi, N.; Kelly, C.; Hughes, C.; Lavery, U.; Richmond, A.; Ijaz, U.Z.; et al. Impact of Commercial Gut Health Interventions on Caecal Metagenome and Broiler Performance. Microbiome 2025, 13, 30. [Google Scholar] [CrossRef]
- Ghiselli, F.; Rossi, B.; Piva, A.; Grilli, E. Assessing Intestinal Health. In Vitro and Ex Vivo Gut Barrier Models of Farm Animals: Benefits and Limitations. Front. Vet. Sci. 2021, 8, 723387. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M. The Livestock Reservoir for Antimicrobial Resistance: A Personal View on Changing Patterns of Risks, Effects of Interventions and the Way Forward. Phil. Trans. R. Soc. B 2015, 370, 20140085. [Google Scholar] [CrossRef]
- Al-Shawi, S.G.; Dang, D.S.; Yousif, A.Y.; Al-Younis, Z.K.; Najm, T.A.; Matarneh, S.K. The Potential Use of Probiotics to Improve Animal Health, Efficiency, and Meat Quality: A Review. Agriculture 2020, 10, 452. [Google Scholar] [CrossRef]
- Arsène, M.M.J.; Davares, A.K.L.; Andreevna, S.L.; Vladimirovich, E.A.; Carime, B.Z.; Marouf, R.; Khelifi, I. The Use of Probiotics in Animal Feeding for Safe Production and as Potential Alternatives to Antibiotics. Vet. World 2021, 14, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Luo, S.; Yan, C. Gut Microbiota Implications for Health and Welfare in Farm Animals: A Review. Animals 2021, 12, 93. [Google Scholar] [CrossRef]
- Grodkowski, G.; Gołębiewski, M.; Slósarz, J.; Grodkowska, K.; Kostusiak, P.; Sakowski, T.; Puppel, K. Organic Milk Production and Dairy Farming Constraints and Prospects under the Laws of the European Union. Animals 2023, 13, 1457. [Google Scholar] [CrossRef]
- Fu, C.; Cheema, W.A.; Mobashar, M.; Shah, A.A.; Alqahtani, M.M. Insects as Sustainable Feed: Enhancing Animal Nutrition and Reducing Livestock Environmental Impression. J. Anim. Physiol. Anim. Nutr. 2025, 109, 280–290. [Google Scholar] [CrossRef]
- Kühl, S.; Bayer, E.; Schulze, M. The Role of Trust, Expectation, and Deception When Buying Organic Animal Products. Anim. Front 2023, 13, 40–47. [Google Scholar] [CrossRef]
- Lazaroiu, G.; Andronie, M.; Uţă, C.; Hurloiu, I. Trust Management in Organic Agriculture: Sustainable Consumption Behavior, Environmentally Conscious Purchase Intention, and Healthy Food Choices. Front. Public Health 2019, 7, 340. [Google Scholar] [CrossRef]
- Arsenault, M.; Lillie, B.; Nadeem, K.; Khafipour, E.; Farzan, A. Progression of Swine Fecal Microbiota during Early Stages of Life and Its Association with Performance: A Longitudinal Study. BMC Microbiol. 2024, 24, 182. [Google Scholar] [CrossRef]
- Barathan, M.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. The Profound Influence of Gut Microbiome and Extracellular Vesicles on Animal Health and Disease. Int. J. Mol. Sci. 2024, 25, 4024. [Google Scholar] [CrossRef] [PubMed]
- Chalvon-Demersay, T.; Luise, D.; Le Floc’h, N.; Tesseraud, S.; Lambert, W.; Bosi, P.; Trevisi, P.; Beaumont, M.; Corrent, E. Functional Amino Acids in Pigs and Chickens: Implication for Gut Health. Front. Vet. Sci. 2021, 8, 663727. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.E. 19 The FarmGTEx Project: Building Reference Omics Datasets for Future Gut Stress Studies. J. Anim. Sci. 2025, 103, 108. [Google Scholar] [CrossRef]
- Vinayamohan, P.; Joseph, D.; Viju, L.S.; Baskaran, S.A.; Venkitanarayanan, K. Efficacy of Probiotics in Reducing Pathogenic Potential of Infectious Agents. Fermentation. 2024, 10, 599. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus Spp. for Gastrointestinal Health: Current and Future Perspectives. Front Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Jena, R.; Choudhury, P.K. coods: A Health Beneficial Outlook. Probiotics Antimicrob Proteins. 2025, 17, 1–22. [Google Scholar] [CrossRef]
- Victoria Obayomi, O.; Folakemi Olaniran, A.; Olugbemiga Owa, S. Unveiling the Role of Functional Foods with Emphasis on Prebiotics and Probiotics in Human Health: A Review. Journal of Functional Foods. 2024, 119, 106337. [Google Scholar] [CrossRef]
- Jovanovic-Malinovska, R.; Kuzmanova, S.; Winkelhausen, E. Oligosaccharide Profile in Fruits and Vegetables as Sources of Prebiotics and Functional Foods. International Journal of Food Properties. 2014, 17, 949–965. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv Nutr. 2021, 13, 492–529. [Google Scholar] [CrossRef] [PubMed]
- Parhi, P.; Liu, S.Q.; Choo, W.S. Synbiotics: Effects of Prebiotics on the Growth and Viability of Probiotics in Food Matrices. Bioactive Carbohydrates and Dietary Fibre. 2024, 32, 100462. [Google Scholar] [CrossRef]

| Nutrient/Additive | Type | Source/Examples | Function/Role | Effect on Gut Health | Impact on Livestock Performance | Potential Risks/Considerations | References |
|---|---|---|---|---|---|---|---|
| Dietary Fibers | Non-starch polysaccharides (NSPs) | Plant-based fibers (e.g., cellulose, hemicellulose, oats, barley) | Stimulate bacterial growth, provide energy for gut microbiota, enhance digestive processes | Improve gut microbiota balance, increase bacterial diversity, and enhance nutrient absorption | Enhanced digestion, improved nutrient absorption, increased weight gain | Excessive fiber may reduce energy intake or cause digestive discomfort | [50,51] |
| Amino Acids | Essential and non-essential | Animal and plant-based proteins (e.g., lysine, methionine, soy, fishmeal) | Essential for protein synthesis, immune response, and gut tissue repair | Support immune function, gut tissue regeneration, and enhance intestinal health | Improved growth rates, stronger immune response | Imbalanced amino acid profiles can affect growth and health | [52,53] |
| Antioxidants | Vitamins A, C, E | Fruits, vegetables, animal products (e.g., liver, carrots) | Protect gastrointestinal lining from oxidative stress and inflammation | Protect gut lining from damage, reduce inflammation, and enhance gut barrier function | Improved immunity, reduced incidence of gastrointestinal diseases | Over-supplementation may disrupt nutrient absorption or cause toxicity | [54,55] |
| Prebiotics | Oligosaccharides, Inulin | Plant fibers, legumes (e.g., chicory, garlic) | Promote the growth of beneficial bacteria by providing a food source for them | Enhance gut microbiota composition, strengthen intestinal barrier, improve gut immune response | Better nutrient absorption, improved growth, and feed conversion | Overfeeding may cause fermentation imbalances or gastrointestinal upset | [56,57] |
| Probiotics | Live beneficial bacteria | Fermented foods, supplements (e.g., Lactobacillus, Bifidobacterium) | Introduce beneficial microbes to balance gut microbiota, suppress harmful bacteria | Restore microbiota balance, improve digestion, suppress pathogenic microorganisms | Improved feed conversion, enhanced immune function, reduced disease incidence | Probiotics must be stored correctly; incorrect strain may not have beneficial effects | [54,55] |
| Synbiotics | Combination of prebiotics and probiotics | Combination of dietary fibers and live beneficial bacteria | Support gut health by combining the benefits of both prebiotics and probiotics | Improve gut microbiome, boost immunity, enhance feed conversion efficiency | Increased growth, enhanced immune function, better feed utilization | Strain and dosage must be appropriately matched for effectiveness | [54,55] |
| Short-Chain Fatty Acids (SCFAs) | Acetic, propionic, butyric acids | Fermentation products (from fiber) | Provide energy to colonocytes, modulate immune responses, lower gut pH to inhibit pathogenic bacteria growth | Support gut epithelial health, reduce inflammation, promote beneficial microbial growth | Improved intestinal health, reduced gastrointestinal distress, enhanced growth | Overproduction of SCFAs could lower gut pH excessively, inhibiting absorption | [56,57] |
| Polyphenols and Flavonoids | Plant-derived compounds | Fruits, vegetables, herbs (e.g., blueberries, apples, green tea) | Possess antimicrobial, antioxidant, and anti-inflammatory properties | Decrease oxidative stress, modulate gut microbiota, improve gut health by strengthening the gut lining | Reduced oxidative stress, better gut lining integrity, improved immune function | High doses may cause digestive upset or interfere with mineral absorption | [56,57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atuahene, D.; Sam, B.A.; Idan, F.; Sana, S.S.; Knop, R.; Suthar, T.; Kumar, H.; Shaikh, A.M. Probiotics, Prebiotics, and Synbiotics in Pigs and Poultry: A Review of Gut Health, Performance, and Environmental Outcomes. Vet. Sci. 2025, 12, 1054. https://doi.org/10.3390/vetsci12111054
Atuahene D, Sam BA, Idan F, Sana SS, Knop R, Suthar T, Kumar H, Shaikh AM. Probiotics, Prebiotics, and Synbiotics in Pigs and Poultry: A Review of Gut Health, Performance, and Environmental Outcomes. Veterinary Sciences. 2025; 12(11):1054. https://doi.org/10.3390/vetsci12111054
Chicago/Turabian StyleAtuahene, David, Bernard Abeiku Sam, Frank Idan, Shaikh Sumayya Sana, Renáta Knop, Tejas Suthar, Harsh Kumar, and Ayaz Mukarram Shaikh. 2025. "Probiotics, Prebiotics, and Synbiotics in Pigs and Poultry: A Review of Gut Health, Performance, and Environmental Outcomes" Veterinary Sciences 12, no. 11: 1054. https://doi.org/10.3390/vetsci12111054
APA StyleAtuahene, D., Sam, B. A., Idan, F., Sana, S. S., Knop, R., Suthar, T., Kumar, H., & Shaikh, A. M. (2025). Probiotics, Prebiotics, and Synbiotics in Pigs and Poultry: A Review of Gut Health, Performance, and Environmental Outcomes. Veterinary Sciences, 12(11), 1054. https://doi.org/10.3390/vetsci12111054









