Targeting the Gut–Mammary Axis for Understanding Mastitis Pathogenesis and Therapeutic Strategies
Simple Summary
Abstract
1. Introduction
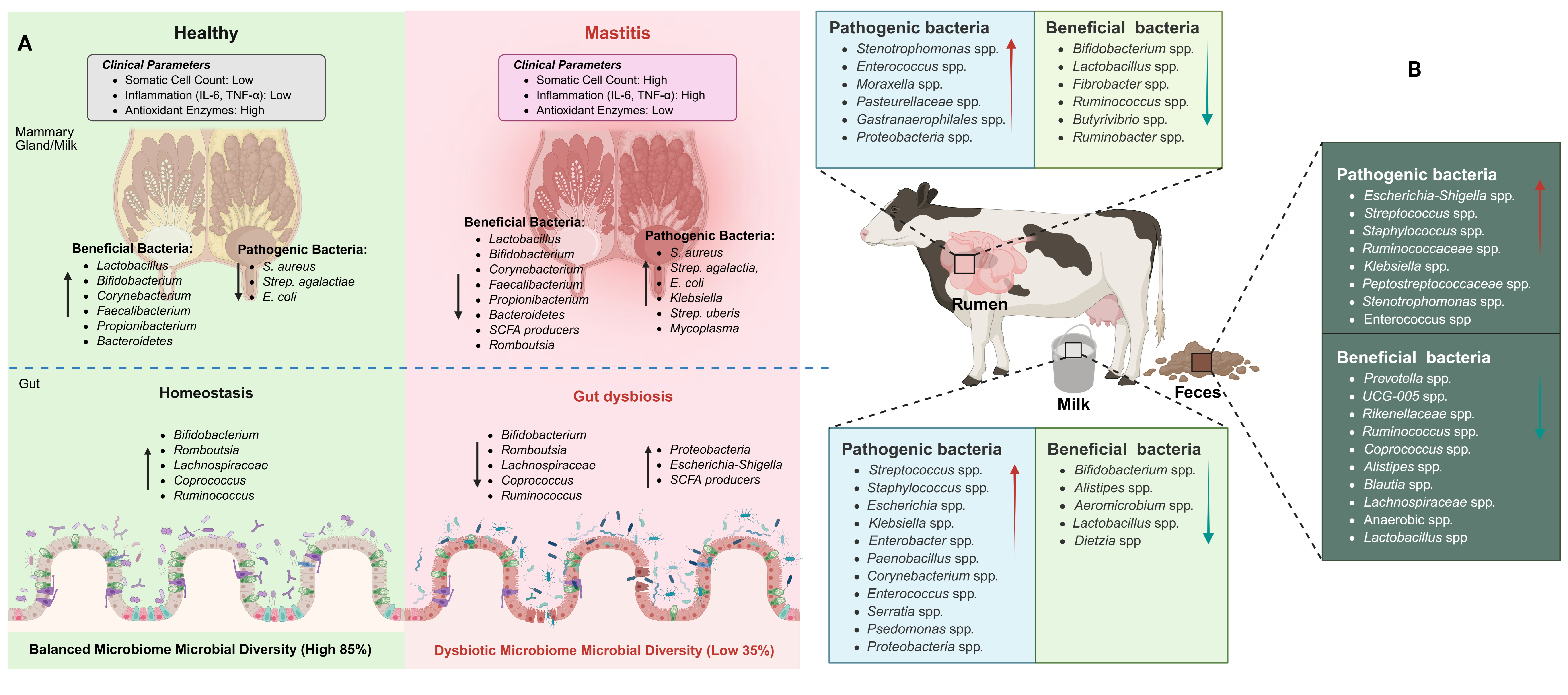
2. Comparative Microbiota of Milk and Gut in Healthy and Mastitis Animals
2.1. Milk Microbiota Association with Mastitis
2.2. Gut Microbiota Association with Mastitis
2.3. Fecal Microbiota Transplantation Causes Mastitis
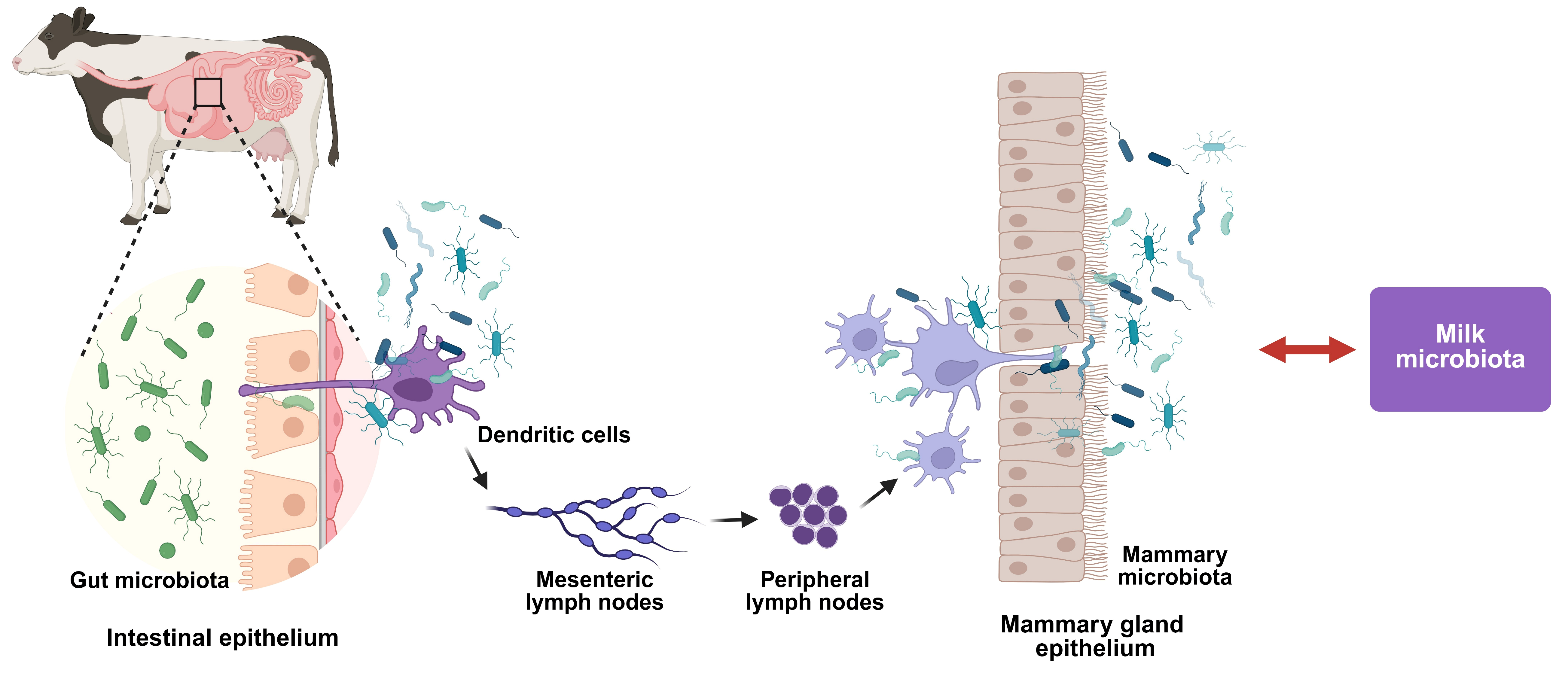
2.4. Functional Correlation Between Gut and Milk Microbiota
3. Enhancement of Microbiota and Their Association with Mastitis Management
3.1. Nutritional Interventions
3.2. Probiotic and Prebiotic Interventions
3.3. Plant-Derived Bioactive Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.Z.; Li, L.; Zhan, Y.; Binjiang, H.; Liu, X.; Kou, X.; Khan, A.; Qadeer, A.; Ullah, Q.; Alzahrani, K.J.; et al. Targeting Nrf2/KEAP1 signaling pathway using bioactive compounds to combat mastitis. Front. Immunol. 2025, 16, 1425901. [Google Scholar] [CrossRef]
- Khan, M.Z.; Wang, J.; Ma, Y.; Chen, T.; Ma, M.; Ullah, Q.; Khan, I.M.; Khan, A.; Cao, Z.; Liu, S. Genetic polymorphisms in immune-and inflammation-associated genes and their association with bovine mastitis resistance/susceptibility. Front. Immunol. 2023, 14, 1082144. [Google Scholar] [CrossRef]
- Tong, X.; Barkema, H.W.; Nobrega, D.B.; Xu, C.; Han, B.; Zhang, C.; Yang, J.; Li, X.; Gao, J. Virulence of Bacteria Causing Mastitis in Dairy Cows: A Literature Review. Microorganisms 2025, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.; Williamson, J.; Lacy-Hulbert, J.; Steele, N.; Eastwood, C. Clinical and subclinical mastitis incidence in pasture-based dairy cows. N. Z. Vet. J. 2025, 73, 316–327. [Google Scholar] [CrossRef]
- Stanek, P.; Żółkiewski, P.; Januś, E. A review on mastitis in dairy cows research: Current status and future perspectives. Agriculture 2024, 14, 1292. [Google Scholar] [CrossRef]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and classification of mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef]
- Martins, L.; Barcelos, M.M.; Cue, R.I.; Anderson, K.L.; Dos Santos, M.V.; Gonçalves, J.L. Chronic subclinical mastitis reduces milk and components yield at the cow level. J. Dairy Res. 2020, 87, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, I.; Van Den Brulle, I.; Geerinckx, K.; D’Anvers, L.; De Vliegher, S.; Aernouts, B. Milk losses linked to mastitis treatments at dairy farms with automatic milking systems. Prev. Vet. Med. 2021, 194, 105420. [Google Scholar] [CrossRef]
- Puerto, M.A.; Shepley, E.; Cue, R.I.; Warner, D.; Dubuc, J.; Vasseur, E. The hidden cost of disease: I. Impact of the first incidence of mastitis on production and economic indicators of primiparous dairy cows. J. Dairy Sci. 2021, 104, 7932–7943. [Google Scholar] [CrossRef] [PubMed]
- Azooz, M.F.; El-Wakeel, S.A.; Yousef, H.M. Financial and economic analyses of the impact of cattle mastitis on the profitability of Egyptian dairy farms. Vet. World 2020, 13, 1750. [Google Scholar] [CrossRef]
- Dalanezi, F.M.; Joaquim, S.F.; Guimarães, F.F.; Guerra, S.T.; Lopes, B.C.; Schmidt, E.M.; Cerri, R.L.; Langoni, H. Influence of pathogens causing clinical mastitis on reproductive variables of dairy cows. J. Dairy Sci. 2020, 103, 3648–3655. [Google Scholar] [CrossRef]
- Dahl, M.O.; De Vries, A.; Galvão, K.N.; Maunsell, F.P.; Risco, C.A.; Hernandez, J.A. Combined effect of mastitis and parity on pregnancy loss in lactating Holstein cows. Theriogenology 2020, 143, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Raboisson, D.; Ferchiou, A.; Pinior, B.; Gautier, T.; Sans, P.; Lhermie, G. The use of meta-analysis for the measurement of animal disease burden: Losses due to clinical mastitis as an example. Front. Vet. Sci. 2020, 7, 149. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Wei, X.J.; Luo, Y.J.; Guo, W.Z.; Zhou, X.Z.; Guo, Z.T. Prevalence and risk factors of subclinical mastitis in lactating cows in Northwest China. Isr. J. Vet. Med. 2019, 74, 17–22. [Google Scholar]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Tang, X.; Nan, X.; Jiang, L.; Wang, H.; Liu, J.; Yang, L.; Yao, J.; Xiong, B. Nutrition, gastrointestinal microorganisms and metabolites in mastitis occurrence and control. Anim. Nutr. 2024, 17, 220–231. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Y.; Kang, X.; Liu, P.; Wang, G. Research progress on the association between mastitis and gastrointestinal microbes in dairy cows and the effect of probiotics. Microb. Pathog. 2022, 173, 105809. [Google Scholar] [CrossRef]
- Gryaznova, M.V.; Syromyatnikov, M.Y.; Dvoretskaya, Y.D.; Solodskikh, S.A.; Klimov, N.T.; Mikhalev, V.I.; Zimnikov, V.I.; Mikhaylov, E.V.; Popov, V.N. Microbiota of cow’s milk with udder pathologies. Microorganisms 2021, 9, 1974. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, X.; Bao, L.; Wu, K.; Feng, L.; Qiu, M.; He, Y.; He, Z.; Zhang, N.; Fu, Y. Aryl hydrocarbon receptor activation by Lactobacillus reuteri tryptophan metabolism alleviates Escherichia coli-induced mastitis in mice. PLoS Pathog. 2021, 17, e1009774. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; He, Y.; Xiang, K.; Zhao, C.; He, Z.; Qiu, M.; Hu, X.; Zhang, N. The role of rumen microbiota and its metabolites in subacute ruminal acidosis (SARA)-induced inflammatory diseases of ruminants. Microorganisms 2022, 10, 1495. [Google Scholar] [CrossRef]
- Bronzo, V.; Lopreiato, V.; Riva, F.; Amadori, M.; Curone, G.; Addis, M.F.; Cremonesi, P.; Moroni, P.; Trevisi, E.; Castiglioni, B. The role of innate immune response and microbiome in resilience of dairy cattle to disease: The mastitis model. Animals 2020, 10, 1397. [Google Scholar] [CrossRef]
- Matera, M.; Palazzi, C.M.; Bertuccioli, A.; Di Pierro, F.; Zerbinati, N.; Cazzaniga, M.; Gregoretti, A.; Cavecchia, I. The Role of Targeted Microbiota Therapy in the Prevention and Management of Puerperal Mastitis. Diseases 2025, 13, 176. [Google Scholar] [CrossRef]
- Zhao, C.; Bao, L.; Qiu, M.; Wu, K.; Zhao, Y.; Feng, L.; Xiang, K.; Zhang, N.; Hu, X.; Fu, Y. Commensal cow Roseburia reduces gut-dysbiosis-induced mastitis through inhibiting bacterial translocation by producing butyrate in mice. Cell Rep. 2022, 41, 108345. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Tang, Y.; Liu, X.; Zhang, H.; Zhou, Y.; Wang, Y.; Xiao, W.; Yu, Y. Testing two somatic cell count cutoff values for bovine subclinical mastitis detection based on milk microbiota and peripheral blood leukocyte transcriptome profile. Animals 2022, 12, 1694. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.C.; Wang, Y.; Li, H.; Wang, X.M.; Wu, Y.; Zhang, D.R.; Gao, S.; Qi, Z.L. Impact of yeast and lactic acid bacteria on mastitis and milk microbiota composition of dairy cows. AMB Express 2020, 10, 22. [Google Scholar] [CrossRef]
- Catozzi, C.; Cusco’, A.; Lecchi, C.; De Carlo, E.; Vecchio, D.; Martucciello, A.; Francino, O.; Grilli, G.; Ceciliani, F. Impact of intramammary inoculation of inactivated Lactobacillus rhamnosus and antibiotics on the milk microbiota of water buffalo with subclinical mastitis. PLoS ONE 2019, 14, e0210204. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, S.; Fu, Y.; Zhang, N. Targeting gut microbiota as a possible therapy for mastitis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, H.; Tang, W.; Zeng, J.; Kulyar, M.F.; Hu, J. Changes in the Microbiome in Yak Mastitis: Insights Based on Full-Length 16S rRNA Sequencing. Vet. Sci. 2024, 11, 335. [Google Scholar] [CrossRef]
- Salman, M.M.; Nawaz, M.; Yaqub, T.; Mushtaq, M.H. Investigation of milk microbiota of healthy and mastitic Sahiwal cattle. BMC Microbiol. 2023, 23, 304. [Google Scholar] [CrossRef]
- Salman, M.M.; Nawaz, M.; Yaqub, T.; Mushtaq, M.H. Exploring the milk microbiota of healthy and Mastitic Nili Ravi Buffalo using 16S rRNA gene base metagenomic analysis. Animals 2023, 13, 2298. [Google Scholar] [CrossRef] [PubMed]
- Secchi, G.; Bisutti, V.; Toscano, A.; Pegolo, S.; Giannuzzi, D.; Cecchinato, A.; Bittante, G.; Franciosi, E. Changes in the milk and fecal microbiota in Holstein cows with subclinical intramammary infection. J. Dairy Sci. 2025, 108, 10220–10236. [Google Scholar] [CrossRef]
- Steinberg, R.S.; Silva e Silva, L.C.; De Souza, M.R.; Reis, R.B.; Da Silva, P.C.; Lacorte, G.A.; Nicoli, J.R.; Neumann, E.; Nunes, Á.C. Changes in bovine milk bacterial microbiome from healthy and subclinical mastitis affected animals of the Girolando, Gyr, Guzera, and Holstein breeds. Int. Microbiol. 2022, 25, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Khasapane, N.G.; Khumalo, Z.T.; Kwenda, S.; Nkhebenyane, S.J.; Thekisoe, O. Characterisation of milk microbiota from subclinical mastitis and apparently healthy dairy cattle in Free State Province, South Africa. Vet. Sci. 2023, 10, 616. [Google Scholar] [CrossRef]
- Alessandri, G.; Sangalli, E.; Facchi, M.; Fontana, F.; Mancabelli, L.; Donofrio, G.; Ventura, M. Metataxonomic analysis of milk microbiota in the bovine subclinical mastitis. FEMS Microbiol. Ecol. 2023, 99, fiad136. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Zhang, H.; Zhang, Y.; Xiong, B.; Jiang, L. Microbiome and metabolome analyses of milk from dairy cows with subclinical Streptococcus agalactiae mastitis—Potential biomarkers. Front. Microbiol. 2019, 10, 2547. [Google Scholar] [CrossRef]
- Burakova, I.; Gryaznova, M.; Smirnova, Y.; Morozova, P.; Mikhalev, V.; Zimnikov, V.; Latsigina, I.; Shabunin, S.; Mikhailov, E.; Syromyatnikov, M. Association of milk microbiome with bovine mastitis before and after antibiotic therapy. Vet. World 2023, 16, 2389. [Google Scholar] [CrossRef]
- Derakhshani, H.; Plaizier, J.C.; De Buck, J.; Barkema, H.W.; Khafipour, E. Composition and co-occurrence patterns of the microbiota of different niches of the bovine mammary gland: Potential associations with mastitis susceptibility, udder inflammation, and teat-end hyperkeratosis. Anim. Microbiome 2020, 2, 11. [Google Scholar] [CrossRef]
- Sokolov, S.; Fursova, K.; Shulcheva, I.; Nikanova, D.; Artyemieva, O.; Kolodina, E.; Sorokin, A.; Dzhelyadin, T.; Shchannikova, M.; Shepelyakovskaya, A.; et al. Comparative analysis of milk microbiomes and their association with bovine mastitis in two farms in Central Russia. Animals 2021, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, P.; Sachivkina, N.; Vatnikov, Y.; Shabunin, S.; Engashev, S.; Kontsevaya, S.; Karamyan, A.; Bokov, D.; Kuznetsova, O.; Vasilieva, E. Role of microorganisms isolated from cows with mastitis in Moscow region in biofilm formation. Vet. World 2021, 14, 40. [Google Scholar] [CrossRef]
- Polveiro, R.C.; Vidigal, P.M.; de Oliveira Mendes, T.A.; Yamatogi, R.S.; da Silva, L.S.; Fujikura, J.M.; Da Costa, M.M.; Moreira, M.A. Distinguishing the milk microbiota of healthy goats and goats diagnosed with subclinical mastitis, clinical mastitis, and gangrenous mastitis. Front. Microbiol. 2022, 13, 918706. [Google Scholar] [CrossRef]
- Maslennikova, I.L.; Nechaeva, Y.I.; Ilina, L.A.; Laptev, G.Y.; Ponomareva, E.S.; Zhdanova, I.N.; Kuznetsova, M.V. A decline in taxonomic diversity of milk microbiome is linked to clinical mastitis and physiological states of cow. Gene Rep. 2025, 39, 102169. [Google Scholar] [CrossRef]
- Hoque, M.N.; Istiaq, A.; Clement, R.A.; Sultana, M.; Crandall, K.A.; Siddiki, A.Z.; Hossain, M.A. Metagenomic deep sequencing reveals association of microbiome signature with functional biases in bovine mastitis. Sci. Rep. 2019, 9, 13536. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Cao, G.; Cui, Y.; Wang, H.; Chen, X.; Xu, F.; Li, X. Microbiota characterization of the cow mammary gland microenvironment and its association with somatic cell count. Vet. Sci. 2023, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Urioste, J.I.; Franzén, J.; Windig, J.J.; Strandberg, E. Genetic relationships among mastitis and alternative somatic cell count traits in the first 3 lactations of Swedish Holsteins. J. Dairy Sci. 2012, 95, 3428–3434. [Google Scholar] [CrossRef] [PubMed]
- Tarrah, A.; Callegaro, S.; Pakroo, S.; Contiero, B.; Magrin, L.; Cassandro, M.; Corich, V. New insights into the raw milk microbiota diversity from animals with a different genetic predisposition for feed efficiency and resilience to mastitis. Sci. Rep. 2022, 12, 13498. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, D.; Zeng, Y.; Cui, J.; Yu, J.; Wang, J.; Li, S.; Huang, Q.; Mansoor, K.M. Clinical characteristics and microbiota analysis of 44 patients with granulomatous mastitis. Front. Microbiol. 2023, 14, 1175206. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, C.; Wang, D.; Wan, P.; Cheng, L.; Yan, X. Integrated microbiome and metabolome analysis reveals altered gut microbial communities and metabolite profiles in dairy cows with subclinical mastitis. BMC Microbiol. 2025, 25, 115. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, T.; Guo, S.; He, T.; Shin, M.K.; Luo, C.; Tong, J.; Zhang, Y. Rumen microbes affect the somatic cell counts of dairy cows by modulating glutathione metabolism. mSystems 2025, 10, e01093-24. [Google Scholar] [CrossRef]
- Chuang, S.T.; Li, K.Y.; Tu, P.W.; Ho, S.T.; Hsu, C.C.; Hsieh, J.C.; Chen, M.J. Investigating the reciprocal interrelationships among the ruminal microbiota, metabolome, and mastitis in early lactating Holstein dairy cows. Animals 2021, 11, 3108. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, X.; Qiu, M.; Bao, L.; Wu, K.; Meng, X.; Zhao, Y.; Feng, L.; Duan, S.; He, Y.; et al. Sialic acid exacerbates gut dysbiosis-associated mastitis through the microbiota-gut–mammary axis by fueling gut microbiota disruption. Microbiome 2023, 11, 78. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Mu, R.; Guo, J.; Zhao, C.; Cao, Y.; Zhang, N.; Fu, Y. The rumen microbiota contributes to the development of mastitis in dairy cows. Microbiol. Spectr. 2022, 10, e02512-21. [Google Scholar] [CrossRef]
- He, Y.; Zhao, C.; Su, N.; Yang, W.; Yang, H.; Yuan, C.; Zhang, N.; Hu, X.; Fu, Y. Disturbances of the gut microbiota-derived tryptophan metabolites as key actors in vagotomy-induced mastitis in mice. Cell Rep. 2024, 43, 114559. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, M.; Wang, H.; Zhang, F.; Xue, F.; Hua, D.; Liu, J.; et al. Rumen microbiome structure and metabolites activity in dairy cows with clinical and subclinical mastitis. J. Anim. Sci. Biotechnol. 2021, 12, 36. [Google Scholar] [CrossRef]
- Hu, X.; Guo, J.; Zhao, C.; Jiang, P.; Maimai, T.; Yanyi, L.; Cao, Y.; Fu, Y.; Zhang, N. The gut microbiota contributes to the development of Staphylococcus aureus-induced mastitis in mice. ISME J. 2020, 14, 1897–1910. [Google Scholar] [CrossRef]
- Pan, N.; Xiu, L.; Xu, Y.; Bao, X.; Liang, Y.; Zhang, H.; Liu, B.; Feng, Y.; Guo, H.; Wu, J.; et al. Mammary γδ T cells promote IL-17A-mediated immunity against Staphylococcus aureus-induced mastitis in a microbiota-dependent manner. iScience 2023, 26, 108489. [Google Scholar] [CrossRef]
- Guo, C.; Liu, J.; Wei, Y.; Du, W.; Li, S. Comparison of the gastrointestinal bacterial microbiota between dairy cows with and without mastitis. Front. Microbiol. 2024, 15, 1332497. [Google Scholar] [CrossRef]
- Chen, K.; Hu, B.; Ren, J.; Deng, X.; Li, Q.; Zhang, R.; Zhang, Y.; Shen, G.; Liu, S.; Zhang, J.; et al. Enhanced protein-metabolite correlation analysis: To investigate the association between Staphylococcus aureus mastitis and metabolic immune pathways. FASEB J. 2024, 38, e23587. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhao, Y.; Yang, F.; Zhang, Q.; Zhao, X.; Yang, Z.; Dao, X.; Laghi, L. Microbiome and metabolome analyses of milk and feces from dairy cows with healthy, subclinical, and clinical mastitis. Front. Microbiol. 2024, 15, 1374911. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Hou, X.; Gao, X.; Liu, P.; Guo, X.; Hu, G.; Li, Q.; Huang, C.; Li, G.; Fang, W.; et al. The 16S rDNA high-throughput sequencing correlation analysis of milk and gut microbial communities in mastitis Holstein cows. BMC Microbiol. 2023, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; An, M.; Zhang, W.; Li, K.; Kulyar, M.F.; Duan, K.; Zhou, H.; Wu, Y.; Wan, X.; Li, J.; et al. Integrated bacteria-fungi diversity analysis reveals the gut microbial changes in buffalo with mastitis. Front. Vet. Sci. 2022, 9, 918541. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, K.; Hao, H.; Zhao, Y.; Bao, L.; Qiu, M.; He, Y.; He, Z.; Zhang, N.; Hu, X.; et al. Gut microbiota-mediated secondary bile acid alleviates Staphylococcus aureus-induced mastitis through the TGR5-cAMP-PKA-NF-κB/NLRP3 pathways in mice. npj Biofilms Microbiomes 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.N.; Rahman, M.S.; Islam, T.; Sultana, M.; Crandall, K.A.; Hossain, M.A. Induction of mastitis by cow-to-mouse fecal and milk microbiota transplantation causes microbiome dysbiosis and genomic functional perturbation in mice. Anim. Microbiome 2022, 4, 43. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, X.; Bao, L.; Wu, K.; Zhao, Y.; Xiang, K.; Li, S.; Wang, Y.; Qiu, M.; Feng, L.; et al. Gut dysbiosis induces the development of mastitis through a reduction in host anti-inflammatory enzyme activity by endotoxemia. Microbiome 2022, 10, 205. [Google Scholar] [CrossRef]
- Kong, C.Y.; Yang, Y.Q.; Han, B.; Chen, H.L.; Mao, Y.Q.; Huang, J.T.; Wang, L.S.; Li, Z.M. Fecal microbiome transplant from patients with lactation mastitis promotes mastitis in conventional lactating mice. Front. Microbiol. 2023, 14, 1123444. [Google Scholar] [CrossRef]
- Tang, R.; Yang, W.; Song, J.; Xiang, K.; Li, S.; Zhao, C.; Zhang, N.; Fu, Y.; Hu, X. The rumen microbiota contributed to the development of mastitis induced by subclinical ketosis. Microb. Pathog. 2024, 187, 106509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Bao, L.; Zhao, Y.; Wu, K.; Qiu, M.; Feng, L.; Zhang, N.; Hu, X.; Fu, Y. A fiber-enriched diet alleviates Staphylococcus aureus-induced mastitis by activating the HDAC3-mediated antimicrobial program in macrophages via butyrate production in mice. PLoS Pathog. 2023, 19, e1011108. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Ye, C.; Zhao, X.; Zou, C.; Tang, R.; Xie, J.; Liu, Y.; Hu, Y.; Hu, X.; Zhang, N.; et al. Succinate exacerbates mastitis in mice via extracellular vesicles derived from the gut microbiota: A potential new mechanism for mastitis. J. Nanobiotechnol. 2024, 22, 712. [Google Scholar] [CrossRef]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef]
- Castro, I.; García-Carral, C.; Furst, A.; Khwajazada, S.; García, J.; Arroyo, R.; Ruiz, L.; Rodríguez, J.M.; Bode, L.; Fernández, L. Interactions between human milk oligosaccharides, microbiota and immune factors in milk of women with and without mastitis. Sci. Rep. 2022, 12, 1367. [Google Scholar] [CrossRef]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.P.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004, 303, 1662–1665. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Z.; Tian, Z.; Jia, H.; Zhang, L.; Mao, Y.; Yang, Z.; Liu, X.; Li, M. Microbial dysbiosis in the gut–mammary axis as a mechanism for mastitis in dairy cows. Int. J. Dairy Technol. 2025, 78, e13150. [Google Scholar] [CrossRef]
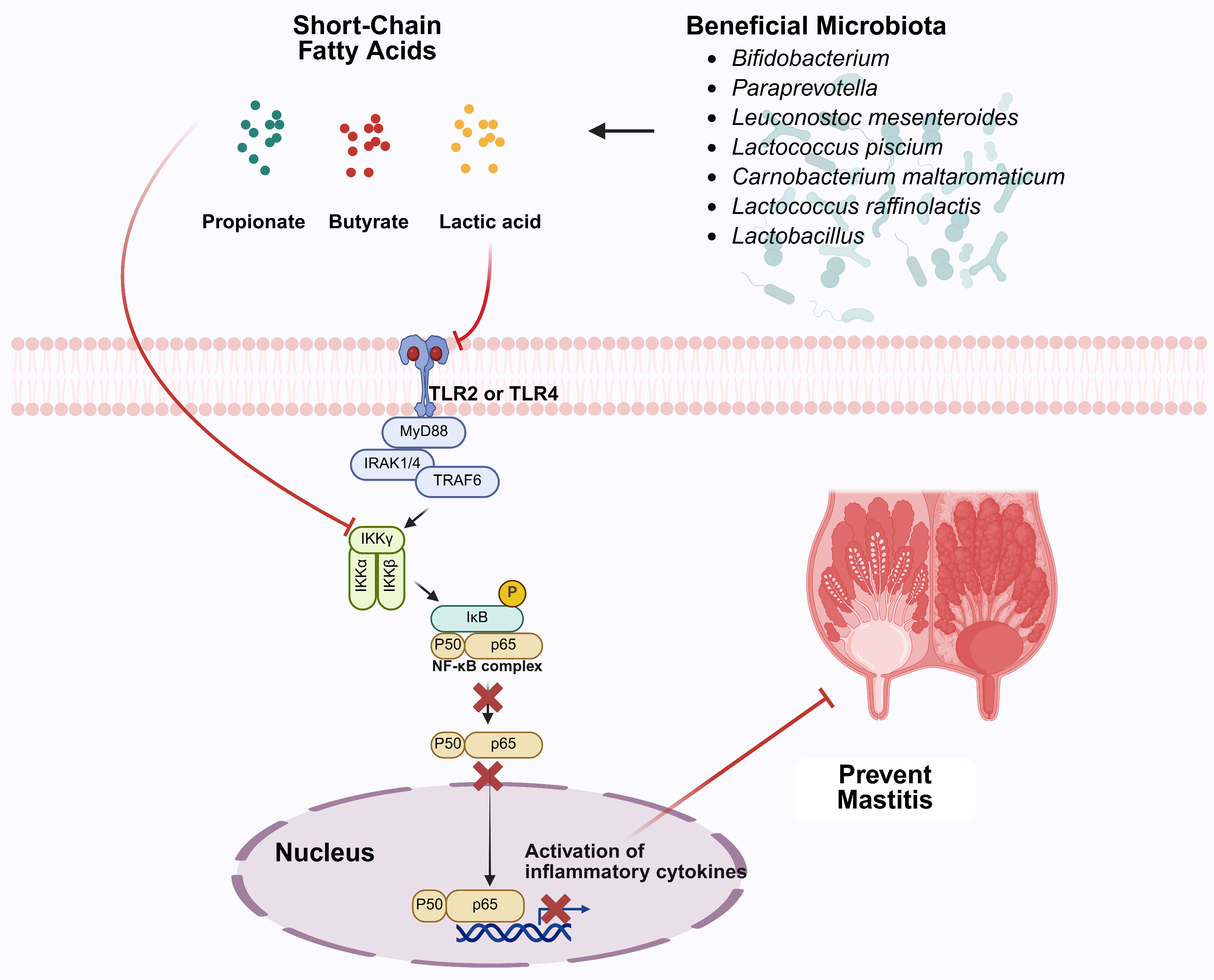
- Duan, H.; Wang, L.; Huangfu, M.; Li, H. The impact of microbiota-derived short-chain fatty acids on macrophage activities in disease: Mechanisms and therapeutic potentials. Biomed. Pharmacother. 2023, 165, 115276. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, N.; Zhang, W.; Lv, Z.; Liu, J.; Shi, H. Bacillus amyloliquefaciens-9 reduces somatic cell count and modifies fecal microbiota in lactating goats. Mar. Drugs 2021, 19, 404. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; He, Z.; Zhao, C.; He, Y.; Qiu, M.; Xiang, K.; Zhang, N.; Fu, Y. Gut/rumen-mammary gland axis in mastitis: Gut/rumen microbiota–mediated “gastroenterogenic mastitis”. J. Adv. Res. 2024, 55, 159–171. [Google Scholar] [CrossRef]
- Li, Y.; Khan, M.Z.; Wang, C.; Ma, Q. The emerging role of milk-derived extracellular vesicles in gut pathology and cancer management. Crit. Rev. Food Sci. Nutr. 2025, 1–8. [Google Scholar] [CrossRef]
- Ma, C.; Sun, Z.; Zeng, B.; Huang, S.; Zhao, J.; Zhang, Y.; Su, X.; Xu, J.; Wei, H.; Zhang, H. Cow-to-mouse fecal transplantations suggest intestinal microbiome as one cause of mastitis. Microbiome 2018, 6, 200. [Google Scholar] [CrossRef]
- Khan, M.Z.; Huang, B.; Kou, X.; Chen, Y.; Liang, H.; Ullah, Q.; Khan, I.M.; Khan, A.; Chai, W.; Wang, C. Enhancing bovine immune, antioxidant and anti-inflammatory responses with vitamins, rumen-protected amino acids, and trace minerals to prevent periparturient mastitis. Front. Immunol. 2024, 14, 1290044. [Google Scholar] [CrossRef]
- Khan, M.Z.; Li, L.; Wang, T.; Liu, X.; Chen, W.; Ma, Q.; Zahoor, M.; Wang, C. Bioactive compounds and probiotics mitigate mastitis by targeting NF-κB signaling pathway. Biomolecules. 2024, 14, 1011. [Google Scholar] [CrossRef]
- Wu, K.; Sun, X.; Xu, J.; Guan, Z.; Yuan, W.; Bao, L.; Zhao, Y.; Shan, R.; Chen, H.; Zhao, C.; et al. Yeast-fermented feed improves high-concentrate diet-induced mastitis in dairy goats by regulating rumen microbiota. Front. Microbiol. 2025, 16, 1582314. [Google Scholar] [CrossRef]
- He, Z.; Li, W.; Yuan, W.; He, Y.; Xu, J.; Yuan, C.; Zhao, C.; Zhang, N.; Fu, Y.; Hu, X. Lactobacillus reuteri inhibits Staphylococcus aureus-induced mastitis by regulating oxytocin releasing and gut microbiota in mice. FASEB J. 2024, 38, e23383. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Chu, B.; Liu, N.; Chen, S.; Wang, J. Lactobacillus rhamnosus GR-1 prevents Escherichia coli-induced apoptosis through PINK1/Parkin-mediated mitophagy in bovine mastitis. Front. Immunol. 2021, 12, 715098. [Google Scholar] [CrossRef]
- Barker, M.; Adelson, P.; Peters, M.D.; Steen, M. Probiotics and human lactational mastitis: A scoping review. Women Birth 2020, 33, e483–e491. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ran, X.; Han, J.; Ding, H.; Wang, X.; Li, Y.; Guo, W.; Li, X.; Guo, W.; Fu, S.; et al. Astragalus polysaccharide alleviates mastitis disrupted by Staphylococcus aureus infection by regulating gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 2025, 286, 138422. [Google Scholar] [CrossRef]
- Ran, X.; Li, Y.; Guo, W.; Li, K.; Guo, W.; Wang, X.; Liu, J.; Bi, J.; Fu, S. Angelica sinensis polysaccharide alleviates Staphylococcus aureus-induced mastitis by regulating the intestinal flora and gut metabolites. J. Agric. Food Chem. 2024, 72, 24504–24517. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, N.; He, G.; Chen, T.; Zong, J.; Shen, C.; Wang, Y.; Li, C.; Yin, X.; Meng, Y.; et al. Akkermansia muciniphila-Derived Outer Membrane Vesicles as a Novel Therapeutic Approach for Mastitis: Insights From In Vitro and Vivo Studies. FASEB J. 2025, 39, e70770. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yao, J.; Yang, L.; et al. Dietary supplementation of inulin ameliorates subclinical mastitis via regulation of rumen microbial community and metabolites in dairy cows. Microbiol. Spectr. 2021, 9, e00105-21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yao, J.; Yang, L.; et al. Consumption of supplementary inulin modulates milk microbiota and metabolites in dairy cows with subclinical mastitis. Appl. Environ. Microbiol. 2022, 88, e02059-21. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yang, L.; Yao, J.; et al. Changes in the profile of fecal microbiota and metabolites as well as serum metabolites and proteome after dietary inulin supplementation in dairy cows with subclinical mastitis. Front. Microbiol. 2022, 13, 809139. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, X.; Xu, D.; He, D.; Wang, J.; Bi, J.; Liu, J.; Fu, S. Hordenine Alleviates Lipopolysaccharide-Induced Mastitis by Suppressing Inflammation and Oxidative Stress, Modulating Intestinal Microbiota, and Preserving the Blood–Milk Barrier. J. Agric. Food Chem. 2024, 72, 21503–21519. [Google Scholar] [CrossRef]
- Gao, Y.; Hao, Z.; Zhang, H.; Liu, J.; Zhou, G.; Wen, H.; Su, Q.; Tong, C.; Huang, S.; Wang, X. Forsythiaside A attenuates lipopolysaccharide-induced mouse mastitis by activating autophagy and regulating gut microbiota and metabolism. Chem. Biol. Interact. 2024, 396, 111044. [Google Scholar] [CrossRef]
- Li, K.; Ran, X.; Zeng, Y.; Li, S.; Hu, G.; Wang, X.; Li, Y.; Yang, Z.; Liu, J.; Fu, S. Maslinic acid alleviates LPS-induced mice mastitis by inhibiting inflammatory response, maintaining the integrity of the blood-milk barrier and regulating intestinal flora. Int. Immunopharmacol. 2023, 122, 110551. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Hu, G.; Guo, W.; Li, K.; Wang, X.; Liu, J.; Fu, S. Hesperetin regulates the intestinal flora and inhibits the TLR4/NF-κB signaling axis to protect the blood-milk barrier and prevent mastitis. Life Sci. 2024, 342, 122533. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ao, Y.; Chen, H.; Deng, T.; Liu, C.; Wang, D.; Wan, P.; Xiang, M.; Cheng, L. Investigating the Alleviating Effects of Dihydromyricetin on Subclinical Mastitis in Dairy Cows: Insights from Gut Microbiota and Metabolomic Analysis. Microorganisms 2025, 13, 1890. [Google Scholar] [CrossRef]
- Sun, W.J.; Wu, E.Y.; Zhang, G.Y.; Xu, B.C.; Chen, X.G.; Hao, K.Y.; Wang, Y.; He, L.Z.; Lv, Q.Z. Total flavonoids of Abrus cantoniensis inhibit CD14/TLR4/NF-κB/MAPK pathway expression and improve gut microbiota disorders to reduce lipopolysaccharide-induced mastitis in mice. Front. Microbiol. 2022, 13, 985529. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Z.; Liu, C.; Wei, Z.; Mo, X.; Zhong, Y.; He, R.; Liang, Z.; He, Y.; He, J. Pulsatilla chinensis extract alleviate Staphylococcus aureus induced mastitis in mice by regulating the inflammatory response and gut microbiota. Front. Vet. Sci. 2025, 12, 1603107. [Google Scholar] [CrossRef]
- Fan, X.; Qadeer, A.; Asiri, M.; Alzahrani, F.M.; Alzahrani, K.; Alsharif, K.F.; Khan, M.Z.; Jiang, X. Traditional Chinese Medicine and Plant-Derived Bioactive Compounds as Sustainable Alternatives to Antibiotics in Bovine Mastitis: A Review. Front. Vet. Sci. 2025, 12, 1642647. [Google Scholar] [CrossRef]
- Zhao, G.; Li, H.; Huang, L.; Cheng, Y.; Liu, J.; Song, R.; Wang, X. Integrated multi-omics analysis reveals that Gongying San ameliorates subclinical mastitis by modulating intestinal microbiota and metabolites in dairy cows. Front. Vet. Sci. 2025, 12, 1589900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Yao, H.; Jiang, L.; Tong, J. Intramammary infusion of matrine-chitosan hydrogels for treating subclinical bovine mastitis—Effects on milk microbiome and metabolites. Front. Microbiol. 2022, 13, 950231. [Google Scholar] [CrossRef]
| Animal Species | Sample Type | Health Status Comparison | Key Microbiota Differences/Associations | Reference |
|---|---|---|---|---|
| Cows/Mice | Gut | Mastitis vs. Healthy | ↑ Escherichia_Shigella, ↓ Roseburia; R. intestinalis protective via butyrate production | [23] |
| Dairy cows | Milk | SCC levels (<100 K vs. 100–200 K vs. >200 K) | SCC < 100 K: ↑ Bifidobacterium, Lachnospiraceae_AC2044; Higher SCC: increased inflammatory gene expression | [24] |
| Yaks | Milk | Healthy vs. Subclinical vs. Clinical mastitis | Healthy: Firmicutes (39.7%), Proteobacteria (60.17%); Mastitis: Proteobacteria ↑ 89.32% (subclinical), 95.36% (clinical); Firmicutes ↓ 10.49%, 2.92% | [28] |
| Sahiwal cattle | Milk | Healthy vs. Subclinical vs. Clinical mastitis | Healthy: Proteobacteria (56.48%), Firmicutes (15.87%); Clinical: Proteobacteria (2.68%), Firmicutes (64%); Subclinical: intermediate patterns | [29] |
| Nili Ravi buffalo | Milk | Healthy vs. Subclinical vs. Clinical mastitis | Healthy: Proteobacteria dominant, Streptococcus (11.60%); Clinical: ↑ Streptococcus (33.96%), Staphylococcus, Corynebacterium | [30] |
| Holstein cows | Milk and Feces | Healthy vs. mastitis | Milk: ↑ Firmicutes, ↑ Cyanobacteria, ↑ Streptococcus, ↓ Macrococcus caseolyticus. Feces: No significant differences | [31] |
| Multiple breeds | Milk | Healthy vs. Subclinical mastitis | ↑ Firmicutes in subclinical; ↑ Proteobacteria in healthy; 45 taxonomic biomarkers identified | [32] |
| Dairy cows | Milk | Non-subclinical vs. Subclinical mastitis | Non-subclinical: Anthropi spp., P. azotoformans, P. fragi dominant; Subclinical: P. azotoformans, Mycobacterium bovis, P. koreensis | [33] |
| Dairy cows | Milk | Healthy vs. Subclinical mastitis | ↑ Corynebacterium bovis, C. xerosis (10-fold), Streptococcus dysgalactiae, S. uberis; Core mastitis microbiota: Lactobacillus acidipiscis, Staphylococcus hominis | [34] |
| Dairy cows | Milk | Healthy vs. GBS subclinical mastitis | ↓ Proteobacteria, Actinobacteria, Acidobacteria; ↑ Firmicutes, Streptococcus, trends toward ↑ Turicibacter, Enterococcus | [35] |
| Dairy cows | Milk | Healthy vs. Subclinical vs. Clinical mastitis | ↑ Hymenobacter, Lachnospiraceae NK4A136 in mastitis; ↓ Ralstonia, Lachnospiraceae NK3A20, Acetitomaculum, Massilia, Atopostipes | [36] |
| Dairy cows | Milk and Teat canal | Healthy vs. Clinical mastitis | Inverse relationship: ↓ milk diversity = ↑ inflammation; Sphingobacterium negatively associated with diversity | [37] |
| Dairy cows | Milk | Healthy vs. Mastitis | Mastitis: S. aureus, Aerococcus spp., Streptococcus spp.; Surprising S. thermophilus in high SCC | [38] |
| Dairy cows | Milk | Clinical mastitis polymicrobial | 486 cultures, 11 genera; 63.6% biofilm-forming; S. aureus most prolific biofilm former (18.8%) | [39] |
| Goats | Milk | Healthy vs. Subclinical vs. Clinical vs. Gangrenous | Staphylococcus dominant all groups; ↑ Mycoplasma in clinical; Novel Escherichia/Shigella-Enterococcus association in gangrenous | [40] |
| Dairy cows | Milk | Healthy vs. Mastitis across lactation | Mastitis: fewer bacterial taxa, ↓ diversity throughout lactation, Proteobacteria (9.1–95.4% vs. 24.0–92.9% healthy), Firmicutes (1.4–50.7% vs. 3.1–35.9% healthy) | [41] |
| Dairy cows | Milk | Clinical mastitis vs. Healthy | Mastitis: 363 vs. 146 strains in healthy; 68% unreported/opportunistic strains; 14 archaeal, 14 viral genera unique | [42] |
| Dairy cows | Milk | SCC levels (<2 × 105 vs. >2 × 105 vs. >5 × 105) | SCC <2 × 105: Actinobacteriota dominant; SCC 2 − 5 × 105: Firmicutes dominant; SCC > 5 × 105: Firmicutes = Proteobacteria | [43] |
| Holstein cows | Milk | High vs. Low resilience to mastitis | Low resilience: ↑ Mycoplana, Rhodococcus; High efficiency: ↑ Aerococcus, Corynebacterium, Facklamia, Psychrobacter | [45] |
| Humans | Breast tissue | Granulomatous vs. Acute mastitis vs. Controls | Corynebacterium >1% in 34.1% GM patients, C. kroppenstedtii predominant species | [46] |
| Dairy cows | Gut | Healthy vs. Subclinical mastitis | ↑ Cyanobacteria, Proteobacteria, Succinivibrio, Lactobacillus_iners; ↓ Paraprevotella, Coprococcus, Succiniclasticum, Desulfovibrio, and Bifidobacterium_pseudolongum | [47] |
| Dairy cows | Rumen | Low vs. High SCC | ↑ Bacteroidetes, Firmicutes, Lachnospiraceae, Prevotella, Rumiclostridium in H-SCC group | [48] |
| Dairy cows | Rumen | Subclinical vs. Clinical mastitis | Clinical: ↑ Lachnospiraceae, Moraxella, Neisseriaceae; ↓ beneficial SCFA producers | [49] |
| Cows/Mice | Rumen/Gut | SARA-mastitis vs. Healthy | ↑ Moraxellaceae, ↓ Prevotellaceae; Sialic acid promotes ↑ Enterobacteriaceae, Akkermansiaceae | [50] |
| Holstein cows | Rumen/Feces/Milk | SARA vs. Controls | ↑ Stenotrophomonas in rumen; Barrier disruption allowing bacterial translocation | [51] |
| Mice | Gut | Vagotomy-induced mastitis | ↓ Firmicutes, Proteobacteria; ↑ Campylobacterota, Rikenellaceae_RC9_gut_group | [52] |
| Dairy cows | Rumen | Healthy vs. Subclinical vs. Clinical mastitis | Clinical: ↑ Lachnospiraceae, Moraxella, Neisseriaceae; ↓ Prevoterotoella_1, Bifidobacterium | [53] |
| Mice | Gut | S. aureus mastitis vs. Controls | ↑ Enterobacter, ↓ short-chain fatty acids (SCFA)-producing bacteria (Firmicutes, Bacteroidetes) | [54] |
| Mice | Gut and Mammary gland | Antibiotic-treated vs. Controls | ↓ Firmicutes, ↓ Lactobacillaceae; ↑ Proteobacteria, Bacteroidota, Campylobacterota | [55] |
| Dairy cows | Rumen and Feces | Healthy vs. Mastitis | Rumen: Moryella characteristic in mastitis; Feces: Aeriscardovia, Lactococcus, Bacillus in healthy | [56] |
| Rats | Gut | Healthy vs. Mastitis | ↑ Proteobacteria phylum triggering metabolic disruptions | [57] |
| Dairy cows | Milk and Feces | Healthy vs. Subclinical vs. Clinical mastitis | Milk: ↑ Proteobacteria, ↓ Firmicutes, Actinobacteriota, Bifidobacterium in mastitis; Feces: ↑ UCG-010, Bacteroides, Prevotella in clinical mastitis | [58] |
| Dairy cows | Milk and Gut | Healthy vs. Mastitis | Milk: ↑ Sphingomonas, Stenotrophomonas; Feces: ↑ Alistipes, Flavonifractor, Agathobacter, Pygmaiobacter | [59] |
| Buffalo | Gut | Healthy vs. Mastitis | ↑ Muribaculaceae, Eubacterium_nodatum, Lachnoclostridium_10, Pichia; ↓ Ruminococcus_2, Candidatus_Stoquefichus, Turicibacter | [60] |
| Cows/Mice | Multiple | Clinical mastitis vs. Healthy | Cows: P. aeruginosa, L. crispatus, K. oxytoca; Mice: Muribaculum, Duncaniella, B. animalis, E. coli, S. aureus | [62] |
| Cows/Mice | Rumen | Clinical mastitis vs. Healthy | ↑ Proteobacteria in rumen; Dysbiosis-derived LPS promotes mastitis via TLR4-cGAS-STING-NF-κB/NLRP3 | [63] |
| Humans/Mice | Gut | Mastitis vs. Controls | ↑ Firmicutes/Bacteroidetes ratio, ↑ Actinobacteria, ↓ Verrucomicrobia, ↓ Ruminococcus, Faecalibacterium; ↑ Parabacteroides | [64] |
| Dairy cows and mice | Feces | Mastitis infected cow’s feces transplantation to mice | Identified key bacterial genera (Chrysobacterium, Christensenellaceae_R-7_group, Prevotella) as biomarkers. Revealed endogenous pathway mediated by rumen microbiota dysbiosis in mastitis development. Transplantation from mastitis cows induced mammary inflammation in mice. | [65] |
| Treatment | Animal Model | Key Findings Associated with Mastitis Treatment | Reference |
|---|---|---|---|
| Fiber-enriched diet | Mice |
| [66] |
| Secondary Bile Acids/Clostridium scindens | Mice |
| [67] |
| Bacillus amyloliquefaciens-9 (GB-9) | Saanen dairy goats |
| [75] |
| Yeast fermentation product (YFF) | Dairy goats |
| [81] |
| Astragalus Polysaccharide (APS) | Mice |
| [85] |
| Angelica sinensis Polysaccharide (ASP) | Mice |
| [86] |
| Akkermansia muciniphila | Dairy cows and mice |
| [87] |
| Inulin (Rumen Microbiome) | Dairy cows |
| [88] |
| Inulin Supplementation | Dairy cows |
| [89,90] |
| Hordenine | Mice |
| [91] |
| Forsythiaside A (FTA) | Mice |
| [92] |
| Maslinic acid | Mice |
| [93] |
| Hesperetin | Mice |
| [94] |
| Abrus cantoniensis total flavonoids (ATF) | Mice |
| [95] |
| Pulsatilla chinensis extract (PCE) | Mice |
| [97] |
| Gongying San (GYS) | Holstein cows |
| [99] |
| Matrine-chitosan Hydrogels | Dairy cows |
| [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, M.; Liu, W.; Geng, M.; Asiri, M.; Alzahrani, F.M.; Alzahrani, K.J.; Ma, Q.; Wang, C.; Khan, M.Z. Targeting the Gut–Mammary Axis for Understanding Mastitis Pathogenesis and Therapeutic Strategies. Vet. Sci. 2025, 12, 1049. https://doi.org/10.3390/vetsci12111049
Li Y, Wang M, Liu W, Geng M, Asiri M, Alzahrani FM, Alzahrani KJ, Ma Q, Wang C, Khan MZ. Targeting the Gut–Mammary Axis for Understanding Mastitis Pathogenesis and Therapeutic Strategies. Veterinary Sciences. 2025; 12(11):1049. https://doi.org/10.3390/vetsci12111049
Chicago/Turabian StyleLi, Yan, Menghan Wang, Wenqiang Liu, Mingyang Geng, Mohammed Asiri, Fuad M. Alzahrani, Khalid J. Alzahrani, Qingshan Ma, Changfa Wang, and Muhammad Zahoor Khan. 2025. "Targeting the Gut–Mammary Axis for Understanding Mastitis Pathogenesis and Therapeutic Strategies" Veterinary Sciences 12, no. 11: 1049. https://doi.org/10.3390/vetsci12111049
APA StyleLi, Y., Wang, M., Liu, W., Geng, M., Asiri, M., Alzahrani, F. M., Alzahrani, K. J., Ma, Q., Wang, C., & Khan, M. Z. (2025). Targeting the Gut–Mammary Axis for Understanding Mastitis Pathogenesis and Therapeutic Strategies. Veterinary Sciences, 12(11), 1049. https://doi.org/10.3390/vetsci12111049







