Comprehensive Characterization of Mycoplasmosis bovis ST52 Strain 16M Reveals Its Pathogenicity and Potential Value in Vaccine Development
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Source
2.2. Isolation and Culture
2.3. Identification of M. bovis
2.4. Drug Resistance Test
2.5. Biofilm Detection
2.6. Pathogenicity and Challenge Study
2.7. Growth Inhibition Experiment
2.8. Vaccine Preparation
2.9. Evaluation of the Immune Effect
2.10. Genome Sequencing and Comprehensive Analysis
2.11. Genetic Stability Testing
2.12. Statistics and Analysis
3. Results
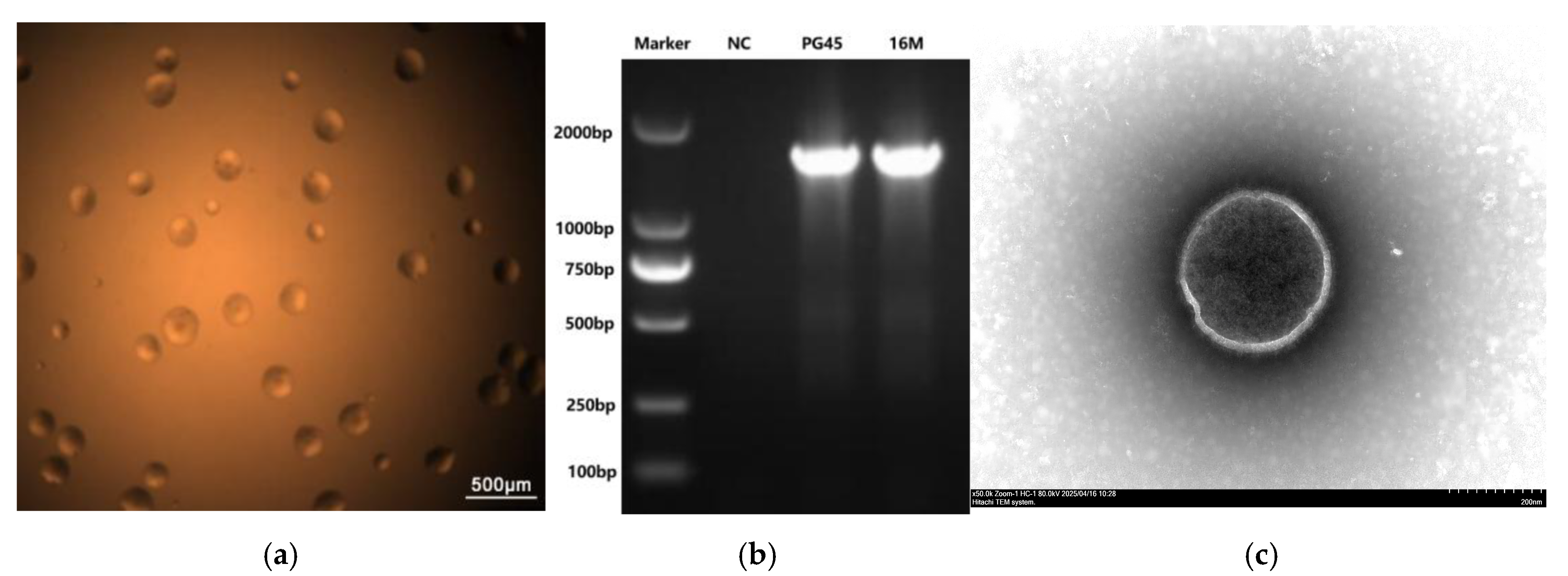
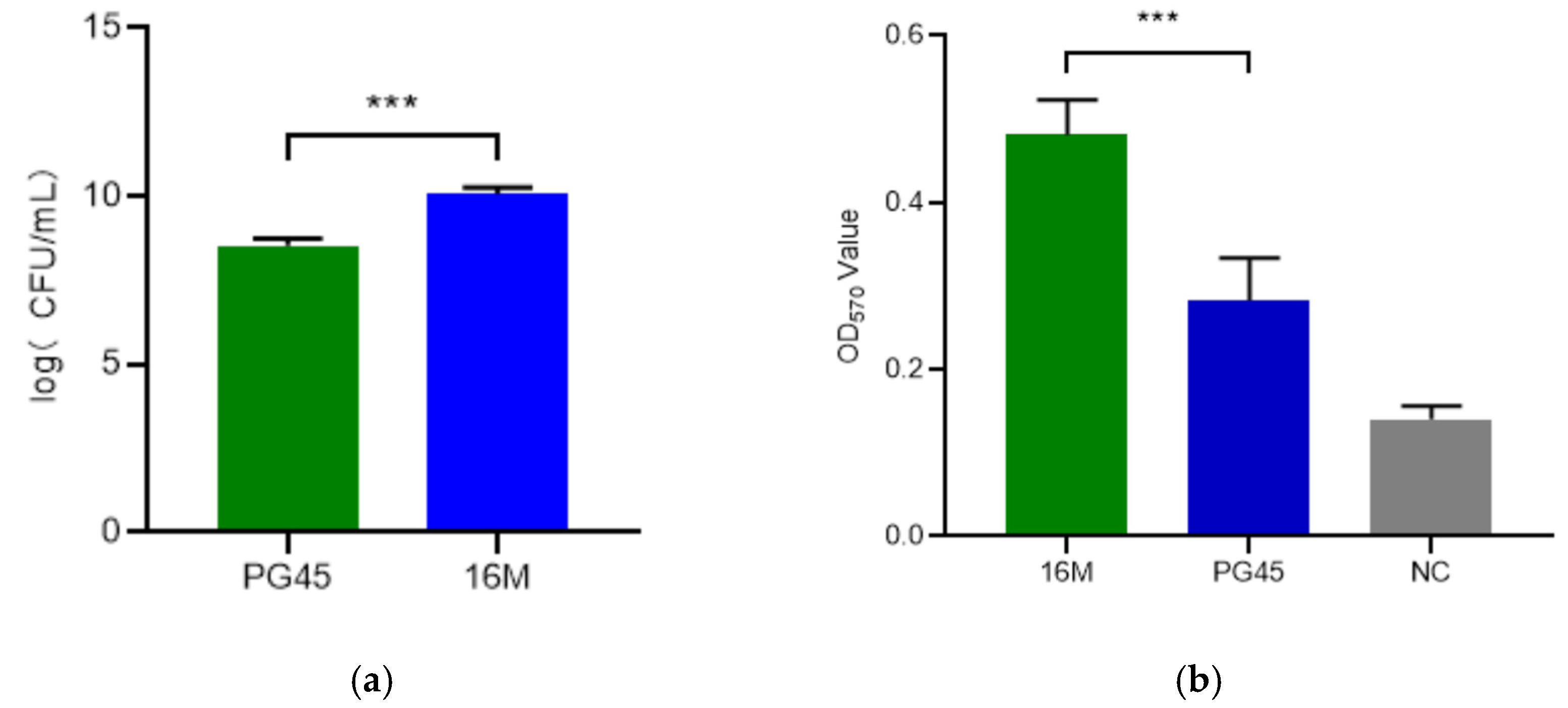
3.1. Molecular and Phenotypic Characteristics of M. bovis Strain 16M
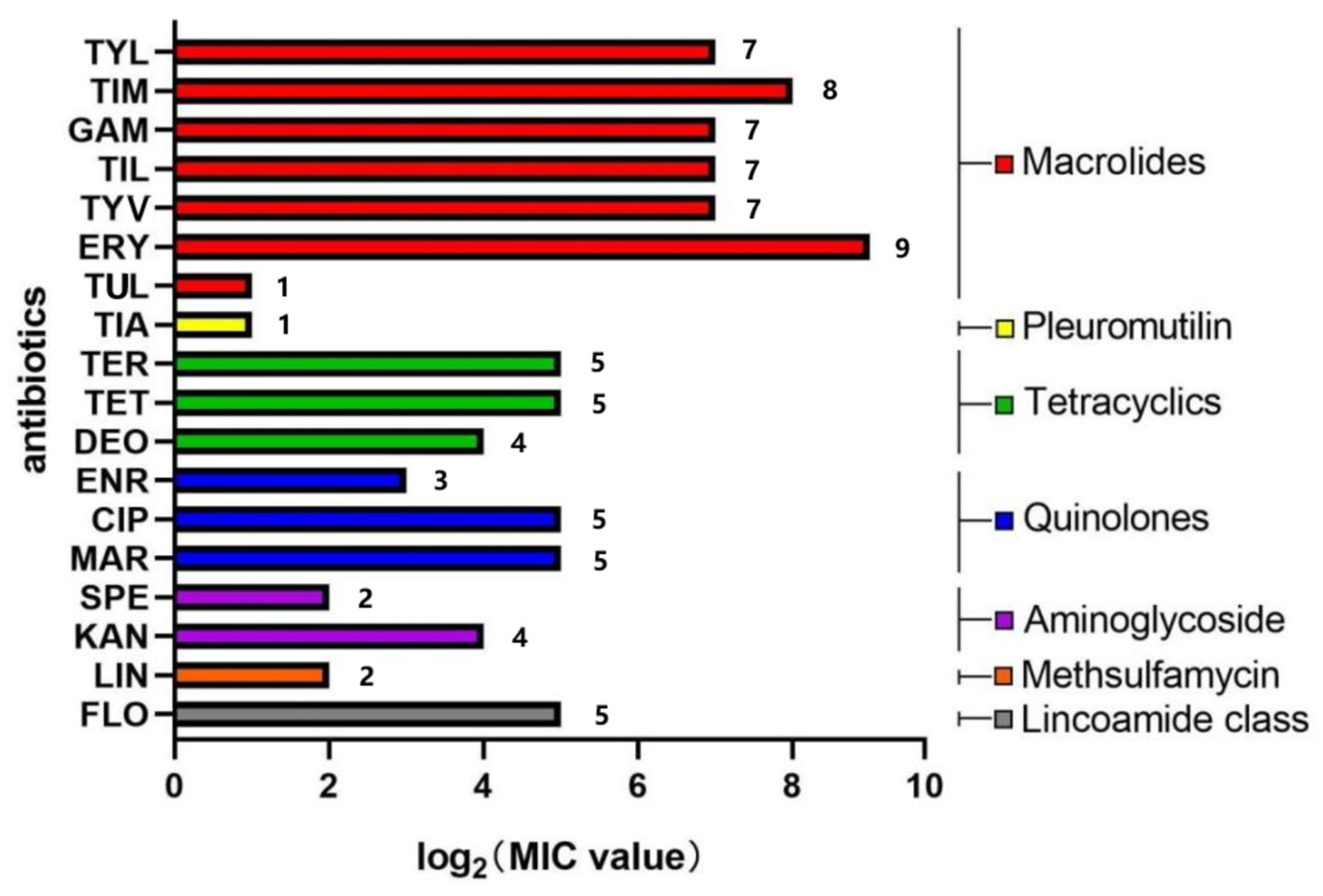
3.2. Drug Susceptibility Analysis
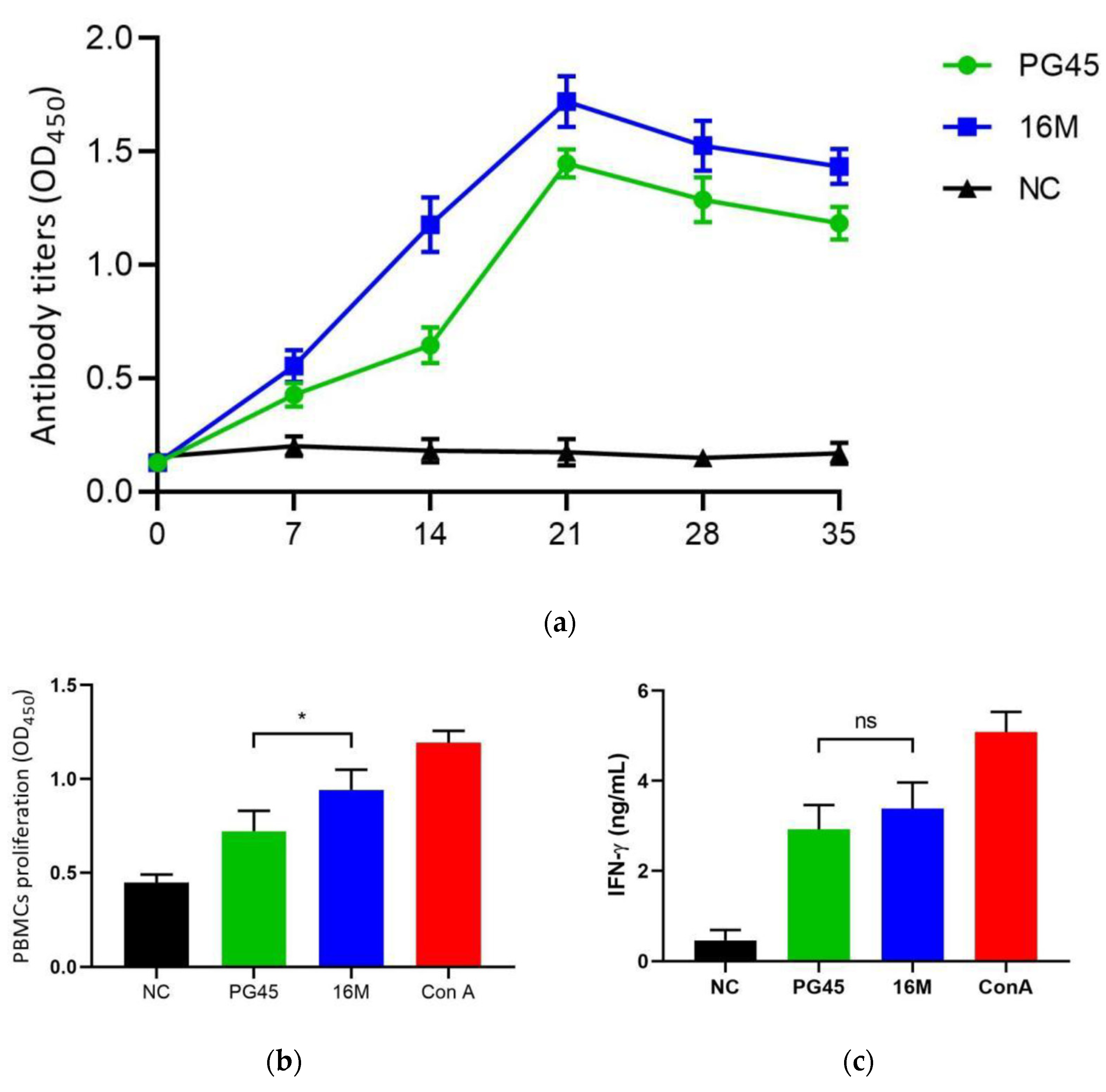
3.3. Immunological Estimate of M. bovis Strain 16M
3.4. Pathogenicity of the M. bovis 16M Strain
3.5. Challenge Model and Vaccine Protection
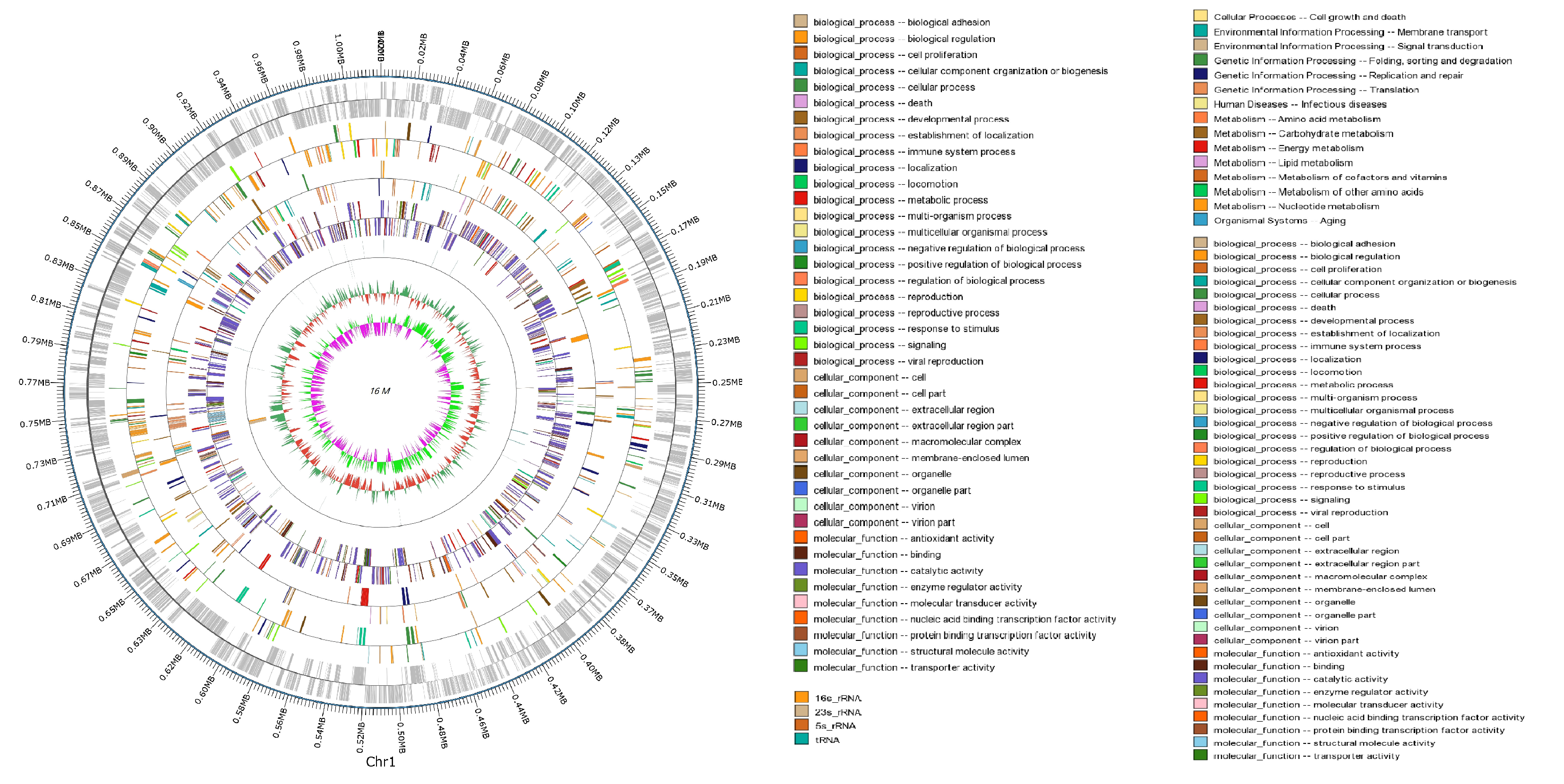
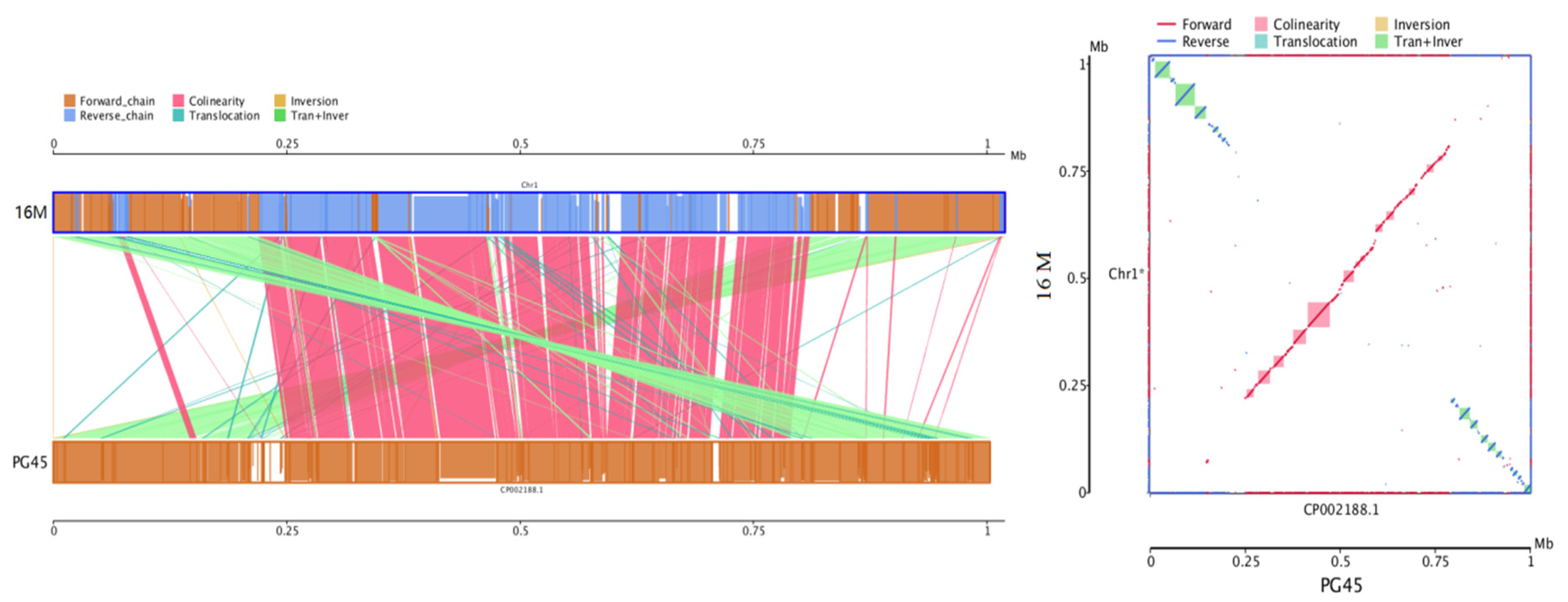
3.6. Comparative and Comprehensive Genome-Wide Analysis
3.7. Genetic Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| M. bovis | Mycoplasmosis bovis |
| PPLO | Pleuropneumonia-like organism |
| DMEM | Dulbecco’s Modified Eagle Medium |
| LD | Linear dichroism |
| CFU | Colony-forming units |
| TEM | Transmission electron microscope |
| CCU | Color-changing units |
| MIC | Minimal inhibitory concentration |
| TYL | Tylosin |
| TIM | Tilmicosin |
| GAM | Gamethromycin |
| TIL | Tildipirosin |
| TYV | Tylvalosin |
| ERY | Erythromycin |
| TUL | Tulathromycin |
| TER | Terramycin |
| TET | Tetracycline |
| DEO | Doxycycline |
| ENR | Enrofloxacin |
| CIP | Ciprofloxacin |
| MAR | Marbofloxacin |
| SPE | Spectinomycin |
| KAN | Kanamycin |
| LIN | Lincomycin |
| FLO | Florfenicol |
| TIA | Tiamulin |
| MOI | Multiplicity of infection |
| ConA | Concanavalin A |
| PBS | Phosphate-buffered solution |
| ELISA | Enzyme-linked immunoadsorption assay |
| FBS | fetal bovine serum |
| PBMC | Peripheral blood mononuclear cells |
| IFN | Interferon |
| COG | Clusters of Orthologous Groups |
| SNP | Single-nucleotide polymorphism |
| SV | Structural variation |
| Indel | insertion and deletion |
| MLST | Multilocus sequence typing |
| PHI | Pathogen Host Interactions |
| VFDB | Virulence Factors of Pathogenic Bacteria |
| ARDB | Antibiotic Resistance Genes Database |
| CARD | Comprehensive Antibiotic Research Database |
References
- Nicholas, R.A.; Fox, L.K.; Lysnyansky, I. Mycoplasma Mastitis in Cattle: To Cull or Not to Cull. Vet. J. 2016, 216, 142–147. [Google Scholar] [CrossRef]
- Adamu, Y.J.; Wawegama, N.K.; Browning, G.F.; Walker, M.J.; Djordjevic, M.A. Membrane Proteins of M. bovis and Their Role in Pathogenesis. Res. Vet. Sci. 2013, 95, 321–325. [Google Scholar] [CrossRef]
- Jose, C.P. Pathogenesis and Virulence of M. bovis. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 269–278. [Google Scholar] [CrossRef]
- Hale, H.H.; Helmboldt, C.F.; Plastridge, W.N.; Stula, J.M. Bovine Mastitis Caused by Mycoplasma Species. Cornell Vet. 1962, 52, 582–591. [Google Scholar] [PubMed]
- Gelgie, A.E.; Desai, S.E.; Gelalcha, B.D.; Kerro Dego, O. M. bovis Mastitis in Dairy Cattle. Front. Vet. Sci. 2024, 11, 1322267. [Google Scholar] [CrossRef]
- Maunsell, F.P.; Woolums, A.R.; Francoz, D.; Rosenbusch, R.F.; Step, D.L.; Wilson, D.J.; Janzen, E.D. M. bovis Infections in Cattle. J. Vet. Intern. Med. 2011, 25, 772–783. [Google Scholar] [CrossRef]
- Wilson, D.J.; Skirpstunas, R.T.; Trujillo, J.D.; Cavender, K.B.; Bagley, C.V.; Harding, R.L. Unusual History and Initial Clinical Signs of M. bovis Mastitis and Arthritis in First-Lactation Cows in a Closed Commercial Dairy Herd. J. Am. Vet. Med. Assoc. 2007, 230, 1519–1523. [Google Scholar] [CrossRef]
- Alberti, A.; Addis, M.F.; Chessa, B.; Cubeddu, T.; Profiti, M.; Rosati, S.; Pittau, M. Molecular and Antigenic Characterization of a M. bovis Strain Causing an Outbreak of Infectious Keratoconjunctivitis. J. Vet. Diagn. Investig. 2006, 18, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Walz, P.H.; Mullaney, T.P.; Render, J.A.; Walker, R.D.; Mosser, T.; Baker, J.C. Otitis Media in Preweaned Holstein Dairy Calves in Michigan Due to M. bovis. J. Vet. Diagn. Investig. 1997, 9, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.E.; Higuchi, H.; Tezuka, E.; Senatore, E.M.; Izaike, Y.; Osawa, T. Mycoplasma Infection in the Uterus of Early Postpartum Dairy Cows and Its Relation to Dystocia and Endometritis. Theriogenology 2013, 79, 180–185. [Google Scholar] [CrossRef]
- Hermeyer, K.; Peters, M.; Brugmann, M.; Jacobsen, B.; Hewicker-Trautwein, M. Demonstration of M. bovis by Immunohistochemistry and In Situ Hybridization in an Aborted Bovine Fetus and Neonatal Calf. J. Vet. Diagn. Investig. 2012, 24, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, E.; Castiglioni, V.; Losa, M.; Nicholas, R.A.J.; Luini, M. Outbreak of Bovine Clinical Mastitis Caused by M. bovis in a North Italian Herd. Res. Vet. Sci. 2011, 91, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Huijben, H.; van Schaik, J.; Benedictus, R.; Lam, T.J.G.M.; van Schaik, G. Prevalence of Mycoplasma Species and Their Associated Clinical Signs in Dairy Herds in the Netherlands. J. Dairy. Sci. 2011, 94, 4557–4565. [Google Scholar] [CrossRef]
- Lysnyansky, I.; Freed, M.; Set, R.; Lifshitz, M.; Levisohn, R. An Overview of M. bovis Mastitis in Israel (2004–2014). Vet. J. 2016, 207, 180–183. [Google Scholar] [CrossRef]
- Dudek, K.; Nicholas, R.A.J.; Szacawa, E.; Bednarek, D. M. bovis Infections—Occurrence, Diagnosis and Control. Pathogens 2020, 9, 640. [Google Scholar] [CrossRef]
- Chen, J.D.; Li, J.S.; Zhang, F.P.; Zhao, H.X.; Xu, R.L.; Zhang, S.Q. Isolation and Identification of Mycoplasma from Dairy Cows in Shanghai. Acta Vet. Zootech. Sin. 1983, 14, 60–66. (In Chinese) [Google Scholar]
- Qi, J.J.; Guo, A.Z.; Cui, P.; Chen, Y.Y.; Mustafa, R.; Ba, X.L.; Hu, C.M.; Bai, Z.D.; Chen, C.; Shi, L.; et al. Comparative geno-plasticity analysis of Mycoplasma bovis HB0801 (Chinese isolate). PLoS ONE 2012, 7, e38239. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, H.; Guo, S.; Lei, Y.; Li, Y.; He, S. Multi-locus sequence typing of Mycoplasma bovis to assess its genetic diversity from 2009 to 2018 in Ningxia Hui Autonomous Region, China. BMC Vet. Res. 2020, 16, 454. [Google Scholar] [CrossRef]
- Lan, S.M.; Liu, S.; Cui, W.J.; Li, Z.C.; Hao, H.F.; Baz, A.A.; Liang, J.J.; Jin, X.R.; Yan, X.M.; Gao, P.C.; et al. Emergence of Novel Fluoroquinolone Resistance Mutations in Mycoplasma bovis, China, 2008–2023. Emerg. Infect. Dis. 2025, 31, 1676–1679. [Google Scholar] [CrossRef]
- Klein, U.; de Jong, A.; Youala, M.; El Garch, F.; Moyaert, H.; Rose, M.; Pridmore, A.; Siem, A. New Antimicrobial Susceptibility Data from Monitoring of M. bovis Isolated in Europe. Vet. Microbiol. 2019, 238, 108432. [Google Scholar] [CrossRef]
- Maunsell, F.P.; Donovan, G.A.; Risco, C.; Brown, M.B. Field Evaluation of a M. bovis Bacterin in Young Dairy Calves. Vaccine 2009, 27, 2781–2788. [Google Scholar] [CrossRef]
- Soehnlen, M.K.; Aydin, A.; Lengerich, E.J.; Houser, B.A.; Fenton, G.D.; Lysnyansky, I.; Bayles, D.O.; Reinhardt, T.A. Blinded, Controlled Field Trial of Two Commercially Available M. bovis Bacterin Vaccines in Veal Calves. Vaccine 2011, 29, 5347–5354. [Google Scholar] [CrossRef]
- Zhang, R.; Han, X.; Chen, Y.; Wei, Y.; Li, X.; Li, H.; Xin, J. Attenuated M. bovis Strains Provide Protection Against Virulent Infection in Calves. Vaccine 2014, 32, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Perez-Casal, J.; Prysliak, T.; Maina, T.; Suleman, M.; Jimbo, S. Status of the development of a vaccine against Mycoplasma bovis. Vaccine 2017, 35, 2902–2907. [Google Scholar] [CrossRef]
- Register, K.B.; Lysnyansky, I.; Jelinski, M.D.; Boatwright, W.D.; Waldner, M.; Bayles, D.O.; Pilo, P.; Alt, D.P. Comparison of Two Multilocus Sequence Typing Schemes for M. bovis and Revision of the PubMLST Reference Method. J. Clin. Microbiol. 2020, 58, e00283-20. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Bednarek, D. Saponin-based Mycoplasma bovis Vaccine Containing Lysozyme Dimer Adjuvant Stimulates Acute Phase Response in Calves. J. Vet. Res. 2018, 62, 269–273. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Y.; Hua, L.; Dong, X.; Li, B.; Shi, C.; He, Q. Genotyping and Biofilm Formation of Mycoplasma hyopneumoniae and Their Association with Virulence. Vet. Res. 2022, 53, 95. [Google Scholar] [CrossRef]
- Wise, K.S.; Calcutt, M.J.; Foecking, M.F.; Madupu, R.; Methe, B.A. Complete Genome Sequence of M. bovis Type Strain PG45 (ATCC 25523). Infect. Immun. 2011, 79, 982–983. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, S.; Li, M.; Zhou, Y.; Xia, L.; Wang, H.; Han, X. Molecular Characteristics and Antibiotic Susceptibility Profiles of M. bovis Associated with Mastitis on Dairy Farms in China. Prev. Vet. Med. 2020, 182, 105106. [Google Scholar] [CrossRef]
- Subramaniam, S.; Bergonier, D.; Poumarat, F.; Capaul, S.; Frey, J. Species Identification of Mycoplasma bovis and Mycoplasma agalactiae Based on the uvrC Genes by PCR. Mol. Cell. Probes. 1998, 12, 161–169. [Google Scholar] [CrossRef]
- Hannan, P.C. Guidelines and Recommendations for Antimicrobial Minimum Inhibitory Concentration (MIC) Testing Against Veterinary Mycoplasma Species. Vet. Res. 2000, 31, 373–395. [Google Scholar] [CrossRef]
- Ammar, A.M.; Abd El-Hamid, M.I.; Mohamed, Y.H.; Mohamed, H.M.; Al-Khalifah, D.H.M.; Hozzein, W.N.; Selim, S.; El-Neshwy, W.M.; El-Malt, R.M.S. Prevalence and Antimicrobial Susceptibility of Bovine Mycoplasma Species in Egypt. Biology 2022, 11, 1083. [Google Scholar] [CrossRef]
- Bokma, J.; Gille, L.; De Bleecker, K.; Callens, J.; Haesebrouck, F.; Pardon, B.; Boyen, F. Antimicrobial Susceptibility of M. bovis Isolates from Veal, Dairy and Beef Herds. Antibiotics 2020, 9, 882. [Google Scholar] [CrossRef]
- Nishi, K.; Gondaira, S.; Hirano, Y.; Ohashi, M.; Sato, A.; Matsuda, K.; Iwasaki, T.; Kanda, T.; Uemura, R.; Higuchi, H. Biofilm characterisation of Mycoplasma bovis co-cultured with Trueperella pyogenes. Vet. Res. 2025, 56, 22. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Liu, G.; Wang, C.; Ji, Y.; Chen, J.; Hu, C.; Chen, X.; Guo, A.; Chen, Y. The Safety and Protective Efficacy Evaluation of an Attenuated M. bovis–BoHV-1 Bivalent Vaccine in Rabbits. Vaccines 2023, 11, 1698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, G.; Wu, W.; Yang, L.; Shirani, I.; Guo, A.; Chen, Y. Investigation of the Optimal Immunization Dose and Protective Efficacy of an Attenuated and Marker M. bovis–Bovine Herpesvirus Type 1 Combined Vaccine in Rabbits. Animals 2024, 14, 748. [Google Scholar] [CrossRef]
- Poveda, J.B.; Nicholas, R. Serological Identification of Mycoplasmas by Growth and Metabolism Inhibition Tests. In Mycoplasma Protocols. Methods in Molecular Biology™; Miles, R., Nicholas, R., Eds.; Humana Press: Totowa, NJ, USA, 1998; Volume 104. [Google Scholar] [CrossRef]
- Clyde, W.A. Mycoplasma species identification based upon growth inhibition by specific antisera. J. Immunol. 1964, 92, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Kleinwort, K.J.H.; De Groote, R.L.; Hirmer, S.; Amann, B.; Hauck, S.M.; Schulze, C.; Mayr, D.; Degroote, R.L. Bovine Peripheral Blood Derived Lymphocyte Proteome and Secretome Show Divergent Reaction of Bovine Immune Phenotypes After Stimulation with Pokeweed Mitogen. Proteomes 2022, 10, 7. [Google Scholar] [CrossRef]
- Urban, M.; Pant, R.; Raghunath, A.; Irvine, A.G.; Pedro, H.; Hammond-Kosack, K.E. The Pathogen-Host Interactions Database (PHI-Base): Additions and Future Developments. Nucleic Acids Res. 2015, 43, D645–D655. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiong, Z.; Sun, L.; Yang, J.; Jin, Q. VFDB 2012 Update: Toward the Genetic Diversity and Molecular Evolution of Bacterial Virulence Factors. Nucleic Acids Res. 2012, 40, D641–D645. [Google Scholar] [CrossRef]
- Liu, B.; Pop, M. ARDB—Antibiotic Resistance Genes Database. Nucleic Acids Res. 2009, 37, D443–D447. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and Model-Centric Curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes Database (CAZy): An Expert Resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Harris, R.S. Improved Pairwise Alignment of Genomic DNA. Ph.D. Thesis, The Pennsylvania State University, University Park, TX, USA, 2007. [Google Scholar]
- Kurtz, S.; Phillippy, A.; Delcher, A.L.; Smoot, M.; Shumway, M.; Antonescu, C.; Salzberg, S.L. Versatile and Open Software for Comparing Large Genomes. Genome Biol. 2004, 5, R12. [Google Scholar] [CrossRef]
- Nicholas, R.A.; Ayling, R.D.; Stipkovits, L.P. An experimental vaccine for calf pneumonia caused by Mycoplasma bovis: Clinical, cultural, serological and pathological findings. Vaccine 2002, 20, 3569–3575. [Google Scholar] [CrossRef]
- Haynes, J.S.; Friebertshauser, S.E.; Stine, D.L. An Experimental Vaccine Composed of Two Adjuvants Gives Protection against Mycoplasma bovis in Calves. Vaccine 2000, 18, 1417–1424. [Google Scholar] [CrossRef]
- Baz, A.A.; Chen, S.; Hao, H.; Jin, X.; Lan, S.; Li, Z.; Jin, S.; Zhang, Y.; Chu, Y. Macrophage extracellular traps are induced by Mycoplasma bovis in bovine macrophages through NADPH oxidase/ROS-dependent manner and their antibacterial efficacy. FASEB J. 2024, 38, e70238. [Google Scholar] [CrossRef]
- Li, B.; Lu, Y.; Feng, Y.; Jiao, X.; Zhang, Q.; Zhou, M.; Zhang, Y.; Xu, J.; Chu, Y.; Ran, D. Mycoplasma bovis Invades Non-Phagocytic Cells by Clathrin-Dependent Endocytic Pathways and Escapes from Phagocytic Vesicles. Pathogens 2024, 13, 1003. [Google Scholar] [CrossRef]
- Dudek, K.; Szacawa, E.; Nicholas, R.A.J. Recent Developments in Vaccines for Bovine Mycoplasmoses Caused by Mycoplasma bovis and Mycoplasma mycoides subsp. mycoides. Vaccines 2021, 9, 549. [Google Scholar] [CrossRef] [PubMed]
- Vulikh, K.; Burrows, D.; Perez-Casal, J.; Tabatabaei, S.; Caswell, J.L. Effects of Inflammatory Stimuli on the Development of Mycoplasma bovis Pneumonia in Experimentally Challenged Calves. Vet. Res. 2024, 55, 102. [Google Scholar] [CrossRef] [PubMed]
- Valeris-Chacin, R.; Powledge, S.; McAtee, T.; Morley, P.S.; Richeson, J. Mycoplasma bovis Is Associated with Mannheimia haemolytica during Acute Bovine Respiratory Disease in Feedlot Cattle. Front. Microbiol. 2022, 13, 946792. [Google Scholar] [CrossRef]
- Simmons, W.L.; Daubenspeck, J.M.; Osborne, J.D.; Balish, M.F.; Waites, K.B.; Dybvig, K. Type 1 and Type 2 Strains of Mycoplasma pneumoniae Form Different Biofilm Structures In Vitro. Front. Microbiol. 2013, 4, 129. [Google Scholar] [CrossRef]
- He, J.; Liu, M.; Ye, Z.; Tan, Y.; Qin, X.; Liu, X. Biofilm Formation and Extracellular Polymeric Substance Synthesis in Mycoplasma gallisepticum. Vet. Microbiol. 2014, 172, 232–240. [Google Scholar] [CrossRef]
- Machado, A.; Sismeiro, R.; Gourlay, L.J.; Franco, A.T.; Varela, C.; Spagnuolo, J.; Legrain, M.; Dessen, A.; Marques, M.V. The Mycoplasma genitalium MG_454 Protein Resists Killing by Antimicrobial Peptides through Biofilm Formation. mBio 2020, 11, e02087-20. [Google Scholar] [CrossRef]
- Chen, S.; Hao, H.; Zhao, P.; Ji, W.; Li, M.; Liu, Y.; Chu, Y. Differential Immunoreactivity to Bovine Convalescent Serum Between Mycoplasma bovis Biofilms and Planktonic Cells Revealed by Comparative Immunoproteomic Analysis. Front. Microbiol. 2018, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Purcell, R.H.; Wong, D.; Chanock, R.M.; Taylor-Robinson, D.; Canchola, J.; Valdesuso, J. Significance of antibody to mycoplasma as measured by metabolic-inhibition techniques. Ann. N. Y. Acad. Sci. 1967, 143, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Li, X.; Zhao, H.R.; Jiang, F.; Wang, Z.H.; Wu, W.X. Antibodies Specific to Membrane Proteins Are Effective in Complement-Mediated Killing of Mycoplasma bovis. Infect. Immun. 2019, 87, e00740-19. [Google Scholar] [CrossRef]
- Sachse, K.; Helbig, J.H.; Lysnyansky, I.; Grajetzki, C.; Müller, W.; Jacobs, E.; Yogev, D. Epitope Mapping of Immunogenic and Adhesive Structures in Repetitive Domains of Mycoplasma bovis Variable Surface Lipoproteins. Infect. Immun. 2000, 68, 680–687. [Google Scholar] [CrossRef]
- Wawegama, N.K.; Browning, G.F.; Kanci, A.; Marenda, M.S.; Markham, P.F. A Surface-Exposed Lipoprotein of Mycoplasma bovis Is a Novel Multifunctional Adhesin. Infect. Immun. 2016, 84, 1100–1111. [Google Scholar] [CrossRef]
- Khan, F.A.; Rasheed, m.a.; Faisal, M.; Menghwar, H.; Zubair, M.; Sadique, U.; Chen, H.C.; Guo, A.Z. Proteomics analysis and its role in elucidation of functionally significant proteins in Mycoplasma bovis. Microb. Pathog. 2017, 111, 50–59. [Google Scholar] [CrossRef]
- Gautier-Bouchardon, A.V.; Ferre, S.; Tardy, F. Immunogenicity of a Trivalent Vaccine Containing Mycoplasma bovis Surface Lipoproteins in Cattle. Vaccine 2020, 38, 1430–1438. [Google Scholar] [CrossRef]
- Maunsell, F.P.; Woolums, A.R.; Francoz, D.; Rosenbusch, R.F.; Step, D.L.; Wilson, D.J.; Janzen, E.D. Mycoplasma bovis: Interactions with the Immune System and Failure to Generate an Effective Immune Response. Vet. Microbiol. 2011, 153, 13–22. [Google Scholar] [CrossRef]
- Gagea, M.I.; Bateman, K.G.; Shanahan, R.A.; van Dreumel, T.; McEwen, B.J.; Carman, S.; Archambault, M.; Caswell, J.L. Cellular and Humoral Immune Responses to Mycoplasma bovis in Calves. Vet. Immunol. Immunopathol. 2006, 109, 141–148. [Google Scholar] [CrossRef]
- Suleman, M.; Cyprian, F.S.; Jimbo, S.; Maina, T.; Prysliak, T.; Windeyer, C.; Perez-Casal, J. Mycoplasma bovis-Induced Inhibition of Bovine Peripheral Blood Mononuclear Cell Proliferation Is Ameliorated after Blocking the Immune-Inhibitory Programmed Death 1 Receptor. Infect. Immun. 2018, 86, e00921-17. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, M.; Li, J.; Wang, X.; Wang, S.; Chen, Q.; He, H. IFN-γ-Producing CD4+ T Cells Are Critical for Host Defense Against Mycoplasma bovis Infection. Front. Immunol. 2020, 11, 585132. [Google Scholar] [CrossRef]
- Sachse, K.; Salam, H.S.; Diller, R.; Schubert, E.; Hoffmann, B.; Hotzel, H. Th1/Th17-Biased Inflammation Dominates Experimental Mycoplasma bovis Pneumonia. Vet. Res. 2019, 50, 18. [Google Scholar] [CrossRef]
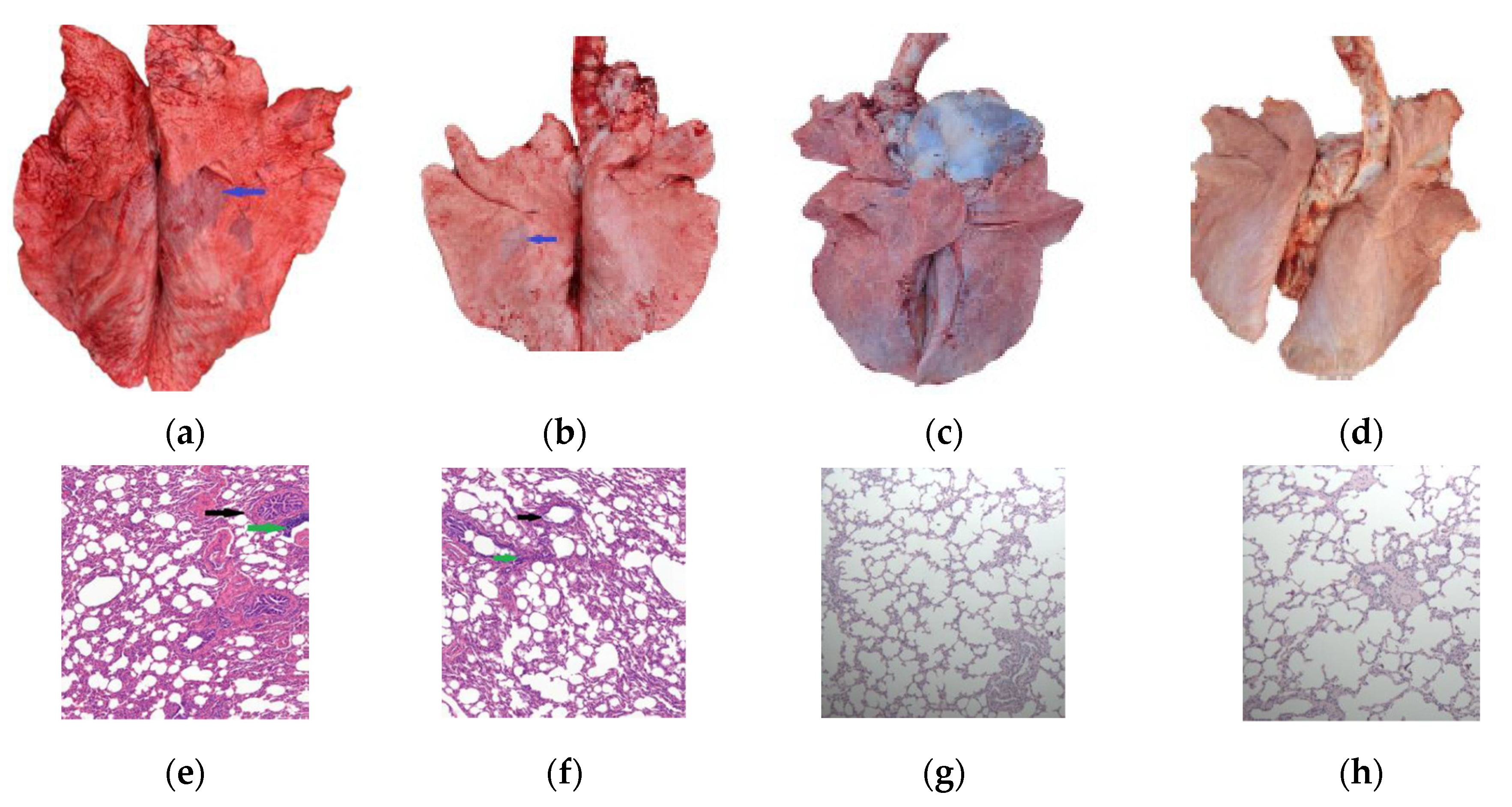
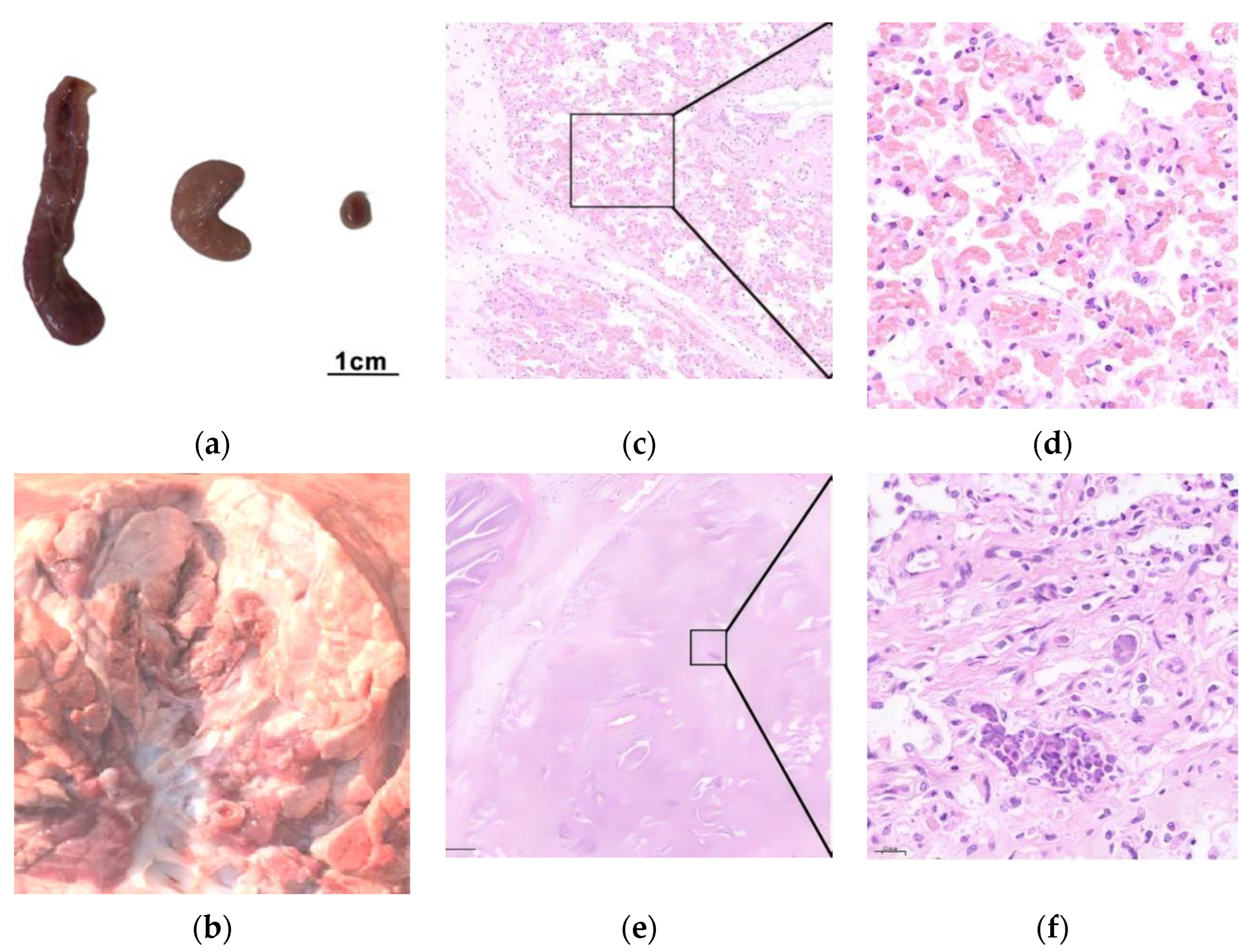
| Condition | PG45 Group | 16M Group | 16M-Vaccinated Group | NC Group | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Clinical signs | 1 | 2 | 2 | 1 | 1 | 4 | 3 | 3 | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Shedding | 2 | 3 | 3 | 2 | 2 | 3 | 4 | 3 | 2 | 3 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| Gross lesions | 2 | 1 | 2 | 1 | 1 | 5 | 3 | 4 | 3 | 3 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Histopathology | 4 | 3 | 4 | 3 | 3 | 5 | 5 | 4 | 3 | 4 | 1 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| Total score | 43 | 67 | 25 | 1 | ||||||||||||||||
| Passage Number | Synonymous Variant | Missense Variant | Stop Gained | Gene Variant | Variant Rate | Biofilm (OD590nm) | Titers (CFU/mL) |
|---|---|---|---|---|---|---|---|
| P | 0 | 0 | 0 | 0 | 0 | 0.481 ± 0.042 | 2.0 × 1010 |
| F50 | 6 | 6 | 1 | 0 | 0.00129% | 0.479 ± 0.025 ns | 2.0 × 1010 |
| F100 | 5 | 12 | 2 | 0 | 0.00189% | 0.389 ± 0.011 *** | 2.0 × 1010 |
| F150 | 7 | 21 | 1 | 6 | 0.00289% | 0.356 ± 0.025 *** | 2.0 × 1010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, T.; Wang, J.; Zhang, Y.; Zhang, T.; Wu, Z.; Wang, W.; Yang, H. Comprehensive Characterization of Mycoplasmosis bovis ST52 Strain 16M Reveals Its Pathogenicity and Potential Value in Vaccine Development. Vet. Sci. 2025, 12, 1044. https://doi.org/10.3390/vetsci12111044
Zhang L, Wang T, Wang J, Zhang Y, Zhang T, Wu Z, Wang W, Yang H. Comprehensive Characterization of Mycoplasmosis bovis ST52 Strain 16M Reveals Its Pathogenicity and Potential Value in Vaccine Development. Veterinary Sciences. 2025; 12(11):1044. https://doi.org/10.3390/vetsci12111044
Chicago/Turabian StyleZhang, Liang, Tingwei Wang, Jilong Wang, Yunfei Zhang, Tianyu Zhang, Zhiyong Wu, Wenhui Wang, and Hongjun Yang. 2025. "Comprehensive Characterization of Mycoplasmosis bovis ST52 Strain 16M Reveals Its Pathogenicity and Potential Value in Vaccine Development" Veterinary Sciences 12, no. 11: 1044. https://doi.org/10.3390/vetsci12111044
APA StyleZhang, L., Wang, T., Wang, J., Zhang, Y., Zhang, T., Wu, Z., Wang, W., & Yang, H. (2025). Comprehensive Characterization of Mycoplasmosis bovis ST52 Strain 16M Reveals Its Pathogenicity and Potential Value in Vaccine Development. Veterinary Sciences, 12(11), 1044. https://doi.org/10.3390/vetsci12111044




