Simple Summary
Rabbit farming is an important source of meat, but a doe’s ability to produce and raise healthy young can be strongly influenced by weather conditions and farm management. In this study, we analyzed 770 litters from a commercial farm in central Mexico between 2015 and 2021 to assess how the year and season of birth affected the number of kits born alive, the number weaned, and the role of genetics. We found that the number of live-born kits varied significantly by year and season—being higher during wetter and semi-dry periods compared to the dry season, and notably greater in 2020–2021 than in earlier years. The number of kits weaned also varied between years but was less clearly linked to season, and survival rates to weaning showed only small seasonal differences. Genetic influence on these traits was very low, suggesting that improvements in farm management and mitigation of heat and drought stress are likely to yield faster and more substantial gains than selective breeding alone. These findings can help farmers and advisors prioritize practical strategies to increase productivity and reduce losses.
Abstract
Reproductive performance in rabbits is highly sensitive to seasonal environmental variation and management practices, while the proportion of variance attributable to additive genetics for litter-level traits is typically low. The objective of this study was to evaluate the effects of year and season on litter size at birth (BR), litter size at weaning (WR), and weaning rate (WT), and to estimate the heritability of these traits in a commercial rabbit farm. A total of 770 kindling events recorded between 2015 and 2021 were analyzed. The mixed model for BR included the fixed effects of year and season, and the random effects of sire and residual error. The model for WR included the same structure, with BR added as a covariate. Least-squares means for fixed effects were used for pairwise comparisons using Tukey’s test. Year and season effects were significant for BR (p < 0.005), and the year effect was also significant for WR (p < 0.021). Litter size at birth ranged from 7.80 (dry season) to 9.21 (year 2020), with higher means observed during the semi-dry (8.52) and humid (8.56) seasons compared to the dry season (7.80). Litter size at weaning varied between 4.65 and 5.81 kits depending on the year. Weaning rate showed interannual variation (56.1–68.2%), but seasonal differences did not reach statistical significance (p < 0.075). Heritability estimates from the sire variance component were low: 0.01 for BR, 0.04 for WR, and 0.05 for WT. These results indicate that phenotypic variation in prolificacy in this population was predominantly driven by interannual and seasonal environmental factors, as well as perinatal management practices, while the additive genetic contribution was marginal.
1. Introduction
Rabbit meat production provides an efficient, small-scale source of high-quality animal protein for many temperate and subtropical regions and remains an important component of mixed and family farming systems where resources and land are limited [1]. Female reproductive efficiency—particularly kindling rate, litter size and pre-weaning survival—is a key determinant of herd productivity and economic return in meat rabbit systems, because prolificacy determines the number of marketable animals produced per doe per year [1]. Rabbit reproduction is highly sensitive to environmental conditions (ambient temperature, humidity, and photoperiod) because rabbits have limited evaporative cooling capacity and rely heavily on vascular ear heat exchange for thermoregulation [2]; therefore, thermal stress can reduce conception, increase embryonic mortality, and negatively affect kit survival and growth [3,4].
Worldwide, rabbit meat production has shown fluctuating trends over the last decade and is strongly concentrated in a few countries; large producers (notably China) account for a major share of global output, while production in several traditional European producers has fallen in recent years [5,6]. Estimates from international databases and recent reviews show that global rabbit meat production is substantially lower than the historical peak of the 2010–2014 period and that production is geographically concentrated, which has implications for global supply and for the comparability of productivity metrics across regions [5,7]. These facts emphasize the need to situate local productivity studies within a global context when interpreting temporal and seasonal effects.
Rabbit meat is recognized for a favorable nutritional profile—high protein content, low total fat and cholesterol, and a high proportion of unsaturated fatty acids—features that have supported its positioning as a healthful red-meat alternative in nutrition and consumer studies [5,8]. Despite these positive attributes, consumer acceptance and market integration vary markedly among countries; this heterogeneity influences the commercial scale of production and the resources available for genetic improvement programs [9,10].
From a production-biology perspective, seasonal variation in reproductive performance results from both direct physiological effects of environmental stressors (heat, humidity, and photoperiod) and indirect effects mediated by changes in feed intake, male fertility—particularly semen quality—milk yield, and management routines [2]. These effects combine to produce predictable seasonal patterns in reproductive output, including the numbers of kits born and successfully weaned in many climates [11,12]. Characterizing local seasonal patterns—and their interaction with management and housing—is therefore central to designing targeted management and mitigation strategies (e.g., cooling/ventilation, nutritional adjustments, timing of breeding) that minimize reproductive losses and stabilize supply [2,4,12].
In rabbits, litter-level reproductive traits (e.g., total born, born alive, or weaned per litter) generally exhibit low additive heritability (h2), indicating that most of the observed variation is attributable to environmental factors and non-additive genetic effects, rather than to additive genetic variance that can be exploited through classical selection [13,14,15,16]. However, heritability estimates reported in the literature are sensitive to factors such as population structure, statistical model (e.g., animal vs. dam models, inclusion of permanent environmental effects), and the stage of measurement—heritability of the decreases from birth to weaning [17,18]. Recent studies on genetic parameters and selection experiments confirm the predominance of environmental variance in survival and litter size traits, but also demonstrate how carefully specified models can enhance estimate accuracy and inform decision-making regarding genetic selection versus management interventions [17,19].
In Mexico, rabbit meat production reached approximately 4520 t in 2023 [20], with over 50% concentrated in the central region, where farming systems are predominantly small- to medium-scale [21,22]. This region is subject to pronounced seasonal and inter-annual climatic variability, transitioning between dry, semi-dry, and humid to temperate periods. Such fluctuations can significantly affect female reproductive performance and preweaning survival [21,23]. Incorporating localized climatic classifications or seasonal indicators into mixed-model analyses improves the identification of high-risk periods for productivity and enables more targeted implementation of mitigation measures (e.g., environmental control, nutritional strategies, and timing of breeding) [24]. Comparable concerns about seasonal stress and fertility have been reported in Mediterranean and North African rabbit production systems, yet analyses tailored to Latin American climates remain limited [25].
In commercial rabbit populations, breed composition and crossbreeding strategies influence both average reproductive performance and sensitivity to environmental factors [26,27]. In Mexico, New Zealand White (NZW), Californian (CA), and their F1 or F2 crosses are widely used in commercial meat production due to their prolificacy, growth rate, and carcass quality [28]. However, local adaptation, genetic background, and crossbreeding history vary across farms and regions, and breed-specific responses to seasonal variation can affect both average litter size and the variance components underlying heritability estimates.
The objective of the present study was to quantify year and season effects on litter size at birth (BR) and at weaning (WR), and on weaning rate (WT), and to estimate the heritabilities of these traits in a commercial rabbit farm in central Mexico during 2015–2021.
2. Materials and Methods
2.1. Farm, Records, and Experimental Unit
Records from 770 kindling events (litters) occurring between 2015 and 2021 were collected on a commercial rabbit farm in Jiquipilco, México, Mexico (19°34′18.777” N 99°43′3.727” W). The experimental unit was the litter. For each kindling event, the following variables were recorded: doe identification, sire identification (when available), kindling date, number of kits born alive per litter (BR), number of kits weaned per litter (WR), and the weaning rate (WT), calculated as WT = (WR/BR) × 100. Stillborn and mummified kits were excluded from BR.
2.2. Genetic Type
The animals were commercial crossbreds. The breeding nucleus primarily used crossbred does mated with New Zealand White (NZW) bucks. Therefore, the population is best characterized as commercial crossbreds with a major contribution from NZW genetics.
2.3. Seasonal Classification and Year Effect
Season of kindling was assigned using Pro.Clima v1.0 [29] (based on farm coordinates and kindling date), which classifies each litter into localized climatic categories—Dry, Semi-dry, or Humid—according to program outputs [29]. Each category classifies the local monthly season based on an aridity index that incorporates rainfall and evapotranspiration, the latter estimated using temperature and solar radiation data [29]. The year effect corresponded to the calendar year of kindling (2015–2021).
2.4. Productive and Reproductive Management
The farm operated a five-week batch production system, consisting of four weeks for gestation and one week for kindling and batch reset. This schedule enabled synchronized parturitions, efficient use of space and labor, and structured reproductive management—standard practice in commercial rabbit production.
Does were managed in cohorts synchronized on a 35-day cycle. Natural mating was employed, with directed mating practices: does were placed with selected bucks under supervision to ensure successful copulation and allow for individual parentage recording.
2.5. Housing, Feeding, and General Management
Does and bucks were housed individually in galvanized metal cages (90 × 60 × 40 cm) located in a naturally ventilated facility with thermal insulation in the roof and walls, and adjustable side windows. Cages were arranged in a flat-deck (single-row) system. Nest boxes were installed five days prior to the expected parturition date.
Each cage was equipped with an English-style hopper feeder (1.5 kg capacity) and an automatic nipple drinker providing freshwater ad libitum.
Animals were fed a commercial pelleted feed (Conejo, Unión® Tepexpan, Acolman, Mexico). The analyzed chemical composition of the diet, on a dry matter basis, was as follows: crude protein, 15.75%; neutral detergent fiber, 49.84%; acid detergent fiber, 39.45%; ash, 8.45%; and organic matter, 91.55%. This formulation met the nutritional requirements for both maintenance and reproduction, supplying adequate levels of protein (12% and 15%, respectively) and fiber (10% and 14%, respectively), in accordance with NRC guidelines [30].
2.6. Sanitary Management
A comprehensive health management protocol was implemented on the farm to prevent and control common infectious and parasitic diseases affecting commercial rabbit production.
Bimonthly deworming was conducted using sulfonamides combined with trimethoprim (e.g., Koryn Triple) to control coccidiosis, a common parasitic disease in rabbits.
Multivitamin complexes (Ruviotic and Vitafort A) were administered to support metabolic function and reproductive performance.
Respiratory issues associated with cold weather or poor ventilation, which can lead to ammonia buildup, were managed by administering ambroxol and doxycycline (Valsyn) in the drinking water at a concentration of approximately 1 g doxycycline per liter, aiming to reduce secondary infections.
Mite infestations (sarcoptic mange) were treated topically with permethrin-based products (e.g., Bravo spray) following the manufacturer’s recommendations.
Diarrhea cases were treated orally with combinations of sulfonamides, trimethoprim, neomycin, and adsorbents such as kaolin–pectin formulations (e.g., Stop-One, Tomo, Kaofin).
Metabolic stimulants (e.g., Catosal) and vitamin A-D-E preparations (e.g., Vigantol) were used to enhance health and reproductive efficiency in breeding does. Vitamin dosages were carefully adjusted—typically ranging from 0.4 to 0.6 mL per doe—to avoid toxicity.
2.7. Ethical Considerations
All management practices and procedures described above reflect routine activities regularly conducted on the commercial rabbit farm. The detailed descriptions are provided solely to offer context for the research. No additional interventions were carried out on the animals specifically for this study. Therefore, ethical approval was not required, as all animals remained under standard farm care and management. The data analyzed were obtained retrospectively from farm records, accessed with permission from the farm management.
2.8. Data Editing and Inclusion/Exclusion Criteria
Records with missing or incomplete BR or WR values were excluded from the analysis. Stillborn and mummified kits were not included in BR. Relevant management events (e.g., feed reformulations, disease outbreaks, major infrastructure changes) were recorded and are discussed qualitatively in the Discussion. When doe or sire identification was missing, the record was retained for litter-level analyses but could not contribute to variance estimation for animal-level random effects.
2.9. Statistical Analysis
All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
After verifying the normality of the variables through a normal quantile plot, a linear mixed model was fitted using PROC MIXED. The most comprehensive model was defined as follows:
where = response variable (e.g., WR), μ = overall mean, Sᵢ = random effect of the i-th sire, Aⱼ = fixed effect of the j-th year of kindling, Eₖ = fixed effect of the k-th season of kindling, β1BRₗ = fixed effect of the l-th BR as a linear covariate (applied only to WR), and εijk = residual error.
2.10. Variance Components and Heritability
Variance components for sires () and the environment () were estimated using the restricted maximum likelihood (REML) procedure. Least-squares means were computed and used for pairwise comparisons, applying the Tukey–Kramer adjustment. Statistical significance was declared at p < 0.05.
Heritabilities (h2) of the evaluated traits were estimated following Falconer and Mackay [31], using the following expression:
where is the phenotypic variance, calculated as , with representing the sire variance and the environmental variance.
3. Results
The effects of year and season of kindling were significant for BR (p = 0.005; Table 1). The effect of year was also significant for WR (p = 0.021), whereas the effect of season on WR was not statistically significant (p = 0.152; Table 1).

Table 1.
Least square means (± standard error) for litter size at birth (BR) and weaning (WR), and weaning rate (WT) of rabbits in Central Mexico by year and season of kindling.
Litters born in 2020 and 2021 had significantly higher BR than those born between 2015 and 2019 (p < 0.05; Figure 1). Regarding season, litters born during the semi-dry and humid periods (8.52 ± 0.18 and 8.56 ± 0.17 kits, respectively) had higher BR than those born in the dry period (7.80 ± 0.20 kits; p < 0.05; Table 1).
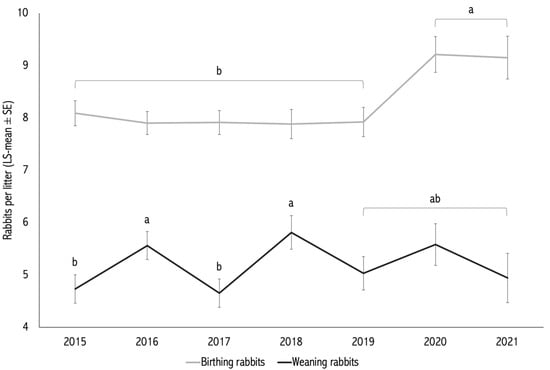
Figure 1.
Annual trends (2015–2021) of adjusted means for number of kits born alive (BR) and number of kits weaned (WR). The effect of year was significant for both BR (p < 0.005) and WR (p < 0.021). Different letters (a,b) above the bars indicate statistically significant differences between seasons (p < 0.05).
For WR, interannual differences were evident: the highest WR means were observed in 2016 (5.56 ± 0.27) and 2018 (5.81 ± 0.32), while other years showed intermediate or lower means (ranging from 4.65 to 5.58). Yearly weaning proportions ranged from 56.1% to 68.2%. Seasonal differences in WT did not reach statistical significance (p = 0.075), although the humid season showed the highest mean WT (65.49 ± 2.58%) (Figure 2).
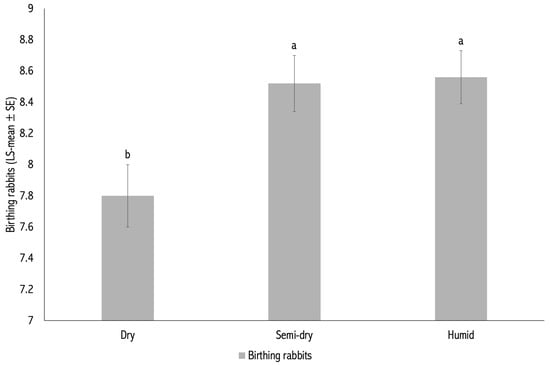
Figure 2.
Number of kits born alive per litter (BR) by season of kindling. Different letters (a,b) above the bars indicate statistically significant differences between seasons (p < 0.05).
Estimates of narrow-sense heritability were low under the current half-sib (sire component) model: BR h2 = 0.01, WR h2 = 0.04, and WT h2 = 0.05, indicating a marginal additive genetic contribution to these traits given the model and available data (Table 2).

Table 2.
Estimated heritabilities (h2) for litter size at birth (BR), litter size at weaning (WR), and weaning rate (WT) obtained from a sire model.
4. Discussion
The results of the present study indicate that phenotypic variation in prolificacy (BR) was primarily driven by interannual and seasonal environmental factors (year and season effects), while the additive genetic contribution, as estimated from the sire variance component, was minimal. This pattern aligns with recent findings in rabbit production and other prolific species. In rabbits, thermal stress and seasonal management conditions negatively impact fertility, increase embryonic loss, and reduce neonatal survival, all of which directly influence the number of live-born kits [27]. Recent studies have reported clear physiological effects of heat on female fertility and male semen quality, as well as hormonal and embryonic disruptions that reduce litter size during and following periods of heat stress [32]. These mechanisms largely explain why the dry season, characterized by more severe environmental stress, is associated with lower BR compared to more favorable climatic periods.
The higher prolificacy observed in 2020–2021 compared to previous years may reflect interannual climatic variation—such as more frequent thermally favorable conditions or fewer extreme events—that temporarily improved doe body condition and fertility [33]. Alternatively, changes in management practices during those years (e.g., nutrition, health protocols, reproductive strategies, or adjustments in batch structure) may have contributed to the observed improvement [34]. Similar combinations of climatic and management-related factors have been reported in other studies to explain interannual variation in reproductive performance [32,33].
The seasonal effect observed can be interpreted considering the interaction among temperature, humidity, and nutritional availability. Under extreme dry and high heat conditions, voluntary feed intake declines, body condition deteriorates, and thermal stress increases—factors that compromise ovulation, early gestation, and early lactation, ultimately leading to smaller litters or increased embryonic mortality [27]. In contrast, temperate conditions with greater feed availability tend to support improved body condition and reproductive performance.
The rabbit’s response to heat stress is complex and non-linear. For example, moderate humidity under non-extreme temperatures may be less detrimental than drought conditions accompanied by feed scarcity—consistent with the patterns observed in this population [4]. These physiological and management-related interactions with the environment are well documented in recent reviews on the effects of heat stress on rabbit reproduction and performance [13,32].
Regarding WR and WT, our results showed a significant year effect for WR, but no statistically significant seasonal effect; WT also did not differ significantly by season. This dissociation supports the idea that pre-weaning survival is strongly influenced by maternal and postnatal management factors—such as housing quality, perinatal care, nutritional supplementation, weaning practices, and herd health—rather than by the season of birth per se [17].
Recent studies indicate that successful weaning depends largely on maternal ability, neonatal management, and litter microenvironment—factors that, when adequately managed, can stabilize survival outcomes across seasons [34,35]. Therefore, the absence of a strong seasonal effect on WR and WT suggests that targeted perinatal interventions—including biosecurity measures, litter management, local temperature control, and nutritional support—are crucial for improving survival rates [36,37].
Moreover, a more comprehensive recording of mortality etiology could enhance survival analysis by contextualizing the observed seasonal and annual variation in the evaluated traits.
Estimated heritabilities were very low, consistent with previous reports of reduced values for reproductive traits measured at the litter level [37]. Litter traits are influenced by a combination of factors, including direct additive genetic effects, permanent maternal effects, temporary environmental influences, and management practices—of which the additive genetic contribution is often minimal [38]. Recent studies in rabbits and other species confirm that h2 estimates for litter traits tend to be low, and that the use of more comprehensive models—such as animal models incorporating permanent maternal permanent effects—can improve parameter estimation and, in some cases, increase the estimated additive component [39].
Consequently, given the low h2 values observed in this study and the available experimental design, the expected response to genetic selection would be very limited. However, for WR, some genetic progress may still be achievable. Improving the accuracy of doe identification and litter recordkeeping would enhance the genetic characterization of the evaluated traits, enabling the inclusion of maternal and maternal environmental effects in the models and providing a more reliable prognosis for genetic selection response.
5. Conclusions
This study demonstrated that both year and season of kindling significantly influenced reproductive performance in rabbits raised under subtropical–semi-dry conditions in central Mexico. Seasonal fluctuations in temperature and humidity were associated with variations in litter size at birth and weaning, confirming the high environmental sensitivity of rabbit prolificacy. These findings underscore the importance of implementing environmental and thermal management strategies to sustain productivity during climatically unfavorable periods.
The low heritability estimates for litter traits suggest that genetic improvement through selection alone is limited, as environmental and maternal effects play a predominant role. Therefore, enhancing reproductive performance will depend primarily on improved environmental control, nutrition, and health management, alongside genetic programs aimed at increasing resilience and maternal ability.
Overall, this study provides baseline information on climate-related reproductive responses in rabbits under subtropical conditions, contributing to the development of more sustainable and adaptable production systems. Future research incorporating climatic indices and genomic tools could further elucidate genotype–environment interactions affecting prolificacy and survival.
Author Contributions
Conceptualization, G.M.P.-B., N.L.-V. and J.F.V.-A.; methodology, L.B.-M., S.E.-J., B.A.-P. and A.G.-M.; software, G.M.P.-B. and N.L.-V.; validation, G.M.P.-B., N.L.-V. and J.F.V.-A.; formal analysis, G.M.P.-B. and N.L.-V.; investigation, L.B.-M., F.S.-D. and S.E.-J.; resources, L.B.-M.; data curation, G.M.P.-B., S.E.-J. and J.F.V.-A.; writing—original draft preparation, G.M.P.-B., N.L.-V. and J.F.V.-A.; writing—review and editing, F.S.-D., S.E.-J., B.A.-P. and A.G.-M.; visualization, S.E.-J. and A.G.-M.; supervision, N.L.-V.; project administration, L.B.-M. and J.F.V.-A.; funding acquisition, L.B.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it was conducted on a farm under standard rearing conditions and in accordance with Mexican regulations.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors thank Cinco Ases Rabbit Farm for providing access to the herd records used in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BR | Number of kits born alive per litter |
| WR | Number of kits weaned per litter |
| WT | Weaning rate, calculated as WT = (WR/BR) × 100 |
| P | Probability |
| h2 | Heritability |
| REML | Restricted maximum likelihood |
References
- Sacarrão-Birrento, L.; Harrison, L.J.S.; Pienaar, R.; Toka, F.N.; Torres-Acosta, J.F.J.; Vilela, V.L.R.; Hernández-Castellano, L.E.; Arriaga-Jordán, C.M.; Soltan, Y.A.; Ungerfeld, R.; et al. Challenges for Animal Health and Production in the Tropics and Mediterranean for the next 55 Years. Trop. Anim. Health Prod. 2024, 56, 381. [Google Scholar] [CrossRef]
- Szendrő, Z.; Szendrő, K.; Zotte, A.D. Management of Reproduction on Small, Medium and Large Rabbit Farms: A Review. Asian Australas. J. Anim. Sci. 2012, 25, 738–748. [Google Scholar] [CrossRef]
- Ezzeroug, R.; Belabbas, R.; Argente, M.J.; Berbar, A.; Diss, S.; Boudjella, Z.; Talaziza, D.; Boudahdir, N.; García, M.D.L.L. Genetic Correlations for Reproductive and Growth Traits in Rabbits. Can. J. Anim. Sci. 2020, 100, 317–322. [Google Scholar] [CrossRef]
- Liang, Z.-L.; Chen, F.; Park, S.; Balasubramanian, B.; Liu, W.-C. Impacts of Heat Stress on Rabbit Immune Function, Endocrine, Blood Biochemical Changes, Antioxidant Capacity and Production Performance, and the Potential Mitigation Strategies of Nutritional Intervention. Front. Vet. Sci. 2022, 9, 906084. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Gerini, F.; Ikram, A.; Saeed, F.; Feng, X.; Chen, Y. Rabbit Meat—Production, Consumption and Consumers’ Attitudes and Behavior. Sustainability 2023, 15, 2008. [Google Scholar] [CrossRef]
- Wu, L. Rabbit Meat Trade of Major Countries: Regional Pattern and Driving Forces. World Rabbit. Sci. 2022, 30, 69–82. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Adli, D.N.; Nugraha, W.S.; Yudhistira, B.; Lavrentev, F.V.; Shityakov, S.; Feng, X.; Nagdalian, A.; Ibrahim, S.A. Social, Ethical, Environmental, Economic and Technological Aspects of Rabbit Meat Production—A Critical Review. Heliyon 2024, 10, e29635. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, N.; Narnoliya, L.K.; Verma, A.K.; Umaraw, P.; Mehta, N.; Ismail-Fitry, M.R.; Kaka, U.; Yong-Meng, G.; Lee, S.-J.; et al. Improving Quality and Consumer Acceptance of Rabbit Meat: Prospects and Challenges. Meat Sci. 2025, 219, 109660. [Google Scholar] [CrossRef] [PubMed]
- Szendrő, K.; Szabó-Szentgróti, E.; Szigeti, O. Consumers’ Attitude to Consumption of Rabbit Meat in Eight Countries Depending on the Production Method and Its Purchase Form. Foods 2020, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.C.D.C.; Costa, R.B.; De Camargo, G.M.F. Consumption of Rabbit Meat in Brazil: Potential and Limitations. Meat Sci. 2022, 191, 108873. [Google Scholar] [CrossRef]
- Huang, D.; Cai, J.; Zhang, C.; Jin, R.; Bai, S.; Yao, F.; Ding, H.; Zhao, B.; Chen, Y.; Wu, X.; et al. Semen Quality and Seminal Plasma Metabolites in Male Rabbits (Oryctolagus cuniculus) under Heat Stress. PeerJ 2023, 11, e15112. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Ayyat, M.S.; Abd El-Monem, U.M. Growth Performance and Reproductive Traits at First Parity of New Zealand White Female Rabbits as Affected by Heat Stress and Its Alleviation under Egyptian Conditions. Trop. Anim. Health Prod. 2001, 33, 451–462. [Google Scholar] [CrossRef]
- Helal, M.; Sameh, J.; Gharib, S.; Merghany, R.M.; Bozhilova-Sakova, M.; Ragab, M. Candidate Genes Associated with Reproductive Traits in Rabbits. Trop. Anim. Health Prod. 2024, 56, 94. [Google Scholar] [CrossRef]
- Belabbas, R.; Ezzeroug, R.; Berbar, A.; De La Luz Garcia, M.; Zitouni, G.; Taalaziza, D.; Boudjella, Z.; Boudahdir, N.; Diss, S.; Argente, M.-J. Genetic Analyses of Rabbit Survival and Individual Birth Weight. Animals 2022, 12, 2695. [Google Scholar] [CrossRef]
- Hill, W.G.; Mulder, H.A. Genetic Analysis of Environmental Variation. Genet. Res. 2010, 92, 381–395. [Google Scholar] [CrossRef]
- Lima, N.H.S.; D’suze, E.Z.L.; Paiva, D.D.A.; Lima, N.D.D.S.; Gomes, T.R.; Paiva, J.T.D. Meta-Analysis of Genetic Parameters for Economic Traits in Rabbit Using a Random-Effects Model. World Rabbit. Sci. 2024, 32, 175–191. [Google Scholar] [CrossRef]
- Nagy, I.; Farkas, J.; Curik, I.; Gorjanc, G.; Gyovai, P.; Szendrő, Z. Estimation of Additive and Dominance Variance for Litter Size Components in Rabbits. Czech J. Anim. Sci. 2014, 59, 182–189. [Google Scholar] [CrossRef]
- Piles, M.; García, M.L.; Rafel, O.; Ramon, J.; Baselga, M. Genetics of Litter Size in Three Maternal Lines of Rabbits: Repeatability versus Multiple-Trait Models. J. Anim. Sci. 2006, 84, 2309–2315. [Google Scholar] [CrossRef]
- Badawy, A.Y.; Peiró, R.; Blasco, A.; Santacreu, M.A. Correlated Responses on Litter Size Traits and Survival Traits after Two-Stage Selection for Ovulation Rate and Litter Size in Rabbits. Animal 2019, 13, 453–459. [Google Scholar] [CrossRef]
- FAO. FAOSTAT: Crops and Livestock Products; FAO: Rome, Italy, 2023; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 October 2025).
- García-López, J.C.; Pro-Martínez, A.; Becerril-Pérez, C.M.; Suárez-Oporta, M.E.; Pinos-Rodríguez, J.M. Technical Note: Rabbit Meat Production under a Small Scale Production System as a Source of Animal Protein in a Rural Area of Mexico. World Rabbit. Sci. 2010, 14, 259–263. [Google Scholar] [CrossRef]
- Vélez Izquierdo, A.; Espinosa García, J.A.; Aguilar Romero, F. Type and Characterization of Rabbit Farmers in Mexico’s Central States. Rev. Mex. Cienc. Pecu. 2021, 12, 469–486. [Google Scholar] [CrossRef]
- Cruz-Bacab, L.E.; Ramírez-Vera, S.; Vázquez-García, M.D.C.; Zapata-Campos, C.C. Reproduction of Rabbits Under Tropical Conditions, Negative Effects and Possible Solutions. CienciaUAT 2018, 13, 135. [Google Scholar] [CrossRef]
- Ji, R.; Shi, S.; Liu, Z.; Wu, Z. Decomposition-Based Multi-Step Forecasting Model for the Environmental Variables of Rabbit Houses. Animals 2023, 13, 546. [Google Scholar] [CrossRef] [PubMed]
- El Sabry, M.I.; Zaki, M.M.; Elgohary, F.A.; Helal, M.M. Sustainable Rabbit Production under the Global Warming Conditions in Southern Mediterranean Region. World’s Veter-J. 2021, 11, 543–548. [Google Scholar] [CrossRef]
- Masara, L.; Jomane, F.; Moyo, S.; Pullen, J.; Mudziwapasi, R.; Mgumba, C.; Moyo, S.; Tokunaga, T.; Mugoti, A. The Efficacy of Crossbreeding on Reproductive and Productive Performance in New Zealand White and Flemish Giant Rabbits. Bulg. J. Anim. Husb. 2024, 61, 18–24. [Google Scholar] [CrossRef]
- Ragab, M.; Elkhaiat, I.; Younis, H.; Ahmed, M.; Helal, M. Genotype by Heat Conditions Interaction Effects on Growth and Litter Traits in Rabbits. Front. Vet. Sci. 2022, 9, 1018625. [Google Scholar] [CrossRef]
- Macias-Fonseca, M.E.; Herrera-Haro, J.G.; Pro-Martínez, A.; Ortega-Cerrilla, M.E.; Ruíz-Sesma, B. Productive Performance and Carcass Characteristics of New Zealand White and California Rabbits and Their Crosses. Agro Prod. 2021, 14, 77–84. [Google Scholar] [CrossRef]
- Herrera-Ojeda, J.B.; Parra-Bracamonte, G.M.; López-Villalobos, N.; Vázquez-Armijo, J.F.; Orozco-Durán, K.E.; Magaña-Monforte, J.G.; Martínez-González, J.C.; Jahuey-Martínez, F.J. Birth Seasons Based on a Climatic Index for Statistical Model Fitting of Live Weight of Bovine Cattle in México. Rev. Mex. Cienc. Pecu. 2018, 9, 647–666. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Rabbits, 2nd ed.; National Academies Press: Washington, DC, USA, 1977; p. 35. ISBN 978-0-309-02607-9. [Google Scholar]
- Falconer, D.S.; Mackay, T. Introduction to Quantitative Genetics, 4th ed.; Pearson Prentice Hall: Harlow, UK, 2009; ISBN 978-0-582-24302-6. [Google Scholar]
- Ebeid, T.A.; Aljabeili, H.S.; Al-Homidan, I.H.; Volek, Z.; Barakat, H. Ramifications of Heat Stress on Rabbit Production and Role of Nutraceuticals in Alleviating Its Negative Impacts: An Updated Review. Antioxidants 2023, 12, 1407. [Google Scholar] [CrossRef]
- Castellini, C.; Dal Bosco, A.; Arias-Álvarez, M.; Lorenzo, P.L.; Cardinali, R.; Rebollar, P.G. The Main Factors Affecting the Reproductive Performance of Rabbit Does: A Review. Anim. Rep. Sci. 2010, 122, 174–182. [Google Scholar] [CrossRef]
- Belabbas, R.; Ezzeroug, R.; De La Luz García, M.; Feknous, N.; Talaziza, D.; Argente, M.J. Genetic and Phenotypic Parameters of Rabbit Individual Body Weight in the Preweaning Period. Vet. Sci. 2023, 11, 14. [Google Scholar] [CrossRef]
- Morgado, J.N.; Lamonaca, E.; Santeramo, F.G.; Caroprese, M.; Albenzio, M.; Ciliberti, M.G. Effects of Management Strategies on Animal Welfare and Productivity under Heat Stress: A Synthesis. Front. Vet. Sci. 2023, 10, 1145610. [Google Scholar] [CrossRef]
- Belabbas, R.; Ezzeroug, R.; García, M.D.L.L.; Berbar, A.; Zitouni, G.; Taalaziza, D.; Boudjella, Z.; Boudahdir, N.; Dis, S.; Argente, M.J. Prenatal Factors Affecting the Probability of Survival between Birth and Weaning in Rabbits. World Rabbit. Sci. 2023, 31, 11–20. [Google Scholar] [CrossRef]
- Ayyat, M.S.; El-Monem, U.M.A.; Moustafa, M.M.A.; Al-Sagheer, A.A.; Mahran, M.D.; El-Attrouny, M.M. Genetic Assessment of Litter Size, Body Weight, Carcass Traits and Gene Expression Profiles in Exotic and Indigenous Rabbit Breeds: A Study on New Zealand White, Californian, and Gabali Rabbits in Egypt. Trop. Anim. Health Prod. 2024, 56, 244. [Google Scholar] [CrossRef]
- Khattab, A.H.; Iraqi, M.M.; Khalil, M.H.; Amin, E.M.; El Nagar, A.G. Heterotic Effects on Litter Traits in Crossbreeding Experiment Involving Egyptian Rabbit Lines. Trop. Anim. Health Prod. 2025, 57, 98. [Google Scholar] [CrossRef]
- Liu, W.; Lu, Q.; Tang, S.; Pu, X.; Wang, Y.; Wu, C.; Hu, X.; Hong, W.; Fu, X. Comparison of Different Animal Models for Estimating Genetic Parameters for Early Growth Traits and Reproductive Traits in Tianmu Sainuo Sheep. Front. Vet. Sci. 2024, 11, 1349790. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
