Integrated 16S rDNA and Metabolomics Analysis Unveils Dietary Fiber-Induced Changes in Small Intestinal Microbiota and Metabolites of Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Haematoxylin-Eosin (H&E) Staining
2.3. 16S rDNA Gene Amplicon Sequencing
2.4. Sequencing Data Analysis
2.5. LC-MS/MS Analysis
2.6. Metabolomic Data Analysis and Metabolite Identification
2.7. Statistical Analysis
3. Results
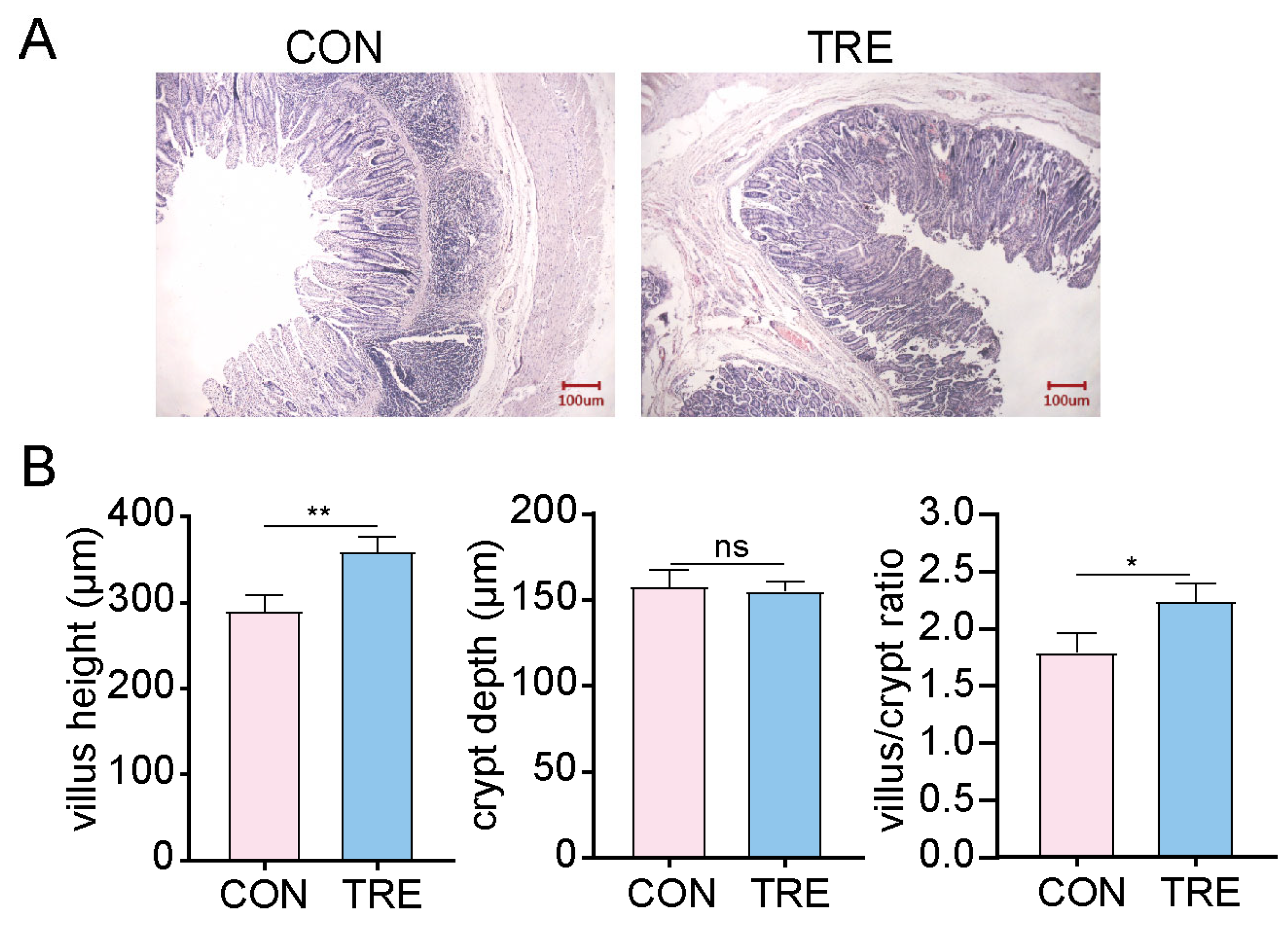
3.1. Effects of Dietary Fiber Supplementation on Small Intestinal Morphology
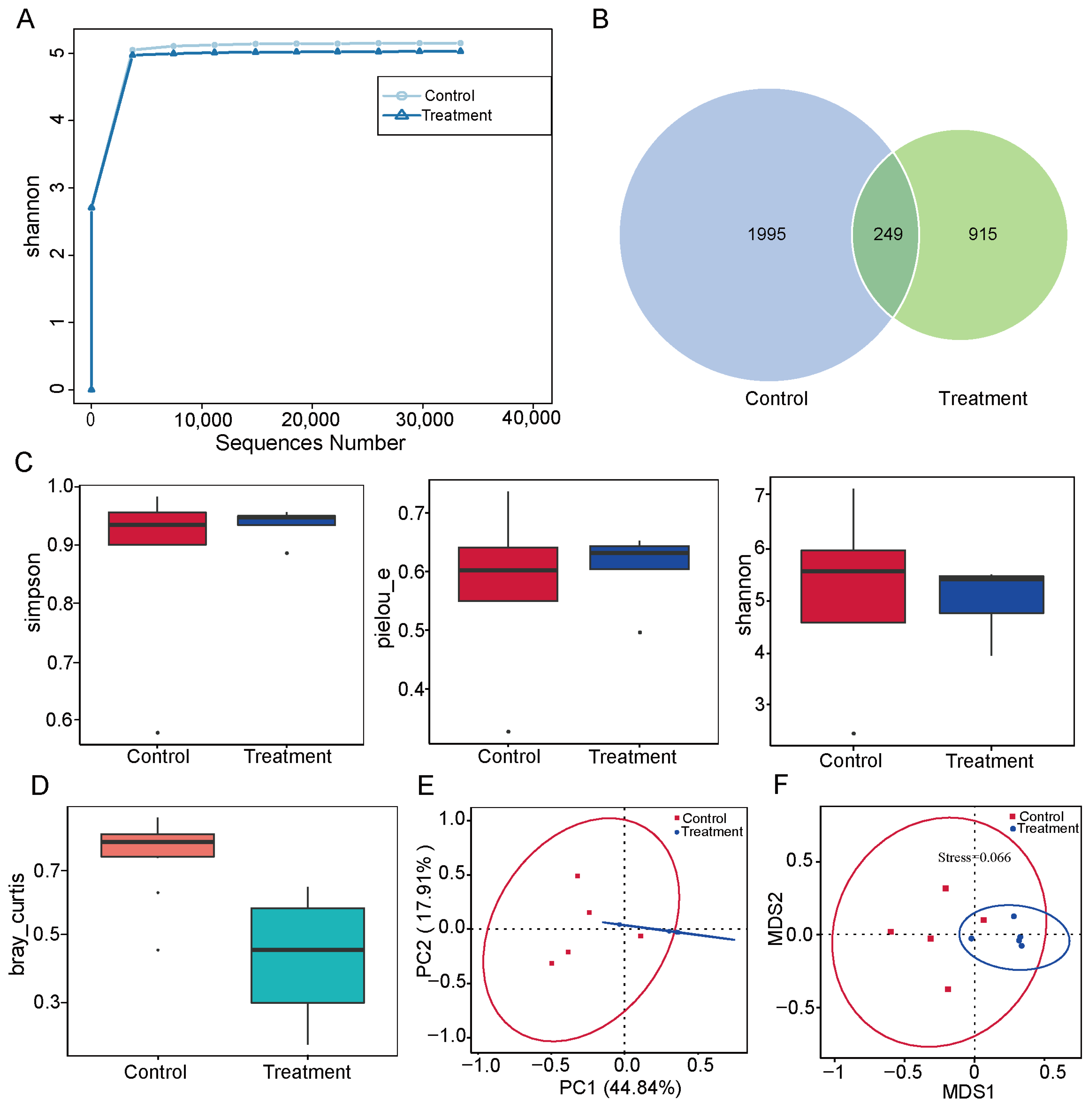
3.2. Effects of Dietary Fiber Supplementation on Microbial Diversity in the Pig Small Intestine
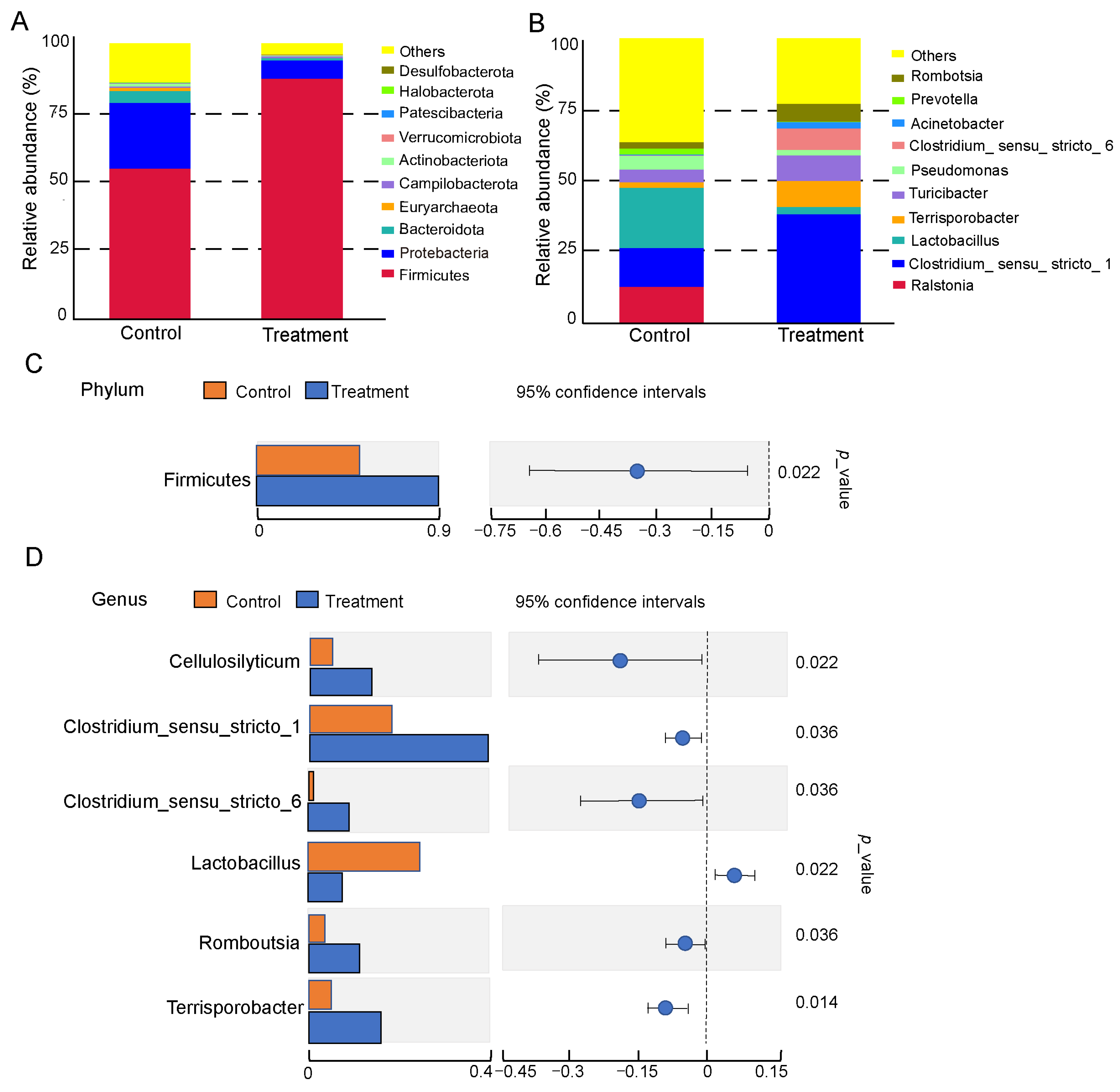
3.3. Effects of Dietary Fiber Supplementation on the Composition of Small Intestinal Microbiota
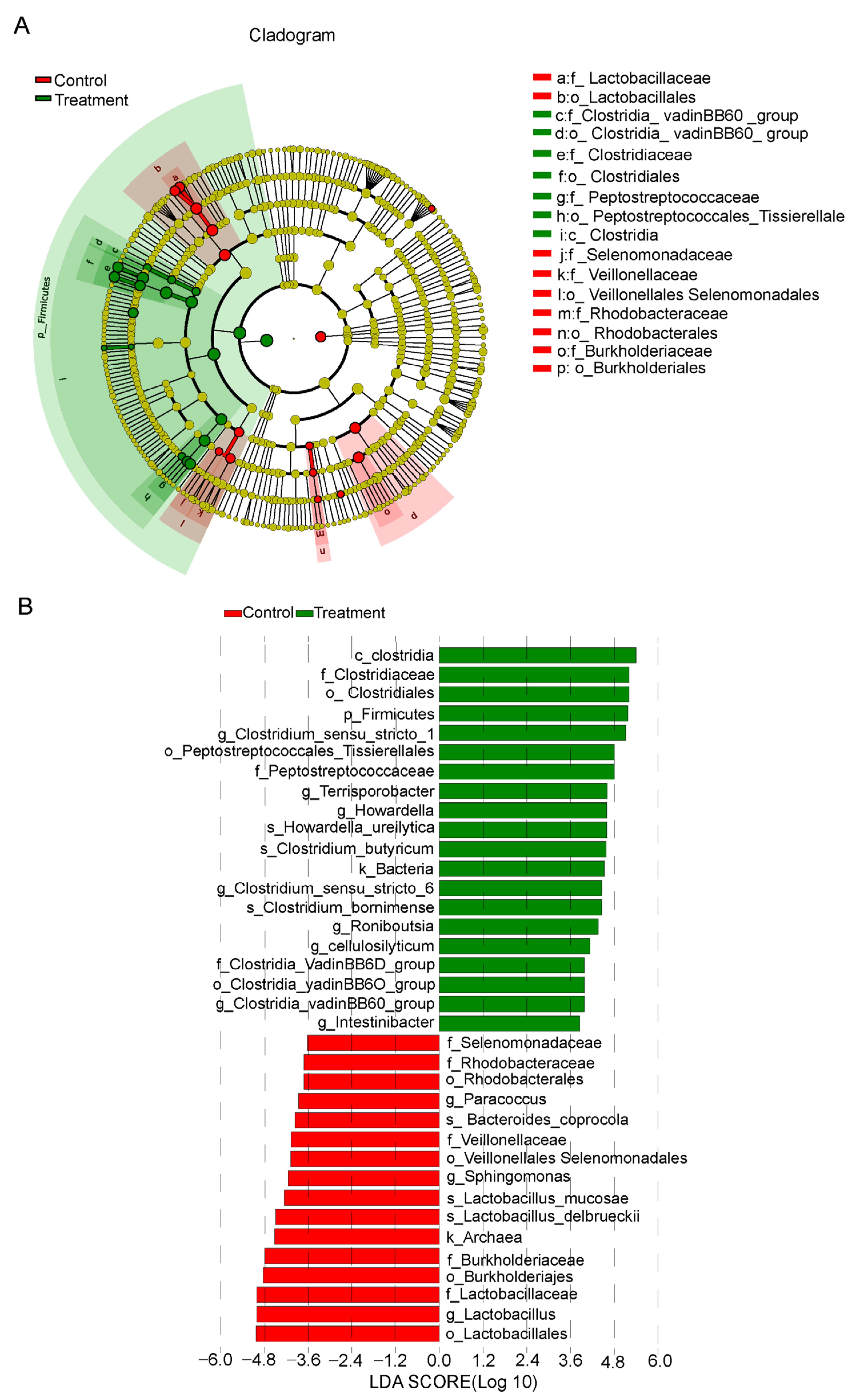
3.4. Identification of Specific Bacteria Related to Fiber Digestion
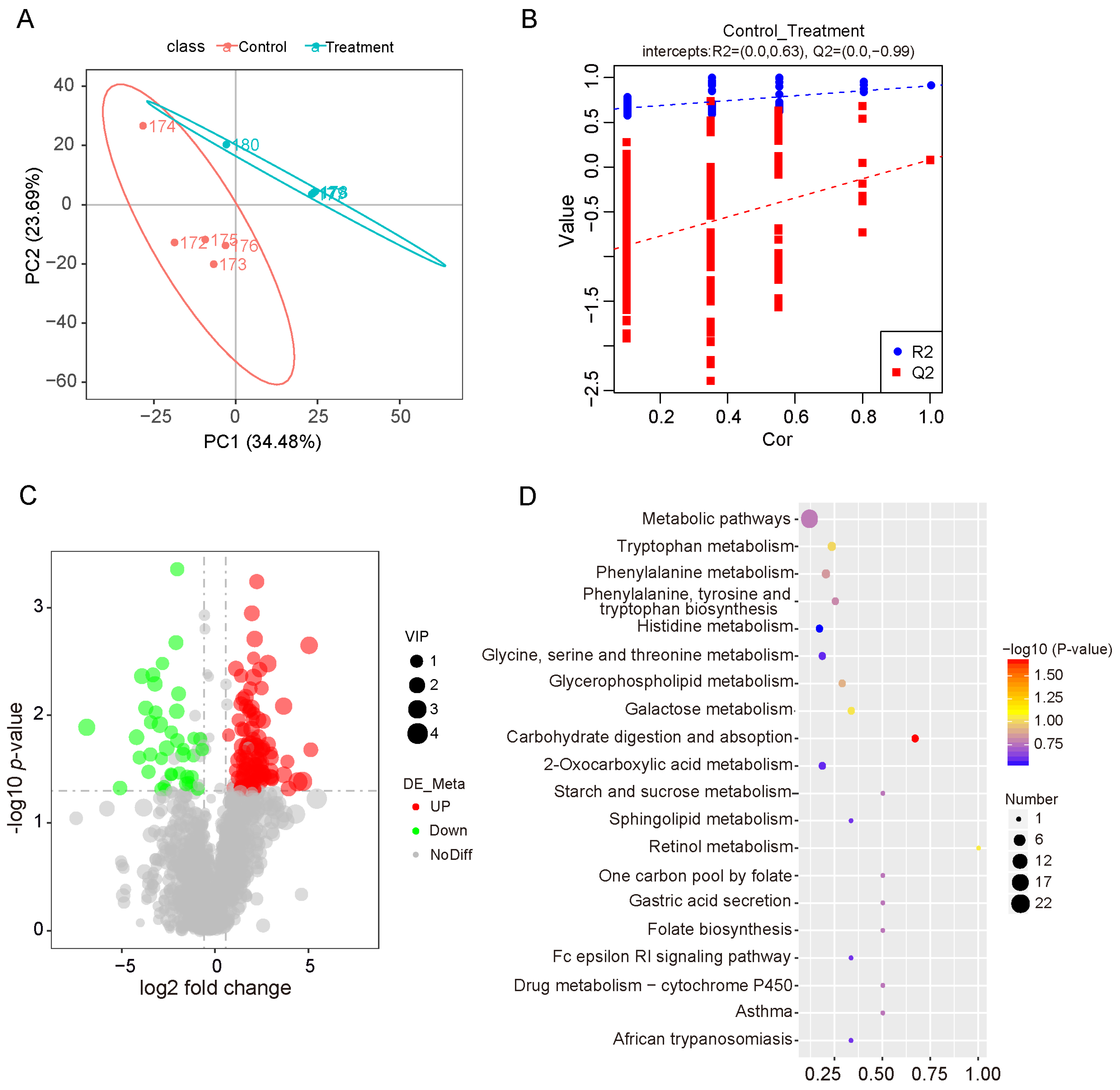
3.5. Effects of Dietary Fiber Supplementation on the Metabolite Profiles of the Small Intestine
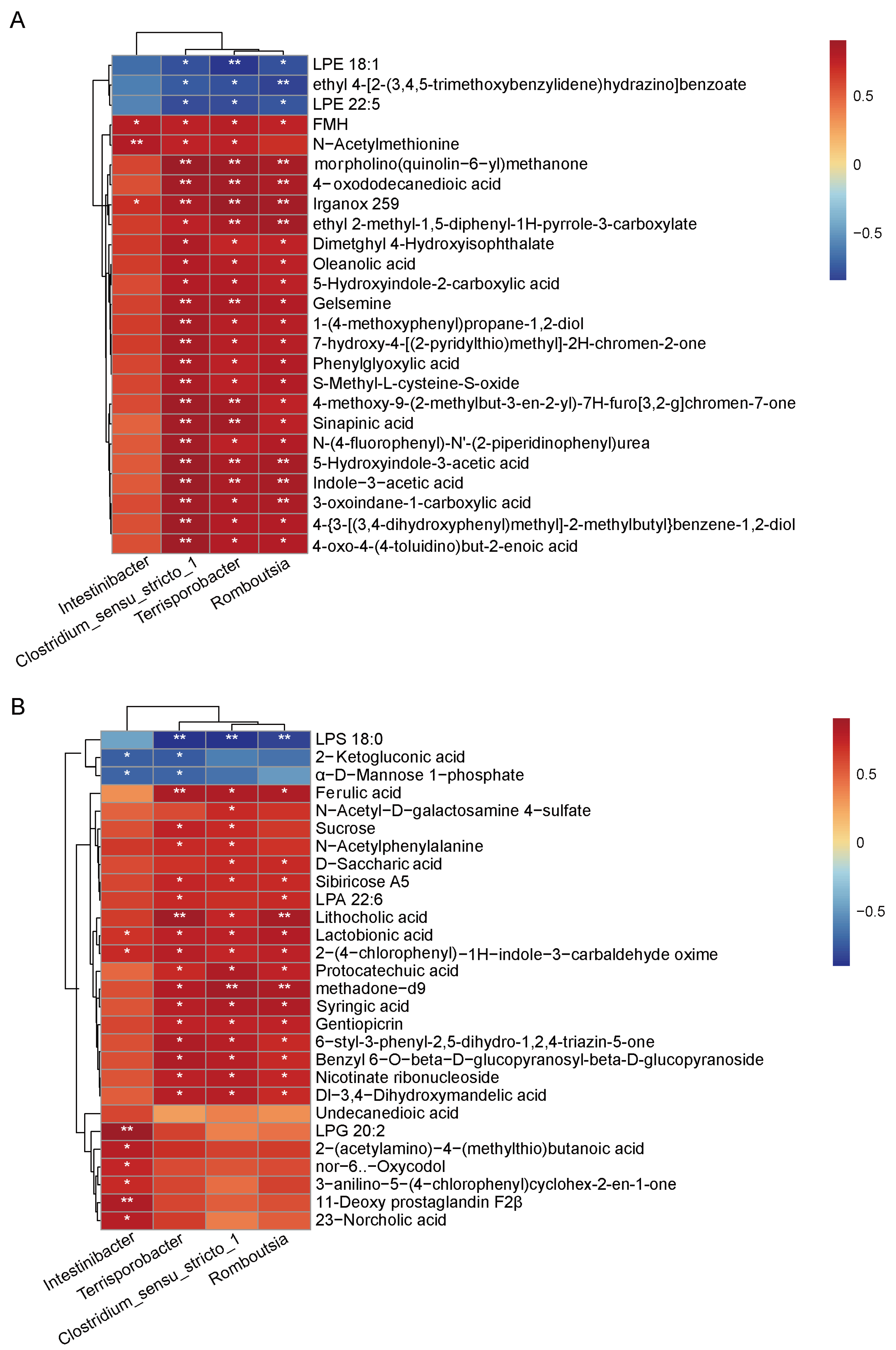
3.6. Integrated Analysis of 16S rRNA Sequencing and Metabolomics Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Yin, J.; Tan, B.; Chen, J.; Zhang, H.; Li, Z.; Ma, X. Physiological function and application of dietary fiber in pig nutrition: A review. Anim. Nutr. 2021, 7, 259–267. [Google Scholar] [CrossRef]
- Pandey, S.; Kim, E.S.; Cho, J.H.; Song, M.; Doo, H.; Kim, S.; Keum, G.B.; Kwak, J.; Ryu, S.; Choi, Y.; et al. Swine gut microbiome associated with non-digestible carbohydrate utilization. Front. Vet. Sci. 2023, 10, 1231072. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, P.; Wu, Y.; Guo, P.; Liu, L.; Ma, N.; Levesque, C.; Chen, Y.; Zhao, J.; Zhang, J.; et al. Dietary Fiber Increases Butyrate-Producing Bacteria and Improves the Growth Performance of Weaned Piglets. J. Agric. Food Chem. 2018, 66, 7995–8004. [Google Scholar] [CrossRef]
- Du, T.; Li, P.; Niu, Q.; Pu, G.; Wang, B.; Liu, G.; Li, P.; Niu, P.; Zhang, Z.; Wu, C.; et al. Effects of Varying Levels of Wheat Bran Dietary Fiber on Growth Performance, Fiber Digestibility and Gut Microbiota in Erhualian and Large White Pigs. Microorganisms 2023, 11, 2474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Y.; Liu, S.; Huang, J.; Zhai, Z.; He, C.; Ding, J.; Wang, J.; Wang, H.; Fan, W.; et al. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS ONE. 2015, 10, e0117441. [Google Scholar] [CrossRef]
- Yao, Q.; Yu, Z.; Meng, Q.; Chen, J.; Liu, Y.; Song, W.; Ren, X.; Zhou, J.; Chen, X. The role of small intestinal bacterial overgrowth in obesity and its related diseases. Biochem. Pharmacol. 2023, 212, 115546. [Google Scholar] [CrossRef]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef]
- Cecchini, D.A.; Laville, E.; Laguerre, S.; Robe, P.; Leclerc, M.; Doré, J.; Henrissat, B.; Remaud-Siméon, M.; Monsan, P.; Potocki-Véronèse, G. Functional metagenomics reveals novel pathways of prebiotic breakdown by human gut bacteria. PLoS ONE 2013, 8, e72766. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.; Xu, X.; Hou, Q.; Ren, J.; Yan, X. Gut microbiota-derived 3-phenylpropionic acid promotes intestinal epithelial barrier function via AhR signaling. Microbiome 2023, 11, 102. [Google Scholar]
- Xie, P.; Luo, M.; Fan, J.; Xiong, L. Multiomics Analysis Reveals Gut Virome-Bacteria-Metabolite Interactions and Their Associations with Symptoms in Patients with IBS-D. Viruses 2024, 16, 1054. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Embleton, N.D.; Marrs, E.C.L.; Smith, D.P.; Fofanova, T.; Nelson, A.; Skeath, T.; Perry, J.D.; Petrosino, J.F.; Berrington, J.E.; et al. Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome 2017, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short readsto improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Estaki, M.; Jiang, L.; Bokulich, N.A.; McDonald, D.; González, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vázquez-Baeza, Y.; et al. QIIME 2 Enables Comprehensive End-to-End Analysis of Diverse Microbiome Data and Comparative Studies with Publicly Available Data. Curr. Protoc. Bioinform. 2020, 70, e100. [Google Scholar] [CrossRef] [PubMed]
- Khleborodova, A.; Gamboa-Tuz, S.D.; Ramos, M.; Segata, N.; Waldron, L.; Oh, S. lefser: Implementation of metagenomic biomarker discovery tool, LEfSe, in R. Bioinformatics 2024, 40, btae707. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Guan, Z.W.; Yu, E.Z.; Feng, Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Zhang, H.; Su, Y.; Zhu, W. Swine gut microbiota and its interaction with host nutrient metabolism. Anim. Nutr. 2020, 6, 410–420. [Google Scholar] [CrossRef]
- Schedle, K.; Pfaffl, M.W.; Plitzner, C.; Meyer, H.H.; Windisch, W. Effect of insoluble fibre on intestinal morphology and mRNA expression pattern of inflammatory, cell cycle and growth marker genes in a piglet model. Arch. Anim. Nutr. 2008, 62, 427–438. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, C.; Niu, J.; Cui, Z.; Zhao, X.; Li, W.; Zhang, Y.; Yang, Y.; Gao, P.; Guo, X.; et al. Impacts of dietary fiber level on growth performance, apparent digestibility, intestinal development, and colonic microbiota and metabolome of pigs. J. Anim. Sci. 2023, 101, skad174. [Google Scholar] [CrossRef]
- Jurburg, S.D.; Buscot, F.; Chatzinotas, A.; Chaudhari, N.M.; Clark, A.T.; Garbowski, M.; Grenié, M.; Hom, E.F.Y.; Karakoç, C.; Marr, S.; et al. The community ecology perspective of omics data. Microbiome 2022, 10, 225. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2021, 63, 727–735. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2023, 63, 12073–12088. [Google Scholar] [CrossRef]
- Wang, C.; Wei, S.; Chen, N.; Xiang, Y.; Wang, Y.; Jin, M. Characteristics of gut microbiota in pigs with different breeds, growth periods and genders. Microb. Biotechnol. 2022, 15, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, B.; Ji, L.; Lv, X.; Feng, Y.; Li, L.; Wu, X. Dietary lysozyme improves growth performance and intestinal barrier function of weaned piglets. Anim. Nutr. 2023, 14, 249–258. [Google Scholar] [CrossRef]
- Li, Y.; Xia, D.; Chen, J.; Zhang, X.; Wang, H.; Huang, L.; Shen, J.; Wang, S.; Feng, Y.; He, D.; et al. Dietary fibers with different viscosity regulate lipid metabolism via ampk pathway: Roles of gut microbiota and short-chain fatty acid. Poult. Sci. 2022, 101, 101742. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, L.; Wang, L.; Ren, S.; Zhang, J.; Ma, Y.; Xu, F.; Wu, G.; Zhang, Y. Multi-Omics Analysis Reveals Dietary Fiber’s Impact on Growth, Slaughter Performance, and Gut Microbiome in Durco × Bamei Crossbred Pig. Microorganisms 2024, 12, 1674. [Google Scholar] [CrossRef]
- Huangfu, W.; Cao, S.; Li, S.; Zhang, S.; Liu, M.; Liu, B.; Zhu, X.; Cui, Y.; Wang, Z.; Zhao, J.; et al. In vitro and in vivo fermentation models to study the function of dietary fiber in pig nutrition. Appl. Microbiol. Biotechnol. 2024, 108, 314. [Google Scholar] [CrossRef]
- Li, Y.; Liu, N.; Ge, Y.; Yang, Y.; Ren, F.; Wu, Z. Tryptophan and the innate intestinal immunity: Crosstalk between metabolites, host innate immune cells, and microbiota. Eur. J. Immunol. 2022, 52, 856–868. [Google Scholar] [CrossRef]
- Augustin, A.; Guennec, A.L.; Umamahesan, C.; Kendler-Rhodes, A.; Tucker, R.M.; Chekmeneva, E.; Takis, P.; Lewis, M.; Balasubramanian, K.; DeSouza, N.; et al. Faecal metabolite deficit, gut inflammation and diet in Parkinson’s disease: Integrative analysis indicates inflammatory response syndrome. Clin. Transl. Med. 2023, 13, e1152. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Huang, J.; Guo, X.; Xia, J.; Hu, M. Romboutsia lituseburensis JCM1404 supplementation ameliorated endothelial function via gut microbiota modulation and lipid metabolisms alterations in obese rats. FEMS Microbiol. Lett. 2023, 370, fnad016. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Qi, L.; Xiao, Y.; Jin, J.; Huang, R.; Wu, S.; Bao, W. Integrated 16S rDNA and Metabolomics Analysis Unveils Dietary Fiber-Induced Changes in Small Intestinal Microbiota and Metabolites of Pigs. Vet. Sci. 2025, 12, 1034. https://doi.org/10.3390/vetsci12111034
Wang H, Qi L, Xiao Y, Jin J, Huang R, Wu S, Bao W. Integrated 16S rDNA and Metabolomics Analysis Unveils Dietary Fiber-Induced Changes in Small Intestinal Microbiota and Metabolites of Pigs. Veterinary Sciences. 2025; 12(11):1034. https://doi.org/10.3390/vetsci12111034
Chicago/Turabian StyleWang, Haifei, Lele Qi, Yeyi Xiao, Jian Jin, Ruihua Huang, Shenglong Wu, and Wenbin Bao. 2025. "Integrated 16S rDNA and Metabolomics Analysis Unveils Dietary Fiber-Induced Changes in Small Intestinal Microbiota and Metabolites of Pigs" Veterinary Sciences 12, no. 11: 1034. https://doi.org/10.3390/vetsci12111034
APA StyleWang, H., Qi, L., Xiao, Y., Jin, J., Huang, R., Wu, S., & Bao, W. (2025). Integrated 16S rDNA and Metabolomics Analysis Unveils Dietary Fiber-Induced Changes in Small Intestinal Microbiota and Metabolites of Pigs. Veterinary Sciences, 12(11), 1034. https://doi.org/10.3390/vetsci12111034









