Protegrin-1 Combats Multidrug-Resistant Porcine ExPEC: Potent Bactericidal Activity and Multimodal Immunometabolic Regulation In Vitro and in a Murine Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. In Vitro Antibacterial Activity Assay
2.3. In Vitro Synergistic Activity of PG-1 and Tetracycline
2.4. Antimicrobial Resistance Induction In Vitro
2.5. Haemolytic Activity Assay
2.6. In Vivo Efficacy of PG-1 in Mouse PCN033 Infection Model
2.7. RNA Isolation
2.8. Transcriptome Analysis
2.9. Eukaryotic Reference-Based Transcriptome Validation Using RT-qPCR
2.10. Control Groups and Procedures
2.11. Statistical Analysis
3. Results
3.1. Antibacterial Activity of PG-1 and Tetracycline
3.2. Synergistic Antibacterial Effect of PG-1 in Combination with TET In Vitro
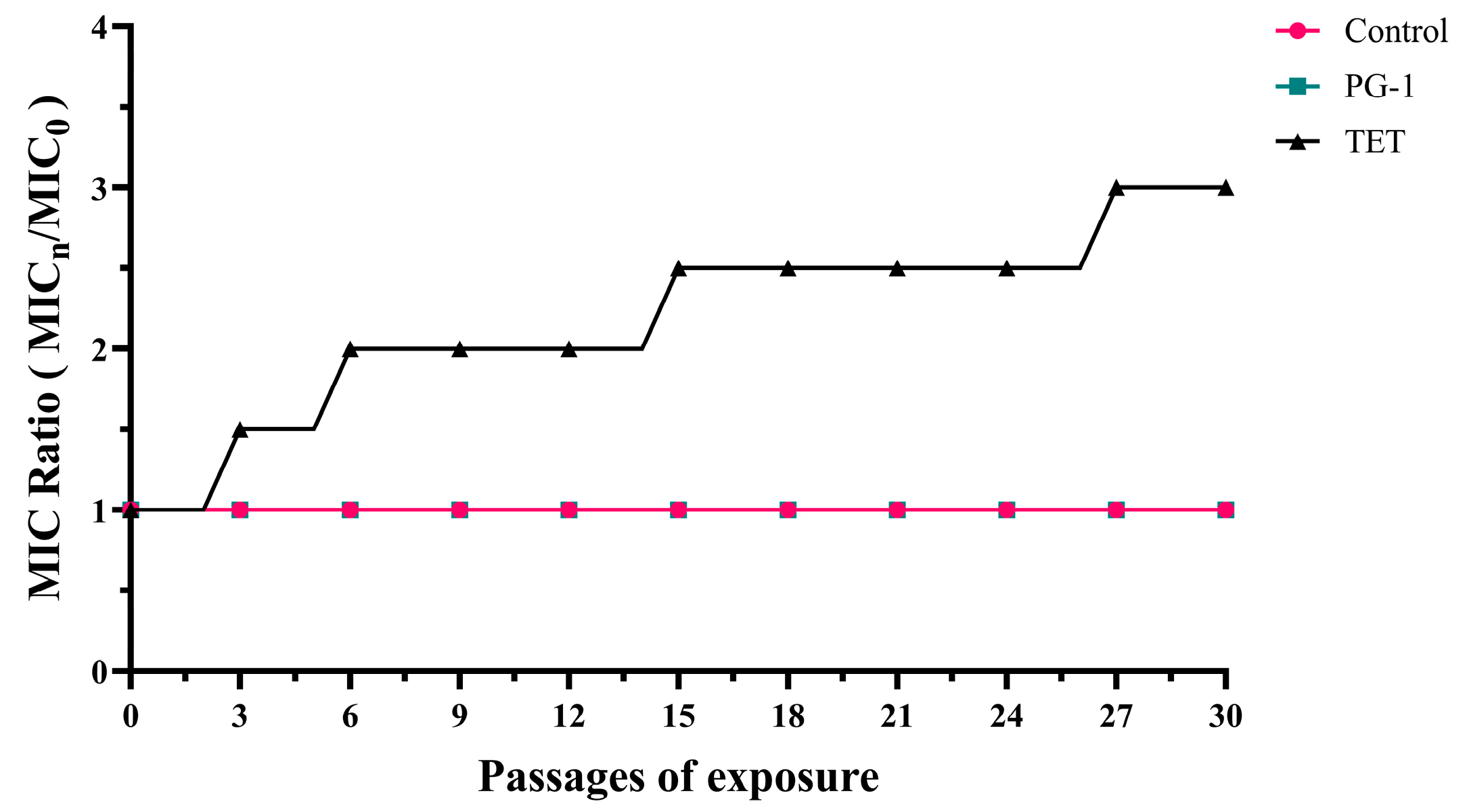
3.3. Induction of Antibiotic Resistance In Vitro
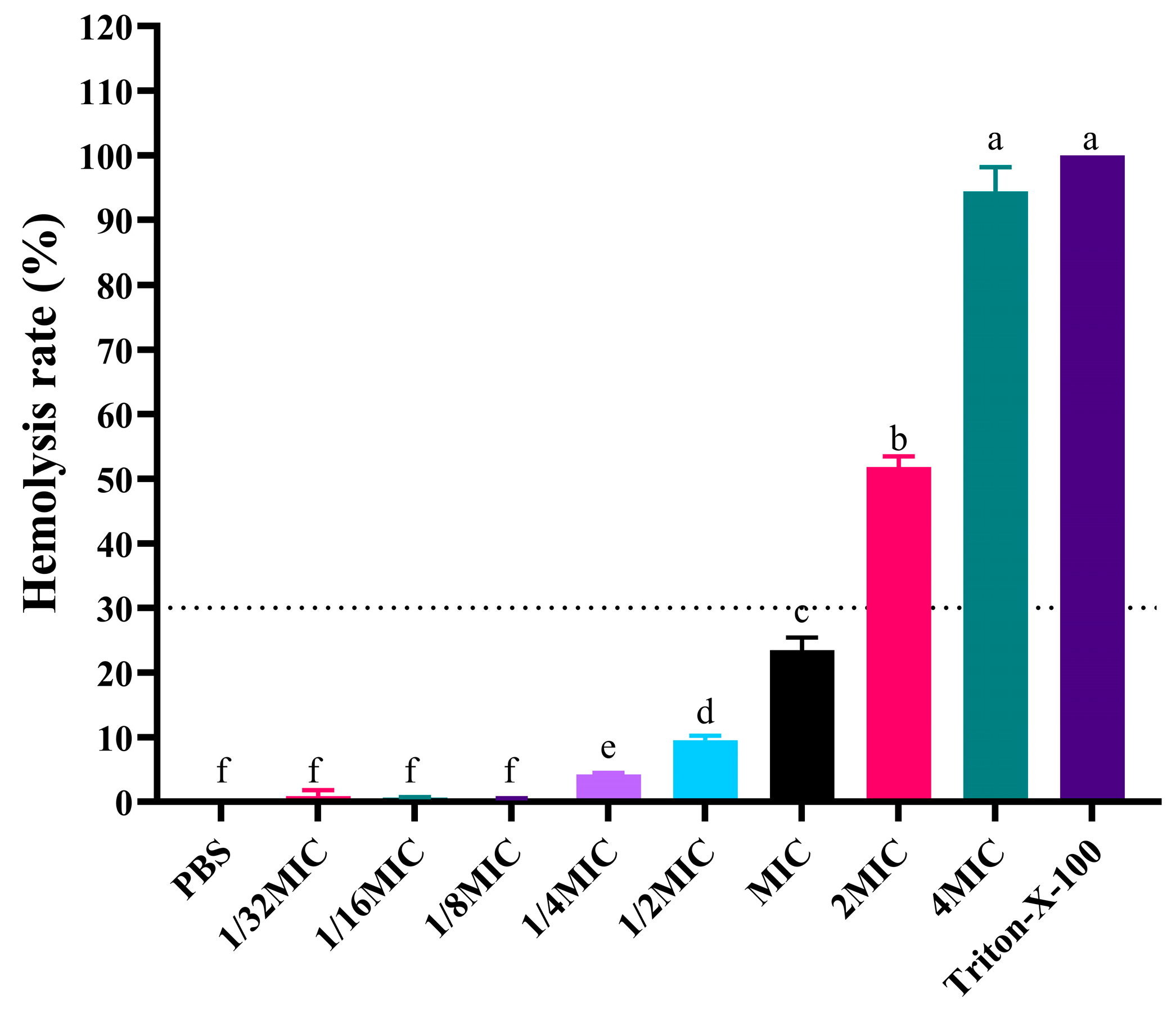
3.4. Hemolytic Analysis
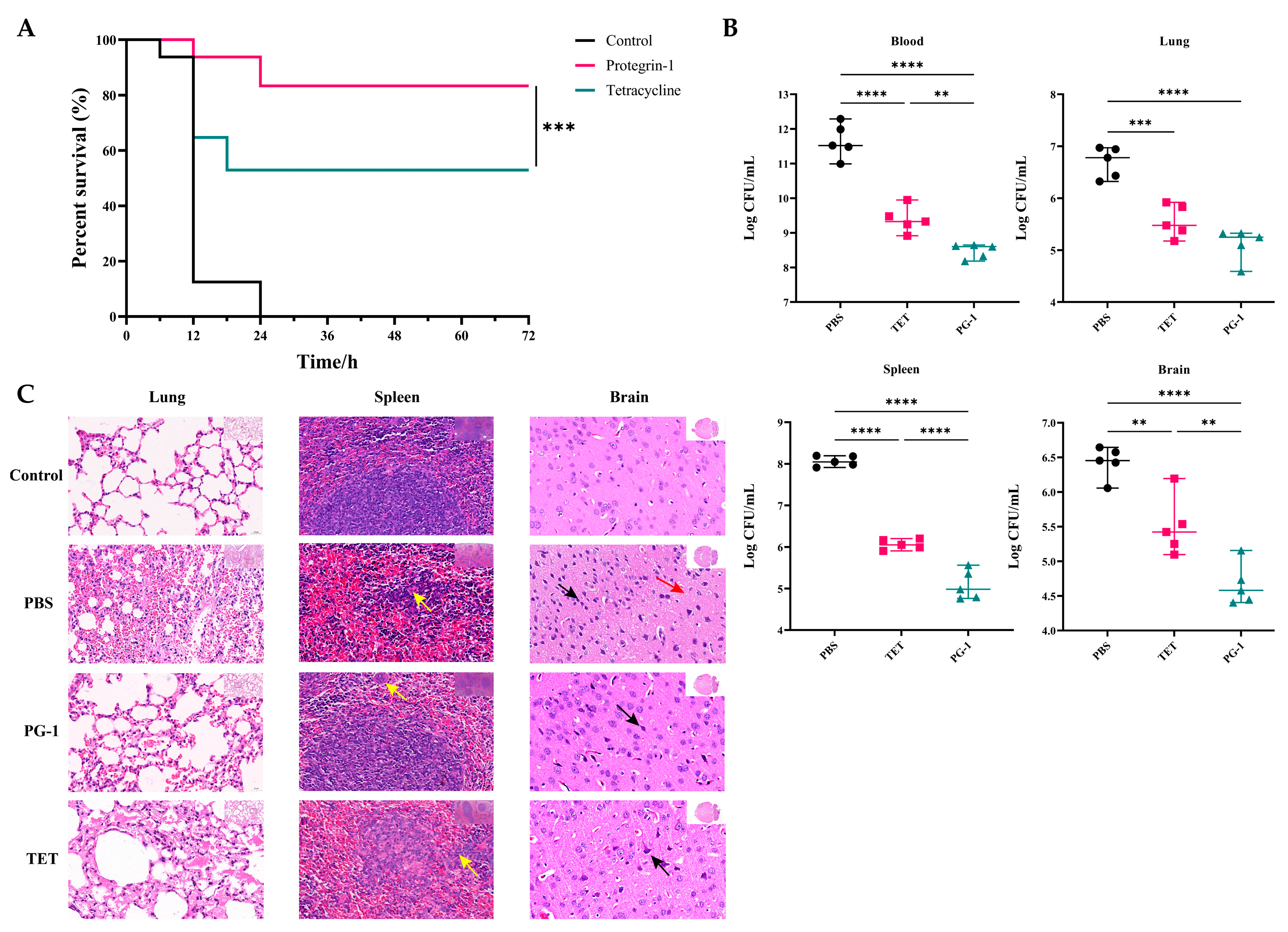
3.5. Anti-Infective Effects of PG-1 and Tetracycline Against PCN033 Infection In Vivo
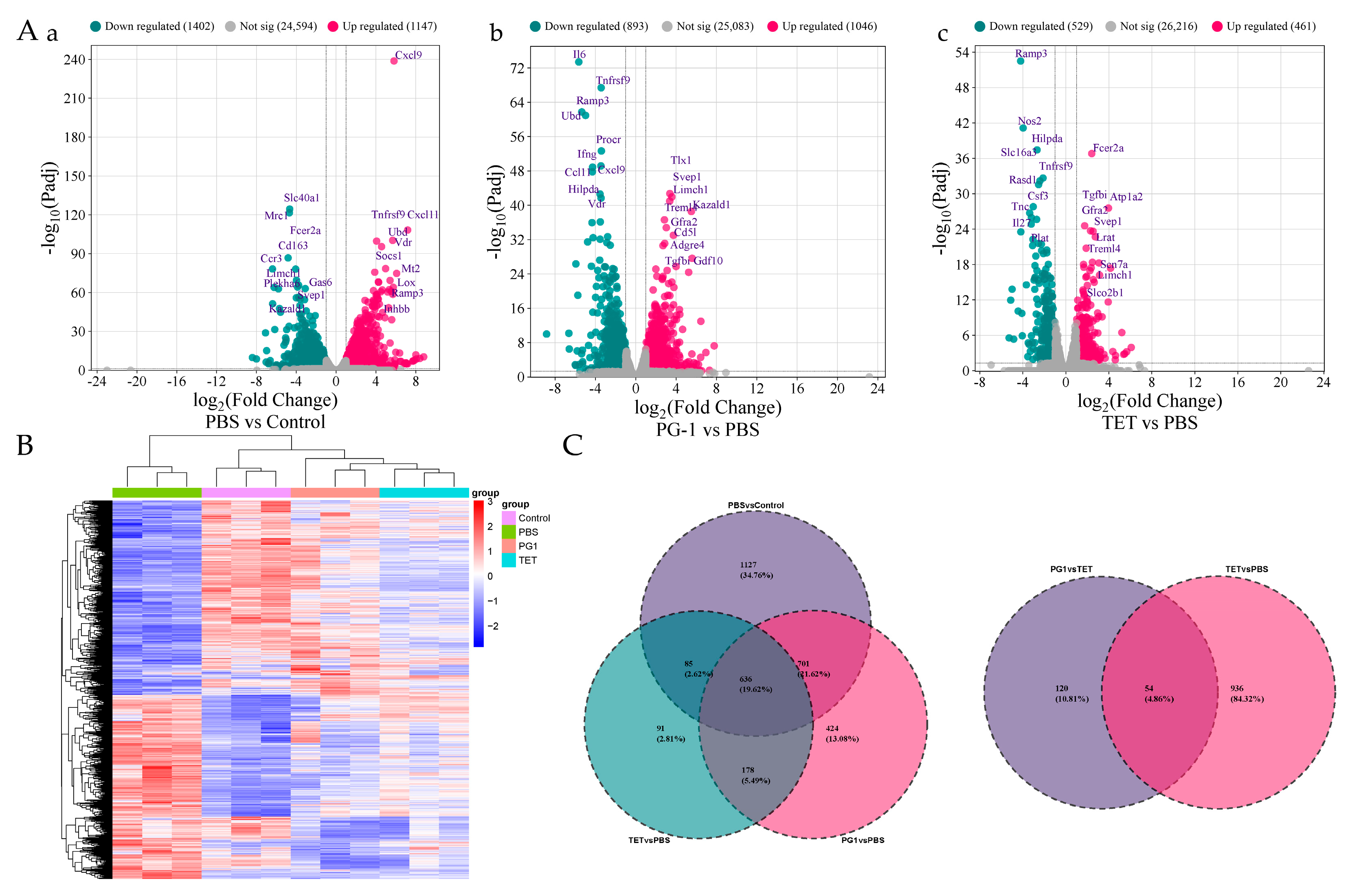
3.6. Transcriptome Sequencing and Read Mapping
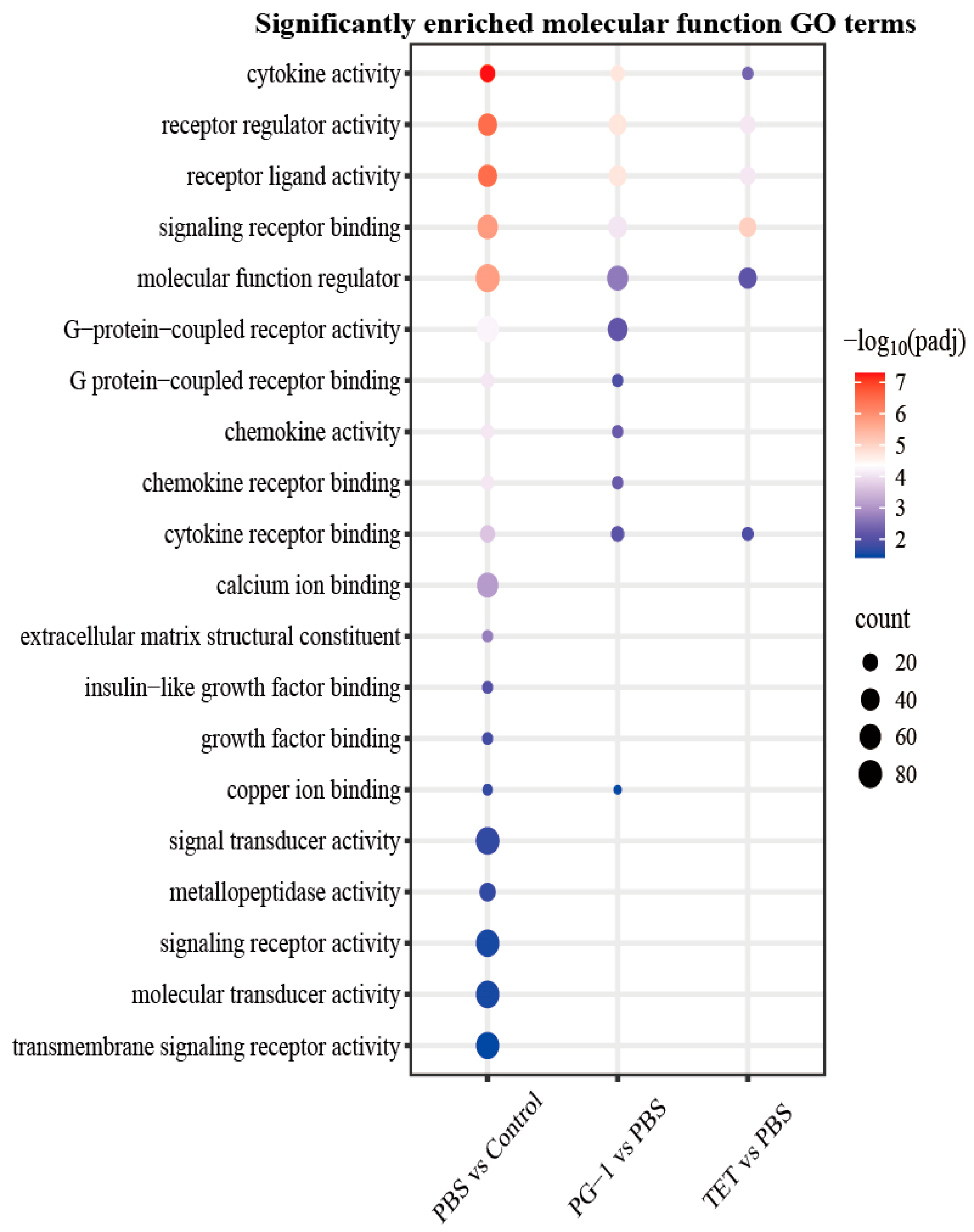
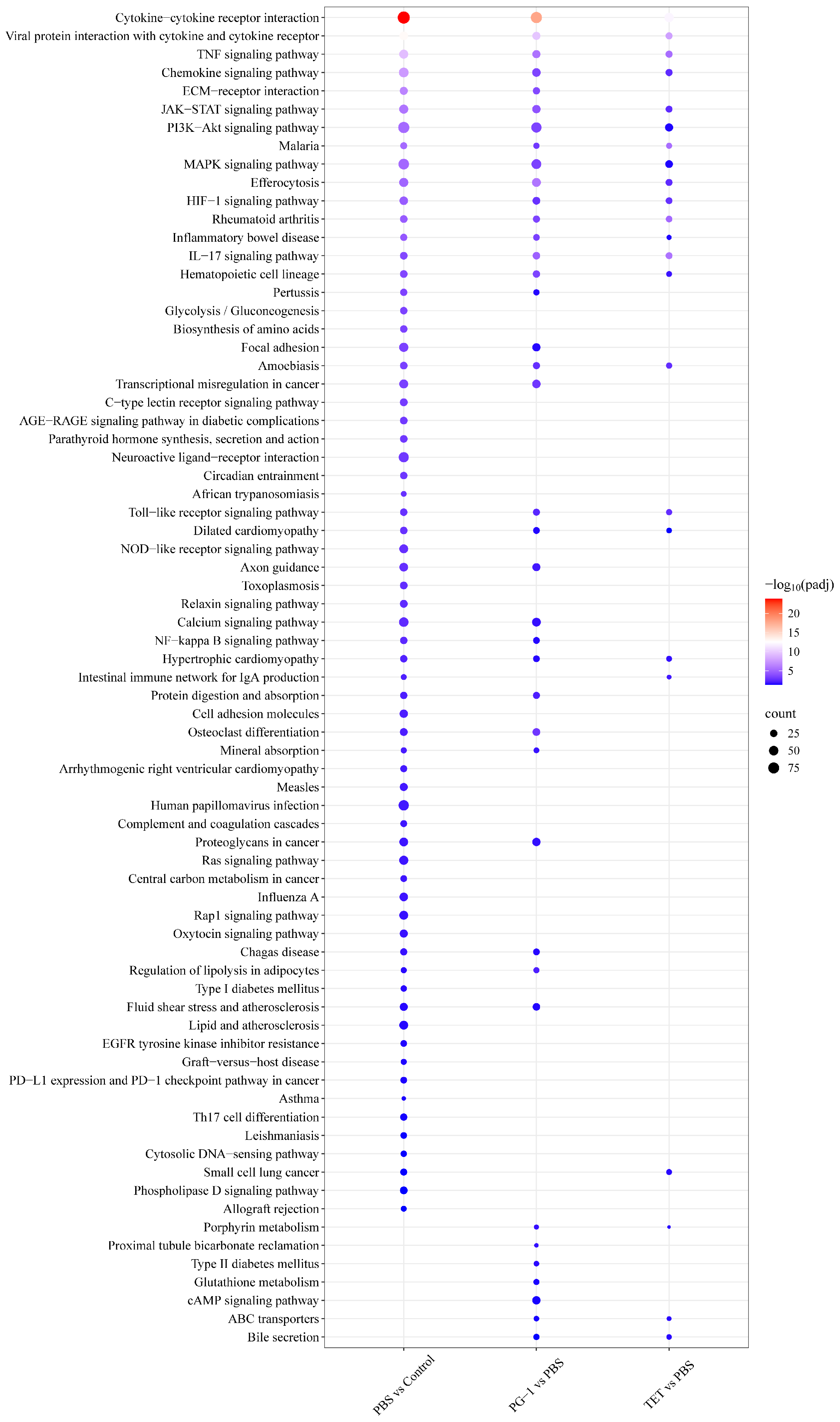
3.7. Analysis of Differentially Expressed Genes (DEGs)
3.8. Validation of RNA-Seq Results by qRT-PCR
4. Discussion
4.1. PG-1 Demonstrates Potent and Resistance-Resistant Bactericidal Activity Against MDR Porcine ExPEC
4.2. PG-1 Achieves Protective Efficacy with an Acceptable Safety Profile and Unique Immunomodulation
4.3. PG-1 Coordinates Bactericidal Activity with Immunomodulation and Metabolic Intervention via Transcriptional Reprogramming
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ExPEC | extraintestinal pathogenic Escherichia coli |
| PG-1 | Protegrin-1 |
| MIC | minimal inhibitory concentration |
| AMP | Antimicrobial peptide |
| MDR | multidrug-resistant |
| MBC | Minimum bactericidal concentration |
| FIC | fractional inhibitory concentrations |
| TET | tetracycline |
| DEGs | Differentially expressed genes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PEG | polyethylene glycol |
References
- Kariuki, S. Global burden of antimicrobial resistance and forecasts to 2050. Lancet 2024, 404, 1172–1173. [Google Scholar] [CrossRef]
- Meena, P.R.; Priyanka, P.; Singh, A.P. Extraintestinal Pathogenic Escherichia coli (ExPEC) Reservoirs, and Antibiotics Resistance Trends: A One-Health Surveillance for Risk Analysis from “Farm-to-Fork”. Lett. Appl. Microbiol. 2023, 76, ovac016. [Google Scholar] [CrossRef]
- Sora, V.M.; Meroni, G.; Martino, P.A.; Soggiu, A.; Bonizzi, L.; Zecconi, A. Extraintestinal Pathogenic Escherichia coli: Virulence Factors and Antibiotic Resistance. Pathogens 2021, 10, 1355. [Google Scholar] [CrossRef]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef]
- Biran, D.; Ron, E.Z. Extraintestinal pathogenic Escherichia coli. Curr. Top. Microbiol. Immunol. 2018, 416, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.S.A.; Motta, R.G.; Martinez, A.C.; Orsi, H.; Hernandes, R.T.; Rall, V.L.M.; Pantoja, J.C.F.; Nardi Júnior, G.D.; Ribeiro, M.G. Virulence-encoding genes related to extraintestinal pathogenic E. coli and multidrug resistant pattern of strains isolated from neonatal calves with different severity scores of umbilical infections. Microb. Pathog. 2023, 174, 105861. [Google Scholar] [CrossRef]
- Belanger, L.; Garenaux, A.; Harel, J.; Boulianne, M.; Nadeau, E.; Dozois, C.M. Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol. Med. Microbiol. 2011, 62, 1–10. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Yang, M.; Xu, Z.; Wang, X.; Wei, L.; Tang, B.; Liu, F.; Zhang, Y.; Ding, Y.; et al. Genome analysis and in vivo virulence of porcine extraintestinal pathogenic Escherichia coli strain PCN033. BMC Genom. 2015, 16, 717. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Zhu, Y.; Wang, T.; Lu, Y.; Wang, X.; Peng, Z.; Sun, M.; Chen, H.; Zheng, J.; et al. Population structure and antibiotic resistance of swine extraintestinal pathogenic Escherichia coli from China. Nat. Commun. 2024, 15, 5811. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Hu, Y.; Bu, Z.; Liu, Y.; Zhang, H.; Hu, Y.; Xue, Y.; Li, S.; Tan, C.; Chen, X.; et al. Genome-wide identification of genes critical for in vivo fitness of multi-drug resistant porcine extraintestinal pathogenic Escherichia coli by transposon-directed insertion site sequencing using a mouse infection model. Virulence 2023, 14, 2158708. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tang, X.; Lu, P.; Wu, B.; Xu, Z.; Liu, W.; Zhang, R.; Bei, W.; Chen, H.; Tan, C. Clonal analysis and virulent traits of pathogenic extraintestinal Escherichia coli isolates from swine in China. BMC Vet. Res. 2012, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; An, F.; Zhang, T.; Lou, M.; Guo, J.; Liu, K.; Zhu, Y.; Wu, J.; Wu, R. Antimicrobial peptides: An alternative to traditional antibiotics. Eur. J. Med. Chem. 2024, 265, 116072. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of antimicrobial peptides: Antimicrobial, anti-inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Bucataru, C.; Ciobanasu, C. Antimicrobial peptides: Opportunities and challenges in overcoming resistance. Microbiol. Res. 2024, 286, 127822. [Google Scholar] [CrossRef]
- Bolintineanu, D.S.; Vivcharuk, V.; Kaznessis, Y.N. Multiscale models of the antimicrobial peptide protegrin-1 on gram-negative bacteria membranes. Int. J. Mol. Sci. 2012, 13, 11000–11011. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Pham, D.S.; Waring, A.J.; Lehrer, R.I.; Lee, K.Y. Insertion selectivity of antimicrobial peptide protegrin-1 into lipid monolayers: Effect of head group electrostatics and tail group packing. Biochim. Biophys. Acta 2006, 1758, 1450–1460. [Google Scholar] [CrossRef]
- Javed, A.; Oedairadjsingh, T.; Ludwig, I.S.; Wood, T.M.; Martin, N.I.; Broere, F.; Weingarth, M.H.; Veldhuizen, E.J.A. Antimicrobial and immunomodulatory activities of porcine cathelicidin protegrin-1. Mol. Immunol. 2024, 173, 100–109. [Google Scholar] [CrossRef]
- Zughaier, S.M.; Svoboda, P.; Pohl, J. Structure-dependent immune modulatory activity of protegrin-1 analogs. Antibiotics 2014, 3, 694–713. [Google Scholar] [CrossRef]
- Guo, C.; Cong, P.; He, Z.; Zhou, J.; Wang, Y.; Liu, X.; Chen, L.; Li, D.; He, Q.; Han, W.; et al. Inhibitory activity and molecular mechanism of protegrin-1 against porcine reproductive and respiratory syndrome virus in vitro. Antivir. Ther. 2015, 20, 573–582. [Google Scholar] [CrossRef]
- Penney, J.; Li, J. Protegrin 1 enhances innate cellular defense via the insulin-like growth factor 1 receptor pathway. Front. Cell. Infect. Microbiol. 2018, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.M.; Hancock, R.E. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef]
- Choi, M.K.; Le, M.T.; Cho, H.S.; Lee, J.; Jeon, H.; Cha, S.-Y.; Na, M.; Chun, T.; Kim, J.-H.; Song, H.; et al. Transgenic mice overexpressing PG1 display corneal opacity and severe inflammation in the eye. Int. J. Mol. Sci. 2021, 22, 1586. [Google Scholar] [CrossRef]
- Lai, J.R.; Huck, B.R.; Weisblum, B.; Gellman, S.H. Design of non-cysteine-containing antimicrobial β-hairpins: Structure-activity relationship studies with linear protegrin-1 analogues. Biochemistry 2002, 41, 12835–12842. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Y.; Li, X.; He, Y.; Bai, Y.; Wang, B.; Chen, S.; Liu, C. Transcriptome profiling reveals transcriptional regulation of protegrin-1 on immune defense and development in porcine granulosa cells. Gene 2024, 890, 147819. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, O.E.; Borregaard, N.; Cole, A.M. Antimicrobial Peptides in Innate Immune Responses. Contrib. Microbiol. 2008, 15, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Osakowicz, C.; Fletcher, L.; Caswell, J.L.; Li, J. Protective and anti-inflammatory effects of protegrin-1 on Citrobacter rodentium intestinal infection in mice. Int. J. Mol. Sci. 2021, 22, 9494. [Google Scholar] [CrossRef]
- Zong, B.; Liu, W.; Zhang, Y.; Wang, X.; Chen, H.; Tan, C. Effect of kpsM on the Virulence of Porcine Extraintestinal Pathogenic Escherichia coli. FEMS Microbiol. Lett. 2016, 363, fnw232. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef]
- Krishnan, S.; Venkatachalam, P.; Shanmugam, S.R.; Paramasivam, N. Fractional inhibitory concentration of bio-actives from agricultural waste disassembles biofilms and quenches virulence of nosocomial pathogens. J. Med. Microbiol. 2025, 74, 1980. [Google Scholar] [CrossRef]
- Jahn, L.J.; Munck, C.; Ellabaan, M.M.H.; Sommer, M.O.A. Adaptive laboratory evolution of antibiotic resistance using different selection regimes lead to similar phenotypes and genotypes. Front. Microbiol. 2017, 8, 816. [Google Scholar] [CrossRef]
- Chang, X.; Ma, Y.; Zhang, X.; Zhang, S.; Bai, B.; Zhang, P.; Sun, G.; Qi, Y.; Ma, S. Design, synthesis and biological evaluation of meucin-18 derived peptides as efficient broad-spectrum antibacterial agents. Bioorganic Chem. 2025, 163, 108621. [Google Scholar] [CrossRef]
- Slaoui, M.; Bauchet, A.; Fiette, L. Tissue Sampling and Processing for Histopathology Evaluation. Methods Mol. Biol. 2017, 1641, 101–114. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.; Mendell, J.T.; Salzberg, S.L. Stringtie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Kwon, B.S. Role of 4-1BB in Immune Responses. Semin. Immunol. 1998, 10, 481–489. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, K.; Sun, M.; Ding, C.; Li, T.; Jia, Y.; Wang, C.; Zhu, X.; Song, X.; Jia, R.; et al. UBD participates in neutrophilic asthma by promoting the activation of IL-17 signaling. Int. J. Biol. Macromol. 2024, 264, 130581. [Google Scholar] [CrossRef]
- Dong, B.; Zhou, Y.; Wang, W.; Scott, J.; Kim, K.; Sun, Z.; Guo, Q.; Lu, Y.; Gonzales, N.M.; Wu, H.; et al. Vitamin D Receptor Activation in Liver Macrophages Ameliorates Hepatic Inflammation, Steatosis, and Insulin Resistance in Mice. Hepatology 2020, 71, 1559–1574. [Google Scholar] [CrossRef]
- Puvača, N.; de Llanos Frutos, R. Antimicrobial Resistance in Escherichia coli Strains Isolated from Humans and Pet Animals. Antibiotics 2021, 10, 69. [Google Scholar] [CrossRef]
- Steinberg, D.A.; Hurst, M.A.; Fujii, C.A.; Kung, A.H.; Ho, J.F.; Cheng, F.C.; Loury, D.J.; Fiddes, J.C. Protegrin-1: A Broad-Spectrum, Rapidly Microbicidal Peptide with in vivo Activity. Antimicrob. Agents Chemother. 1997, 41, 1738–1742. [Google Scholar] [CrossRef]
- Jarosiewicz, M.; Garbacz, K.; Neubauer, D.; Kamysz, W. In vitro Efficiency of Antimicrobial Peptides against Staphylococcal Pathogens Associated with Canine Pyoderma. Animals 2020, 10, 470. [Google Scholar] [CrossRef]
- Gupta, P.; Mishra, P.; Prasad, R.; Poluri, K.M. Dissecting the Therapeutic Potency of Antimicrobial Peptides Against Microbial Biofilms. Curr. Protein Pept. Sci. 2021, 22, 336–360. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Lu, Y.; Liu, Q.; Chen, R.; Xu, Y.; Li, G.; Hu, X.; Ye, C.; Peng, L.; Fang, R. Antibacterial activity of the antimicrobial peptide PMAP-36 in combination with tetracycline against porcine extraintestinal pathogenic Escherichia coli in vitro and in vivo. Vet. Res. 2024, 55, 35. [Google Scholar] [CrossRef] [PubMed]
- Morroni, G.; Simonetti, O.; Brenciani, A.; Brescini, L.; Kamysz, W.; Kamysz, E.; Neubauer, D.; Caffarini, M.; Orciani, M.; Giovanetti, E.; et al. In vitro activity of protegrin-1, alone and in combination with clinically useful antibiotics, against acinetobacter baumannii strains isolated from surgical wounds. Med. Microbiol. Immunol. 2019, 208, 877–883. [Google Scholar] [CrossRef]
- Zharkova, M.S.; Orlov, D.S.; Golubeva, O.Y.; Chakchir, O.B.; Eliseev, I.E.; Grinchuk, T.M.; Shamova, O.V. Application of antimicrobial peptides of the innate immune system in combination with conventional antibiotics-a novel way to combat antibiotic resistance? Front. Cell. Infect. Microbiol. 2019, 9, 128. [Google Scholar] [CrossRef]
- Bolosov, I.A.; Panteleev, P.V.; Sychev, S.V.; Khokhlova, V.A.; Safronova, V.N.; Toropygin, I.Y.; Kombarova, T.I.; Korobova, O.V.; Pereskokova, E.S.; Borzilov, A.I.; et al. Design of Protegrin-1 Analogs with Improved Antibacterial Selectivity. Pharmaceutics 2023, 15, 2047. [Google Scholar] [CrossRef] [PubMed]
- Maystrenko, A.; Feng, Y.; Akhtar, N.; Li, J. The Addition of a Synthetic LPS-Targeting Domain Improves Serum Stability While Maintaining Antimicrobial, Antibiofilm, and Cell Stimulating Properties of an Antimicrobial Peptide. Biomolecules 2020, 10, 1014. [Google Scholar] [CrossRef]
- Yu, W.; Wang, J.; Wang, Z.; Li, L.; Li, W.; Song, J.; Zhang, S.; Shan, A. Pegylation of the antimicrobial peptide pg-1: A link between propensity for nanostructuring and capacity of the antitrypsin hydrolytic ability. J. Med. Chem. 2021, 64, 10469–10481. [Google Scholar] [CrossRef]
- Cheung, Q.C.K.; Turner, P.V.; Song, C.; Wu, D.; Cai, H.Y.; Macinnes, J.I.; Li, J. Enhanced resistance to bacterial infection in protegrin-1 transgenic mice. Antimicrob. Agents Chemother. 2008, 52, 1812–1819. [Google Scholar] [CrossRef]
- Soundrarajan, N.; Somasundaram, P.; Kim, D.; Cho, H.; Jeon, H.; Ahn, B.; Kang, M.; Song, H.; Park, C. Effective Healing of Staphylococcus aureus-Infected Wounds in Pig Cathelicidin Protegrin-1-Overexpressing Transgenic Mice. Int. J. Mol. Sci. 2023, 24, 11658. [Google Scholar] [CrossRef]
- Tan, C.; Tang, X.; Zhang, X.; Ding, Y.; Zhao, Z.; Wu, B.; Cai, X.; Liu, Z.; He, Q.; Chen, H. Serotypes and virulence genes of extraintestinal pathogenic Escherichia coli isolates from diseased pigs in China. Vet. J. 2012, 192, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Qian, J.; Jin, Q.; Liu, F.; Zhang, G.; Gu, H.; Fu, L.; Wang, Y.; Zhang, X.; Yu, Y.; et al. Expression and Analyses of CXCL9/10/11 and CXCR3 in Ulcerative Cutaneous Tuberculosis. J. Interferon Cytokine Res. 2022, 42, 180–190. [Google Scholar] [CrossRef]
- Li, J.; Jing, Q.; Hu, Z.; Wang, X.; Hu, Y.; Zhang, J.; Li, L. Mycobacterium tuberculosis-specific memory T cells in bronchoalveolar lavage of patients with pulmonary tuberculosis. Cytokine 2023, 171, 156374. [Google Scholar] [CrossRef]
- Zong, B.; Xiao, Y.; Ren, M.; Wang, P.; Fu, S.; Qiu, Y. Baicalin weakens the porcine ExPEC-induced inflammatory response in 3D4/21 cells by inhibiting the expression of NF-κB/MAPK signaling pathways and reducing NLRP3 inflammasome activation. Microorganisms 2023, 11, 2126. [Google Scholar] [CrossRef]
- Knight, M.; Stanley, S. HIF-1α as a Central Mediator of Cellular Resistance to Intracellular Pathogens. Curr. Opin. Immunol. 2019, 60, 111–116. [Google Scholar] [CrossRef]
- Li, Z.; Ding, L.; Ma, R.; Zhang, Y.; Zhang, Y.; Ni, W.; Tang, T.; Wang, G.; Wang, B.; Lv, L.; et al. Activation of HIF-1α C-terminal transactivation domain protects against hypoxia-induced kidney injury through hexokinase 2-mediated mitophagy. Cell Death Dis. 2023, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Minchenko, O.; Opentanova, I.; Caro, J. Hypoxic regulation of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene family (PFKFB-1-4) expression in vivo. FEBS Lett. 2003, 554, 264–270. [Google Scholar] [CrossRef]
- Thompson, A.P.; O’Neill, I.; Smith, E.J.; Catchpole, J.; Fagan, A.; Burgess, K.E.V.; Carmody, R.J.; Clarke, D.J. Glycolysis and pyrimidine biosynthesis are required for replication of adherent–invasive Escherichia coli in macrophages. Microbiology 2016, 162, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Beebout, C.J.; Robertson, G.L.; Reinfeld, B.I.; Blee, A.M.; Morales, G.H.; Brannon, J.R.; Chazin, W.J.; Rathmell, W.K.; Rathmell, J.C.; Gama, V.; et al. Uropathogenic Escherichia coli subverts mitochondrial metabolism to enable intracellular bacterial pathogenesis in urinary tract infection. Nat. Microbiol. 2022, 7, 1348–1360. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB signaling in macrophages: Dynamics, crosstalk, and signal integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Xie, L.; Huang, Z.; Meng, H.; Shi, X.; Xie, J. Immunomodulation Effect of Polysaccharides from Liquid Fermentation of Monascus purpureus 40269 via Membrane TLR-4 to Activate the MAPK and NF-κB Signaling Pathways. Int. J. Biol. Macromol. 2022, 201, 480–491. [Google Scholar] [CrossRef]
- Lucas, R.M.; Luo, L.; Stow, J.L. ERK1/2 in Immune Signalling. Biochem. Soc. Trans. 2022, 50, 1341–1352. [Google Scholar] [CrossRef]
- Hardy, R.R.; Hayakawa, K. B cell development pathways. Annu. Rev. Immunol. 2001, 19, 595–621. [Google Scholar] [CrossRef] [PubMed]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M. Tumour necrosis factor signalling in health and disease. F1000Research 2019, 8, 111. [Google Scholar] [CrossRef]
- Joshi, A.R.T.; Chung, C.; Song, G.Y.; Lomas, J.; Priester, R.A.; Ayala, A. NF-κB activation has tissue-specific effects on immune cell apoptosis during polymicrobial sepsis. Shock 2002, 18, 380–386. [Google Scholar] [CrossRef]
- Gupta, S.; Gollapudi, S. Molecular mechanisms of TNF-α-induced apoptosis in naïve and memory T cell subsets. Autoimmun. Rev. 2006, 5, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Suh, J.W.; Lee, K.H.; Kang, J.L.; Woo, S. IL-17 and IL-22 enhance skin inflammation by stimulating the secretion of IL-1β by keratinocytes via the ROS-NLRP3-caspase-1 pathway. Int. Immunol. 2012, 24, 147–158. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Primer Sequence (5′-3′) | Product Length | GenBank Number |
|---|---|---|---|
| GAPDH | F: AATTCAACGGCACAGTCAAGG | 137 | 14433 |
| R: ACCAGTAGACTCCACGACATAC | |||
| β-actin | F: CCTGTATGCCTCTGGTCGTA | 339 | 11461 |
| R: CGCTCGTTGCCAATAGTGAT | |||
| Kazald1 | F: CTGACAAATCCAGTTGTTGAGG | 448 | 107250 |
| R: GTCCTTGCTACTACCATTATTG | |||
| Tlx1 | F: AGTGAGGTTAGCGTCCAG | 138 | 21908 |
| R: CTCTCACCCTTCACTGTAAC | |||
| Svep1 | F: GTACTTCTGGCGGTGGAA | 330 | 64817 |
| R: GAAGGTGCGGATTACAGAT | |||
| Limch1 | F: CGTGCCATAGAGGAAGTG | 156 | 77569 |
| R: GATGATATGTCCGCACGAA | |||
| Atp1a2 | F: GCTCCAACTCTTCCTGAAT | 101 | 98660 |
| R: ATATCTAGGTATCGTGCTAG | |||
| Cxcl9 | F: TGTTCTTGAAGGAGTCCAT | 178 | 17329 |
| R: CTACACTGAAGAACGGAGAT | |||
| IL27 | F: CTTGAACGACGACGACTT | 116 | 246779 |
| R: GGAAGAGGAGGAAGAAGAAG | |||
| Vdr | F: AGTGAAGGAGCTGGTAGG | 140 | 22337 |
| R: CATTGCCGAACACCTCTA | |||
| Ramp3 | F: GTCGGAGTTCATCGTGTATT | 226 | 56089 |
| R: AGCCATAGCCACAGTCAG | |||
| Il6 | F: TAGTCCTTCCTACCCCAATTTCC | 76 | 16193 |
| R: TTGGTCCTTAGCCACTCCTTC | |||
| Cxcl11 | F: TACTGCAATCCGGACCAGTG | 122 | 56066 |
| R: GATGTTCGTGTGCCTCGTGA | |||
| Abca8a | F: TATTGGCTTCTCCGAATGAA | 152 | 217258 |
| R: GTGGTCCTGATGCTCCTT | |||
| Ccr3 | F: CTATGTTCTGTGGAATGAGTG | 291 | 1277 |
| R: TCTGGATAGCGAGGACTG | |||
| Gdf10 | F: GGCTCGTGAGGTATTCTG | 100 | 14560 |
| R: AAGACTGCGGAAGATTAGG | |||
| Tlr5 | F: CGAAGACTGCGATGAAGA | 358 | 53791 |
| R: CTGGATGTTGGAGATATGGT | |||
| Serpina3a | F: GTTCTCCTTATCACAAGACTAC | 101 | 74069 |
| R: CTCCAGTGATCCCAGACA |
| Antimicrobial Agents | PCN033 | E. coli ATCC25922 | ||
|---|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | |
| PG-1 | 32 | 32 | 32 | 64 |
| TET | 512 | 512 | 0.125 | 0.25 |
| Antimicrobial Agents | MIC (μg/mL) | FIC | |||
|---|---|---|---|---|---|
| PG-1 | TET | ||||
| Single Drug | Combination Therapy | Single Drug | Combination Therapy | ||
| E. coli ATCC25922 | 32 | 32 | 0.25 | 0.03125 | 1.125 |
| PCN033 | 32 | 32 | 512 | 16 | 1.03125 |
| Sample | Raw Reads | Clean Reads | Clean Bases | Q30 (%) | GC (%) | Total Mapped Reads |
|---|---|---|---|---|---|---|
| PBS1 | 46,004,704 | 44,834,110 (97.46%) | 6.73 G | 97.28 | 49.74 | 39,676,052 (88.5%) |
| PBS2 | 49,219,132 | 48,065,826 (97.66%) | 7.21 G | 97.19 | 49.93 | 42,553,506 (88.53%) |
| PBS3 | 44,484,664 | 43,692,736 (98.22%) | 6.55 G | 97.38 | 50.3 | 38,977,111 (89.21%) |
| PG1-1 | 40,395,778 | 39,509,588 (97.81%) | 5.93 G | 97.41 | 48.9 | 35,492,724 (89.83%) |
| PG1-2 | 48,340,560 | 47,100,692 (97.44%) | 7.07 G | 97.45 | 49.41 | 42,595,633 (90.44%) |
| PG1-3 | 52,695,198 | 50,631,020 (96.08%) | 7.59 G | 97.3 | 49.8 | 45,094,025 (89.06%) |
| TET1 | 50,118,192 | 48,757,348 (97.28%) | 7.31 G | 97.33 | 49.74 | 42,893,924 (87.97%) |
| TET2 | 41,649,796 | 40,517,388 (97.28%) | 6.08 G | 97.25 | 50.09 | 35,941,515 (88.71%) |
| TET3 | 47,035,952 | 45,557,334 (96.86%) | 6.83 G | 97.31 | 49.75 | 40,320,159 (88.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; He, Y.; Liang, Z.; Chen, S.; Tang, B.; Su, F.; Liu, C. Protegrin-1 Combats Multidrug-Resistant Porcine ExPEC: Potent Bactericidal Activity and Multimodal Immunometabolic Regulation In Vitro and in a Murine Model. Vet. Sci. 2025, 12, 1030. https://doi.org/10.3390/vetsci12111030
Xu J, He Y, Liang Z, Chen S, Tang B, Su F, Liu C. Protegrin-1 Combats Multidrug-Resistant Porcine ExPEC: Potent Bactericidal Activity and Multimodal Immunometabolic Regulation In Vitro and in a Murine Model. Veterinary Sciences. 2025; 12(11):1030. https://doi.org/10.3390/vetsci12111030
Chicago/Turabian StyleXu, Jing, Yinlin He, Zihao Liang, Shengfeng Chen, Biao Tang, Fei Su, and Canying Liu. 2025. "Protegrin-1 Combats Multidrug-Resistant Porcine ExPEC: Potent Bactericidal Activity and Multimodal Immunometabolic Regulation In Vitro and in a Murine Model" Veterinary Sciences 12, no. 11: 1030. https://doi.org/10.3390/vetsci12111030
APA StyleXu, J., He, Y., Liang, Z., Chen, S., Tang, B., Su, F., & Liu, C. (2025). Protegrin-1 Combats Multidrug-Resistant Porcine ExPEC: Potent Bactericidal Activity and Multimodal Immunometabolic Regulation In Vitro and in a Murine Model. Veterinary Sciences, 12(11), 1030. https://doi.org/10.3390/vetsci12111030






