In Vitro Effect of Elevated Ammonia and Urea Levels on Post-Thawed Bull Semen Sperm Characteristics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Source and Preparation
2.2. Experimental Treatments and Incubation
2.3. pH Measurement
2.4. Sperm Cell Characteristic Analysis
2.4.1. Viability
2.4.2. Reactive Oxygen Species (ROS)
2.4.3. Mitochondrial Membrane Potential (MMP)
2.4.4. Sperm Chromatin Structure Assay
2.5. Statistical Analysis
3. Results
3.1. pH of the Prepared Incubation Media
3.2. Sperm Motility Variables
3.2.1. Incubation with Ammonia
3.2.2. Incubation with Urea
3.3. Flow Cytometric Analysis
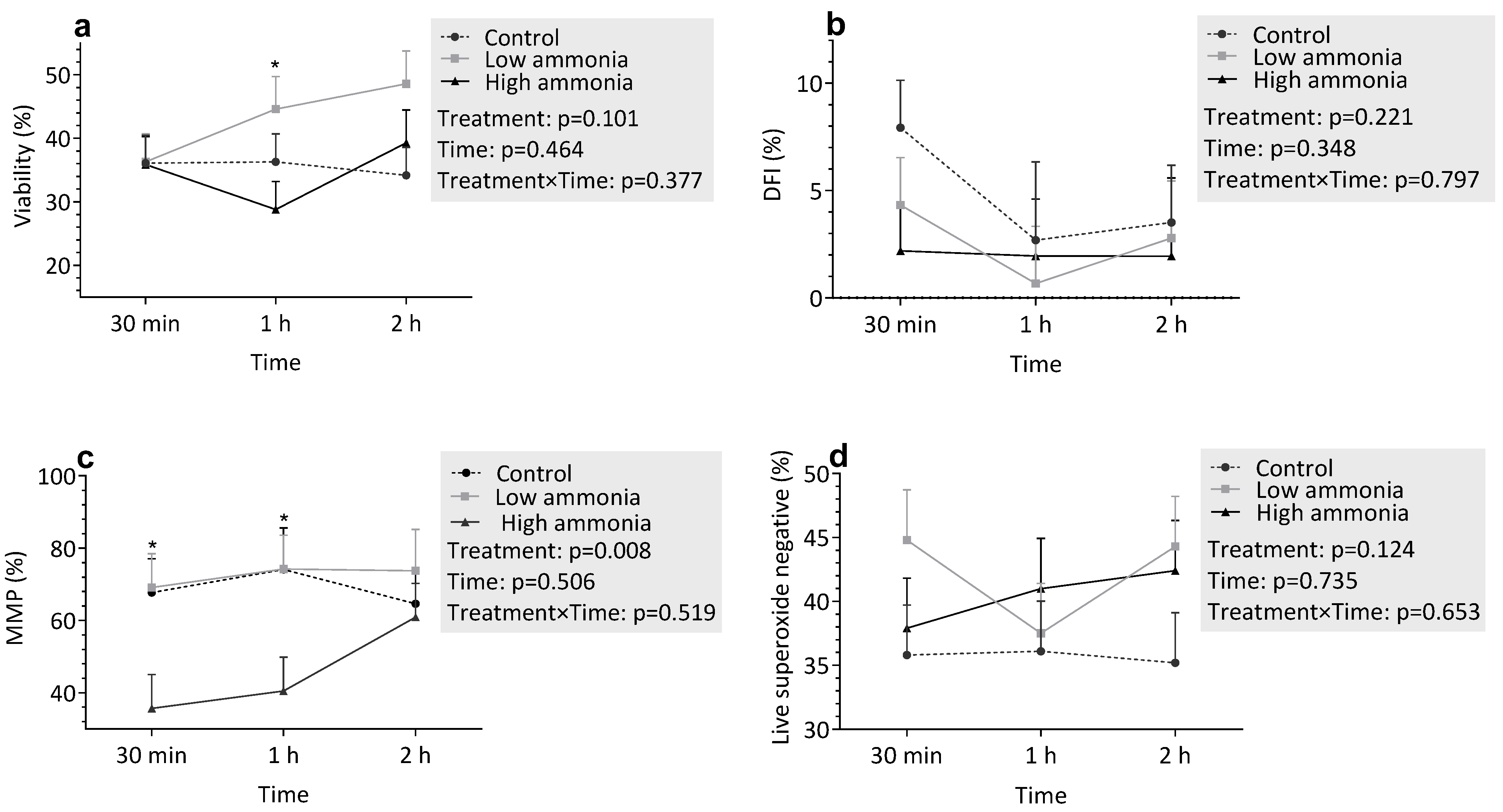
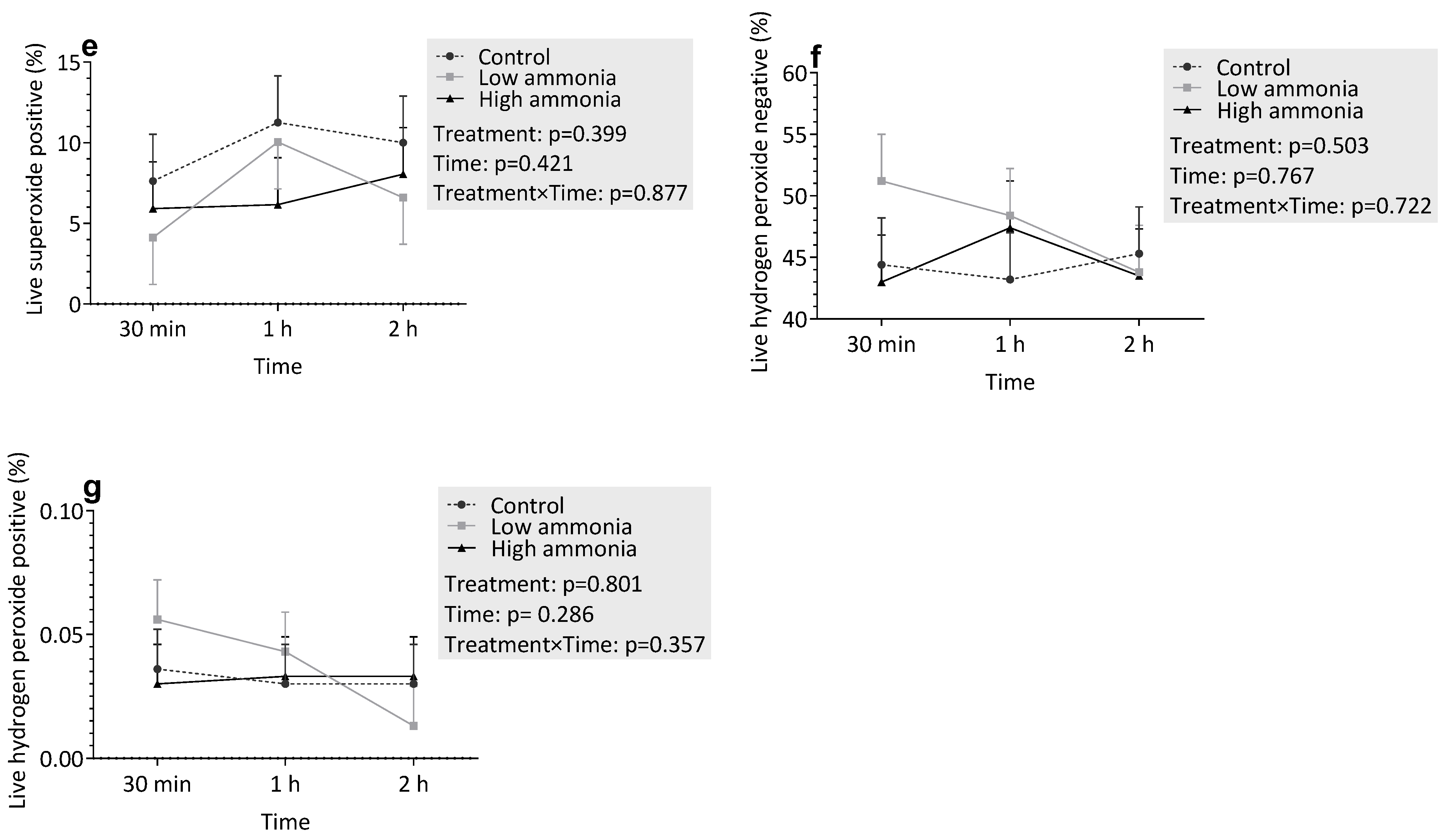
3.3.1. Incubation with Ammonia
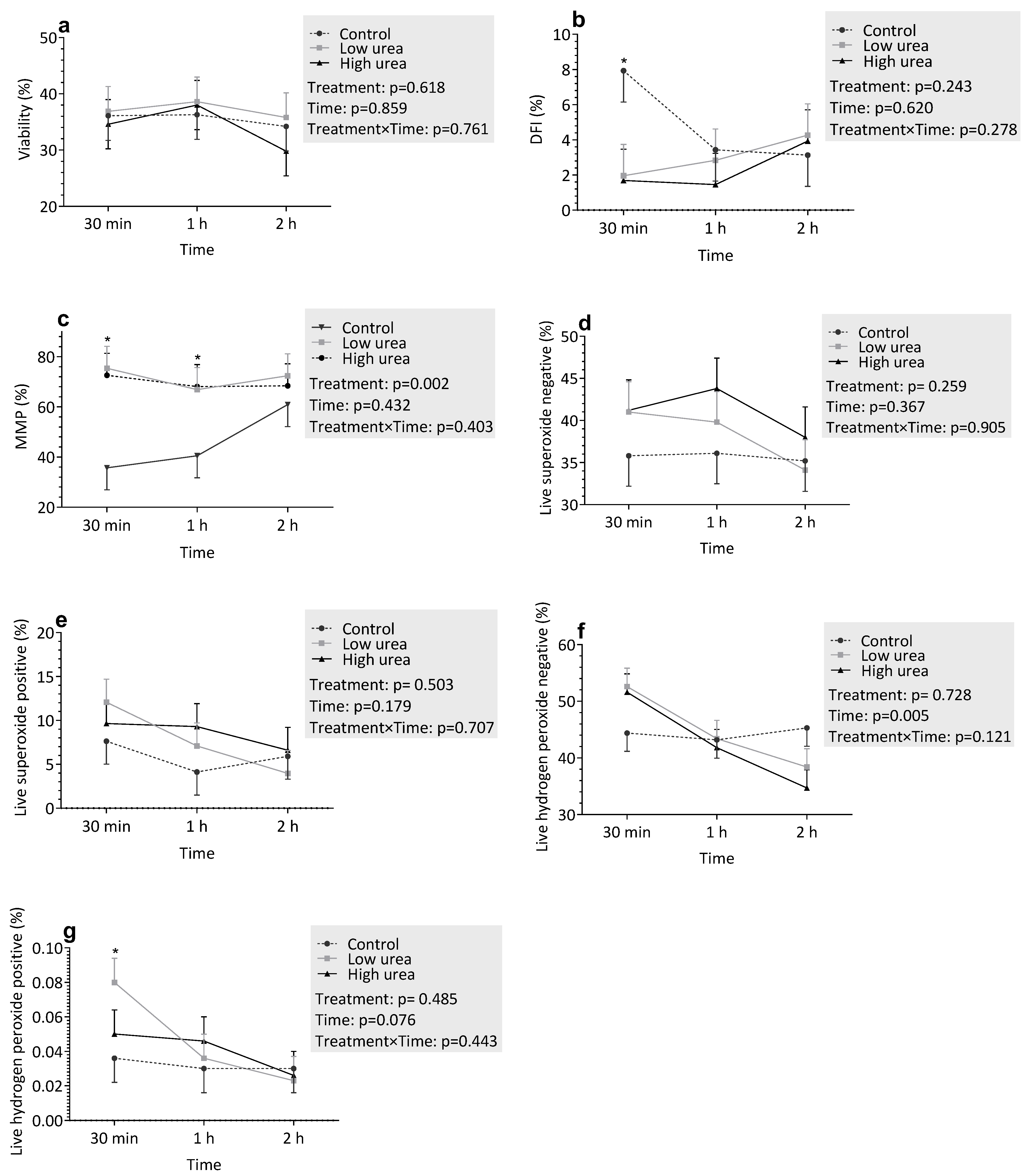
3.3.2. Incubation with Urea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; Satter, L.D. Milk Production During the Complete Lactation of Dairy Cows Fed Diets Containing Different Amounts of Protein. J. Dairy Sci. 2000, 83, 1042–1051. [Google Scholar] [CrossRef]
- Bisinotto, R.S.; Greco, L.F.; Ribeiro, E.S.; Martinez, N.; Lima, F.S.; Staples, C.R.; Thatcher, W.W.; Santos, J.E.P. Influences of nutrition and metabolism on fertility of dairy cows. Anim. Reprod. (AR) 2018, 9, 260–272. [Google Scholar]
- Rajala-Schultz, P.J.; Saville, W.J.A.; Frazer, G.S.; Wittum, T.E. Association Between Milk Urea Nitrogen and Fertility in Ohio Dairy Cows. J. Dairy Sci. 2001, 84, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Melendez, P.; Donovan, A.; Hernandez, J.; Bartolome, J.; Risco, C.A.; Staples, C.; Thatcher, W.W. Milk, plasma, and blood urea nitrogen concentrations, dietary protein, and fertility in dairy cattle. Timely Top. Nutr. 2003, 223, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Albaaj, A.; Foucras, G.; Raboisson, D. Changes in milk urea around insemination are negatively associated with conception success in dairy cows. J. Dairy Sci. 2017, 100, 3257–3265. [Google Scholar] [CrossRef]
- Raboisson, D.; Albaaj, A.; Nonne, G.; Foucras, G. High urea and pregnancy or conception in dairy cows: A meta-analysis to define the appropriate urea threshold. J. Dairy Sci. 2017, 100, 7581–7587. [Google Scholar] [CrossRef]
- Butler, W.; Calaman, J.; Beam, S.W. Plasma and milk urea nitrogen in relation to pregnancy rate in lactating dairy cattle. J. Anim. Sci. 1996, 74, 858–865. [Google Scholar] [CrossRef]
- Westwood, C.T.; Lean, I.J.; Kellaway, R.C. Indications and implications for testing of milk urea in dairy cattle: A quantitative review. Part 1. Dietary protein sources and metabolism. N. Z. Vet. J. 1998, 46, 87–96. [Google Scholar] [CrossRef]
- Chaveiro, A.E.; Andrade, M.; Borba, A.E.S.; Moreira da Silva, F. Association between Plasma and Milk Urea on the Insemination Day and Pregnancy Rate in Early Lactation Dairy Cows. J. Physiol. Pharmacol. Adv. 2014, 1, 9–14. [Google Scholar]
- Hammon, D.S.; Holyoak, G.R.; Dhiman, T.R. Association between blood plasma urea nitrogen levels and reproductive fluid urea nitrogen and ammonia concentrations in early lactation dairy cows. Anim. Reprod. Sci. 2005, 86, 195–204. [Google Scholar] [CrossRef]
- Howard, H.J.; Aalseth, E.P.; Adams, G.D.; Bush, L.J.; McNew, R.W.; Dawson, L.J. Influence of dietary protein on reproductive performance of dairy cows. J. Dairy Sci. 1987, 70, 1563–1571. [Google Scholar] [CrossRef]
- Allahkarami, S.; Atabakhsh, M.; Moh Moradi, M.N.; Ghasemi, H.; Bahmanzadeh, M.; Tavilani, H. Correlation of Uric Acid, Urea, Ammonia and Creatinine of Seminal Plasma With Semen Parameters and Fertilization Rate in Infertile Couples. Avicenna J. Med. Biochem. 2017, 5, 76–80. [Google Scholar] [CrossRef]
- Lavanya, M.; Swathi, D.; Archana, S.S.; Ramya, L.; Ranjithkumaran, R.; Krishnaswamy, N.; Singh, S.K.; Krishnappa, B.; Rajendran, D.; Kumar, H.; et al. Supraphysiological concentration of urea affects the functional competence of Holstein-Friesian (Bos taurus) sperm. Theriogenology 2021, 176, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.S. The Effects of High Levels of Crude Protein on Ram Fertility and the In Vitro Effect of Urea and Ammonia on Spermatozoa Penetration of Synthetic Mucus. Bachelor’s Thesis, University of Connecticut, Amherst, MA, USA, 1989. Available online: https://www.proquest.com/openview/bc736b85a9201eeaf21ca611aeeb319d/1?cb (accessed on 9 November 2024).
- Kowsar, R.; Iranshahi, V.N.; Sadeghi, N.; Riasi, A.; Miyamoto, A. Urea influences amino acid turnover in bovine cumulus-oocyte complexes, cumulus cells and denuded oocytes, and affects in vitro fertilization outcome. Sci. Rep. 2018, 8, 12191. [Google Scholar] [CrossRef] [PubMed]
- De Wit, A.A.C.; Cesar, M.L.F.; Kruip, T.A.M. Effect of urea during in vitro maturation on nuclear maturation and embryo development of bovine cumulus-oocyte-complexes. J. Dairy Sci. 2001, 84, 1800–1804. [Google Scholar] [CrossRef]
- Tareq, K.; Miah, A.G.; Salma, U.; Yoshida, M.; Tsujii, H. Effect of amino acids and dipeptides on accumulation of ammonia in the medium during in vitro maturation and fertilization of porcine oocytes. Reprod. Med. Biol. 2007, 6, 165–170. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M. Amino Acids and Ammonium Regulate Mouse Embryo Development in Culture. Biol. Reprod. 1993, 48, 377–385. [Google Scholar] [CrossRef]
- Golchin, A.; Asadpour, R.; Roshangar, L.; Jafari-Jozani, R. The Effect of Ammonium Chloride Concentration in In Vitro Maturation Culture on Ovine Embryo Development. J. Reprod. Infertil. 2016, 17, 144–150. [Google Scholar]
- Lima-Verde, I.; Johannisson, A.; Ntallaris, T.; Al-Essawe, E.; Al-Kass, Z.; Nongbua, T.; Dórea, F.; Lundeheim, N.; Kupisiewicz, K.; Edman, A.; et al. Effect of freezing bull semen in two non-egg yolk extenders on post-thaw sperm quality. Reprod. Domest. Anim. 2018, 53, 127–136. [Google Scholar] [CrossRef]
- Morrell, J.M.; Valeanu, A.S.; Lundeheim, N.; Johannisson, A. Sperm quality in frozen beef and dairy bull semen. Acta Vet. Scand. 2018, 60, 41. [Google Scholar] [CrossRef]
- Kumaresan, A.; Johannisson, A.; Al-Essawe, E.M.; Morrell, J.M. Sperm viability, reactive oxygen species, and DNA fragmentation index combined can discriminate between above- and below-average fertility bulls. J. Dairy Sci. 2017, 100, 5824–5836. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.; Jost, L. Sperm chromatin structure assay is useful for fertility assessment. Methods Cell Sci. 2000, 22, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Contri, A.; Valorz, C.; Faustini, M.; Wegher, L.; Carluccio, A. Effect of semen preparation on casa motility results in cryopreserved bull spermatozoa. Theriogenology 2010, 74, 424–435. [Google Scholar] [CrossRef] [PubMed]
- McKnight, K.; Hoang, H.D.; Prasain, J.K.; Brown, N.; Vibbert, J.; Hollister, K.A.; Moore, R.; Ragains, J.R.; Reese, J.; Miller, M.A. Neurosensory perception of environmental cues modulates sperm motility critical for fertilization. Science 2014, 344, 754–757. [Google Scholar] [CrossRef]
- Gillan, L.; Evans, G.; Maxwell, W.M.C. Flow cytometric evaluation of sperm parameters in relation to fertility potential. Theriogenology 2005, 63, 445–457. [Google Scholar] [CrossRef]
- Christensen, P.; Labouriau, R.; Birck, A.; Boe-Hansen, G.B.; Pedersen, J.; Borchersen, S. Relationship between semen quality and the proportion of sperm with major morphological abnormalities in bulls. Anim. Reprod. Sci. 2011, 125, 23–29. [Google Scholar]
- Amann, R.P.; Waberski, D. Computer-assisted sperm analysis (CASA): Capabilities and potential developments. Theriogenology 2014, 81, 5–17.e3. [Google Scholar]
- Amundson, O.L.; Larimore, E.L.; McNeel, A.K.; Chase Jr, C.C.; Cushman, R.A.; Freetly, H.C.; Perry, G.A. Uterine environment and pregnancy rate of heifers with elevated plasma urea nitrogen. Anim. Reprod. Sci. 2016, 173, 56–62. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.-D.; Liu, X.-Q.; Zhang, P.-F.; Hao, Y.-N.; Li, L.; Chen, L.; Shen, W.; Tang, X.-F.; Min, L.-J.; et al. Hydrogen Sulfide and/or Ammonia Reduces Spermatozoa Motility through AMPK/AKT Related Pathways. Sci. Rep. 2016, 6, 37884. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef]
- Kasai, T.; Ogawa, K.; Mizuno, K.; Nagai, S.; Uchida, Y.; Ohta, S.; Fujie, M.; Suzuki, K.; Hirata, S.; Hoshi, K. Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian J. Androl. 2002, 4, 97–103. [Google Scholar]
- Agnihotri, S.K.; Agrawal, A.K.; Hakim, B.A.; Vishwakarma, A.L.; Narender, T.; Sachan, R.; Sachdev, M. Mitochondrial membrane potential (MMP) regulates sperm motility. In Vitro Cell Dev. Biol. Anim. 2016, 52, 953–960. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Zhang, P.; Hao, Y.; Yu, S.; Min, L.; Li, L.; Ma, D.; Chen, L.; Yi, B.; et al. Decrease in male mouse fertility by hydrogen sulfide and/or ammonia can Be inheritable. Chemosphere 2018, 194, 147–157. [Google Scholar] [CrossRef]
- Massy, Z.A.; Stenvinkel, P.; Drueke, T.B. Progress in Uremic Toxin Research: The Role of Oxidative Stress in Chronic Kidney Disease. Semin. Dial. 2009, 22, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Dashty, M. A quick look at biochemistry: Carbohydrate metabolism. Clin. Biochem. 2013, 46, 1339–1352. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N. On the possible origins of DNA damage in human spermatozoa. Mol. Hum. Reprod. 2010, 16, 3–13. [Google Scholar] [CrossRef]
| Treatment | pH |
|---|---|
| Control | 7.60 ± 0.14 |
| HA | 7.82 ± 0.1 |
| LA | 7.78 ± 0.06 |
| HU | 7.80 ± 0.07 |
| LU | 7.73 ± 0.01 |
| Sperm Motility Descriptors 1 | Class 2 | Time | SE 3 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 h | 2 h | 4 h | Treatment | Time | T × T 4 | |||
| % Mot | Control | 34.5 a | 41.4 | 52.9 a | 41.2 | 5.56 | 0.008 | 0.929 | 0.199 |
| LA | 46.5 ab | 36.0 | 35.1 b | 35.1 | |||||
| HA | 29.1 ac | 30.4 | 27.6 b | 32.0 | |||||
| % Prog Mot | Control | 34.2 a | 49.8 a | 52.3 a | 39.5 | 4.97 | 0.0002 | 0.721 | 0.035 |
| LA | 45.8 a | 35.5 b | 34.7 b | 34.6 | |||||
| HA | 28.4 b | 29.9 b | 27.1 b | 30.9 | |||||
| VAP (µm/s) | Control | 43.5 a | 64.5 a | 59.9 a | 35.1 | 6.22 | 0.0001 | 0.002 | 0.010 |
| LA | 60.4 b | 41.3 b | 38.0 b | 30.4 | |||||
| HA | 37.2 a | 36.6 b | 19.2 c | 24.0 | |||||
| VCL (µm/s) | Control | 83.6 a | 125.7 a | 116.7 a | 66.8 | 12.22 | 0.0005 | 0.004 | 0.003 |
| LA | 121.5 b | 74.1 b | 72.5 b | 64.2 | |||||
| HA | 72.5 a | 74.8 b | 58.0 b | 54.9 | |||||
| VSL (µm/s) | Control | 37.1 a | 56.3 a | 49.8 a | 30.5 | 5.61 | 0.0001 | 0.001 | 0.024 |
| LA | 51.1 b | 35.7 b | 32.0 b | 24.9 | |||||
| HA | 31.4 a | 29.9 b | 24.0 b | 19.7 | |||||
| STR | Control | 0.84 | 0.86 | 0.83 | 0.85 | 0.02 | 0.612 | 0.618 | 0.398 |
| LA | 0.79 | 0.86 | 0.83 | 0.81 | |||||
| HA | 0.84 | 0.81 | 0.88 | 0.82 | |||||
| LIN | Control | 0.44 | 0.44 | 0.42 | 0.44 | 0.02 | 0.128 | 0.239 | 0.610 |
| LA | 0.42 | 0.48 | 0.44 | 0.39 | |||||
| HA | 0.43 | 0.40 | 0.40 | 0.36 | |||||
| WOB | Control | 0.52 | 0.53 | 0.51 | 0.51 a | 0.02 | 0.177 | 0.089 | 0.567 |
| LA | 0.49 | 0.55 | 0.52 | 0.47 ab | |||||
| HA | 0.51 | 0.49 | 0.50 | 0.43 b | |||||
| ALH (µm) | Control | 0.96 a | 1.37 a | 1.42 a | 0.82 | 0.13 | 0.0003 | 0.037 | 0.005 |
| LA | 1.28 b | 0.88 b | 0.88 b | 0.85 | |||||
| HA | 0.81 a | 0.87 b | 0.70 b | 0.71 | |||||
| BCF (Hz) | Control | 8.83 a | 9.61 a | 9.89 a | 8.60 | 0.97 | 0.0001 | 0.262 | 0.127 |
| LA | 9.89 ab | 6.66 b | 6.97 b | 7.21 | |||||
| HA | 6.92 ac | 6.29 b | 5.90 b | 6.71 | |||||
| Sperm Motility Descriptors 1 | Class 2 | Time | SE 3 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 h | 2 h | 4 h | Treatment | Time | T × T 4 | |||
| % Mot | Control | 34.5 | 41.4 | 52.9 | 41.2 | 6.70 | 0.378 | 0.494 | 0.507 |
| LU | 35.9 | 33.5 | 42.6 | 36.9 | |||||
| HU | 43.2 | 38.8 | 35.3 | 29.0 | |||||
| % Prog Mot | Control | 34.2 | 49.8 a | 52.3 a | 39.5 | 6.17 | 0.091 | 0.274 | 0.235 |
| LU | 35.5 | 33.0 b | 42.2 ab | 35.9 | |||||
| HU | 42.8 | 38.2 ab | 34.5 b | 27.8 | |||||
| VAP (µm/s) | Control | 43.5 | 64.5 a | 59.9 a | 35.1 | 7.67 | 0.083 | 0.001 | 0.241 |
| LU | 48.0 | 41.6 b | 45.4 ab | 26.8 | |||||
| HU | 54.3 | 51.2 ab | 36.5 b | 20.5 | |||||
| VCL (µm/s) | Control | 83.6 | 125.7 a | 116.7 a | 66.8 | 13.6 | 0.106 | 0.0008 | 0.154 |
| LU | 95.9 | 83.8 b | 84.5 b | 57.2 | |||||
| HU | 107.9 | 101.6 ab | 71.0 b | 43.7 | |||||
| VSL (µm/s) | Control | 37.1 | 56.3 a | 49.8 | 30.5 | 6.84 | 0.114 | 0.002 | 0.328 |
| LU | 41.8 | 35.0 b | 38.8 | 22.5 | |||||
| HU | 46.8 | 42.9 ab | 33.1 | 17.3 | |||||
| STR | Control | 0.84 | 0.86 | 0.83 | 0.85 | 0.02 | 0.986 | 0.966 | 0.874 |
| LU | 0.85 | 0.84 | 0.85 | 0.83 | |||||
| HU | 0.86 | 0.83 | 0.85 | 0.83 | |||||
| LIN | Control | 0.44 | 0.44 | 0.42 | 0.44 | 0.02 | 0.671 | 0.344 | 0.732 |
| LU | 0.41 | 0.41 | 0.42 | 0.39 | |||||
| HU | 0.43 | 0.42 | 0.46 | 0.38 | |||||
| WOB | Control | 0.52 | 0.53 | 0.51 | 0.51 | 0.02 | 0.385 | 0.114 | 0.690 |
| LU | 0.49 | 0.49 | 0.53 | 0.47 | |||||
| HU | 0.50 | 0.50 | 0.54 | 0.46 | |||||
| ALH (µm) | Control | 0.96 | 1.37 a | 1.42 a | 0.87 | 0.15 | 0.065 | 0.002 | 0.138 |
| LU | 1.05 | 0.94 b | 1.06 ab | 0.75 | |||||
| HU | 1.17 | 1.11 ab | 0.87 b | 0.58 | |||||
| BCF (Hz) | Control | 8.83 | 9.61 | 9.89 a | 8.60 | 1.14 | 0.022 | 0.419 | 0.378 |
| LU | 8.43 | 7.25 | 8.09 ab | 8.05 | |||||
| HU | 9.20 | 7.69 | 6.57 b | 6.07 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelli, A.; Besbaci, M.; Al-Kass, Z.; Iguer-Ouada, M.; Morrell, J.M. In Vitro Effect of Elevated Ammonia and Urea Levels on Post-Thawed Bull Semen Sperm Characteristics. Vet. Sci. 2025, 12, 997. https://doi.org/10.3390/vetsci12100997
Abdelli A, Besbaci M, Al-Kass Z, Iguer-Ouada M, Morrell JM. In Vitro Effect of Elevated Ammonia and Urea Levels on Post-Thawed Bull Semen Sperm Characteristics. Veterinary Sciences. 2025; 12(10):997. https://doi.org/10.3390/vetsci12100997
Chicago/Turabian StyleAbdelli, Amine, Mohamed Besbaci, Ziyad Al-Kass, Mokrane Iguer-Ouada, and Jane M. Morrell. 2025. "In Vitro Effect of Elevated Ammonia and Urea Levels on Post-Thawed Bull Semen Sperm Characteristics" Veterinary Sciences 12, no. 10: 997. https://doi.org/10.3390/vetsci12100997
APA StyleAbdelli, A., Besbaci, M., Al-Kass, Z., Iguer-Ouada, M., & Morrell, J. M. (2025). In Vitro Effect of Elevated Ammonia and Urea Levels on Post-Thawed Bull Semen Sperm Characteristics. Veterinary Sciences, 12(10), 997. https://doi.org/10.3390/vetsci12100997







