Feline Lymphoma in Focus: Examining the Patterns and Types in Croatia’s Pathological Records
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Diagnosis of Lymphoma
2.3. Immunohistochemistry (IHC) Staining
2.4. Statistical Analysis
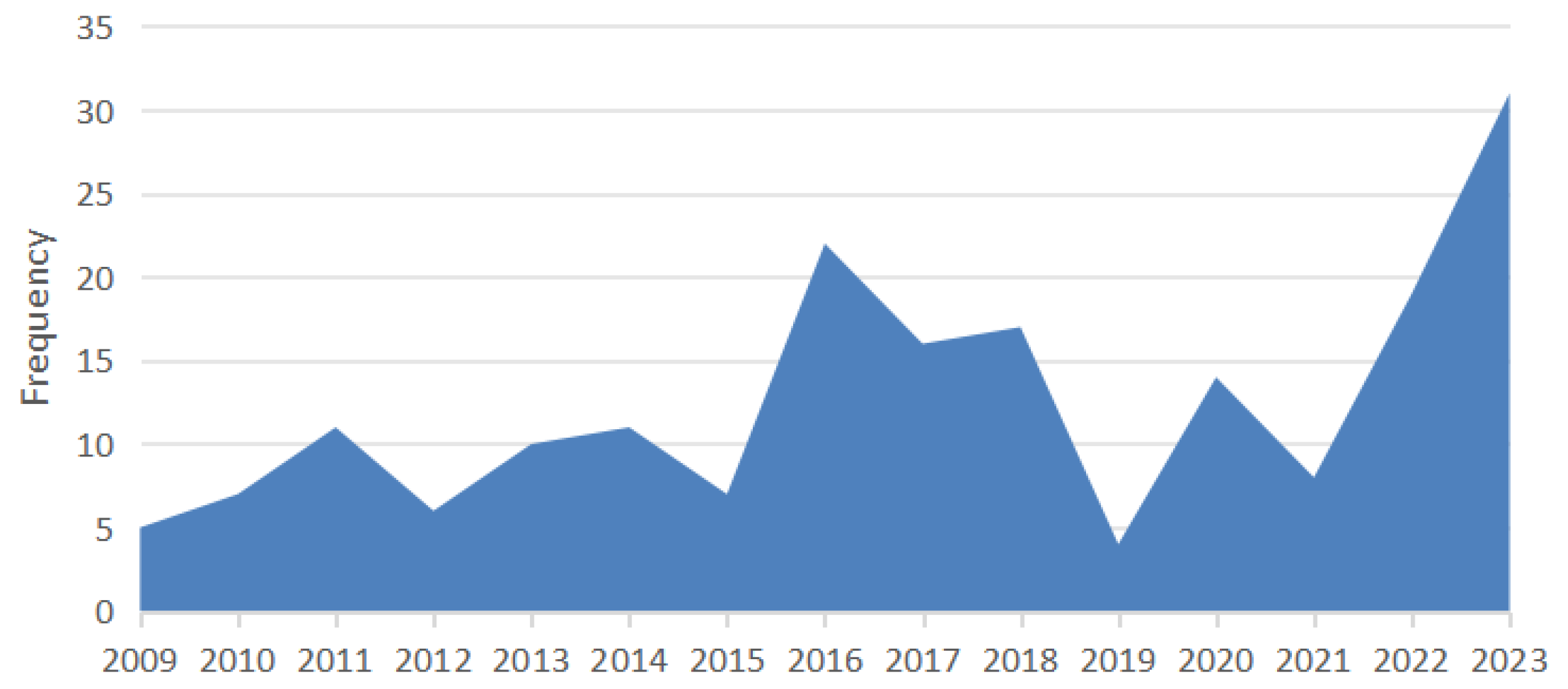
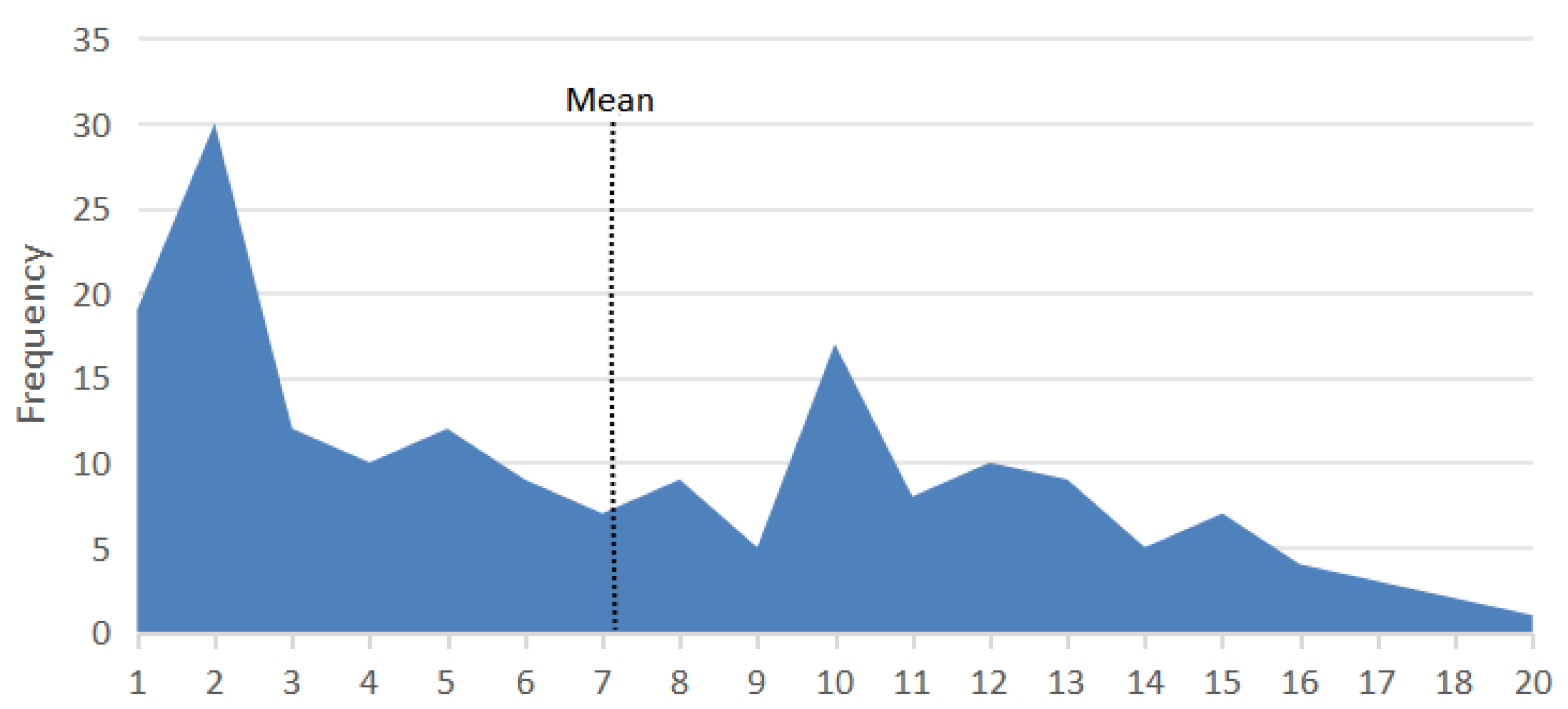
3. Results
3.1. Age
3.2. Sex Distribution
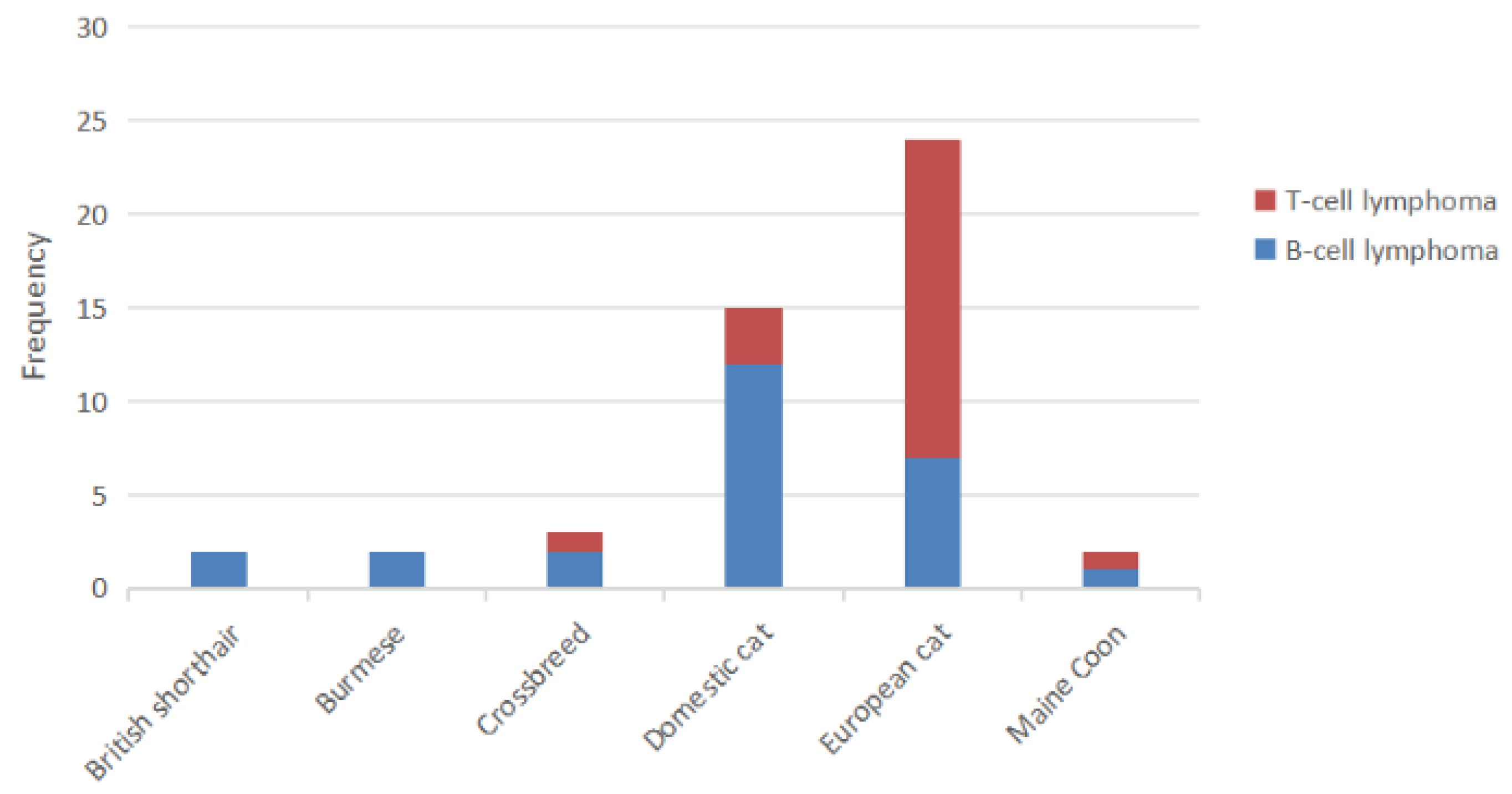
3.3. Breed
3.4. FeLV/FIV Status
3.5. Immunophenotyping of Lymphoma
3.6. Anatomical Distribution of Lymphoma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IHC | Immunohistochemistry |
| FeLV | Feline leukemia virus |
| FFPE | Formalin-fixed, paraffin-embedded |
| CV | Coefficient of Variation |
References
- Manuali, E.; Forte, C.; Vichi, G.; Genovese, D.A.; Mancini, D.; De Leo, A.A.P.; Cavicchioli, L.; Pierucci, P.; Zappulli, V. Tumours in European Shorthair Cats: A Retrospective Study of 680 Cases. J. Feline Med. Surg. 2020, 22, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Almendros, A.; Chan, L.K.; dos Santos Horta, R.; Nekouei, O.; Hill, F.; Giuliano, A. Description and Characterization of Different Types of Lymphoma in Cats in Hong Kong. Animals 2024, 14, 1654. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.F.; Rodriguez-Bertos, A. Immunohistochemistry for Diagnosis of Lymphoproliferative Diseases in the Cat. Vet. Pathol. 2013, 50, 478–488. [Google Scholar]
- Sato, H.; Fujino, Y.; Chino, J.; Takahashi, M.; Fukushima, K.; Goto-Koshino, Y.; Uchida, K.; Ohno, K.; Tsujimoto, H. Prognostic Analyses on Anatomical and Morphological Classification of Feline Lymphoma. J. Vet. Med. Sci. 2014, 76, 807–811. [Google Scholar] [CrossRef]
- Louwerens, M.; London, C.A.; Pedersen, N.C.; Lyons, L.A. Feline lymphoma in the post-feline leukemia virus era. J. Vet. Intern. Med. 2005, 19, 329–335. [Google Scholar] [CrossRef]
- Brodey, R.S. Canine and Feline Neoplasia. Adv. Vet. Sci. Comp. Med. 1970, 14, 309–354. [Google Scholar]
- Vail, D.M.; Moore, A.S.; Ogilvie, G.K.; Volk, L.M. Feline Lymphoma (145 Cases): Proliferation Indices, Cluster of Differentiation 3 Immunoreactivity, and Their Association with Prognosis in 90 Cats. J. Vet. Intern. Med. 1998, 12, 349–354. [Google Scholar] [CrossRef]
- Ettinger, S.N. Principles of Treatment for Feline Lymphoma. Clin. Tech. Small Anim. Pract. 2003, 18, 98–102. [Google Scholar] [CrossRef]
- Blackwood, L. Cats with Cancer: Where to Start. J. Feline Med. Surg. 2013, 15, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Versteegh, H.; Zandvliet, M.M.J.M.; Feenstra, L.R.; van der Steen, F.E.M.M.; Teske, E. Feline Lymphoma: Patient Characteristics and Response Outcome of the COP-Protocol in Cats with Malignant Lymphoma in The Netherlands. Animals 2023, 13, 2667. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; Jacobs, R.M.; Norris, A.; Couto, C.G.; Morrison, W.B.; McCaw, D.; Cotter, S.; Ogilvie, G.; Moore, A. The Histologic Classification of 602 Cases of Feline Lymphoproliferative Disease Using the National Cancer Institute Working Formulation. J. Vet. Diagn. Investig. 2000, 12, 295–306. [Google Scholar] [CrossRef]
- Taylor, S.S.; Goodfellow, M.R.; Browne, W.J.; Walding, B.; Murphy, S.; Tzannes, S.; Gerou-Ferriani, M.; Schwartz, A.; Dobson, J.M. Feline Extranodal Lymphoma: Response to Chemotherapy and Survival in 110 Cats. J. Small Anim. Pract. 2009, 50, 584–592. [Google Scholar] [CrossRef]
- Bound, N.J.; Priestnall, S.L.; Cariou, M.P. Lingual and Renal Lymphoma in a Cat. J. Feline Med. Surg. 2011, 13, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Gabor, L.J.; Malik, R.; Canfield, P.J. Clinical and Anatomical Features of Lymphosarcoma in 118 Cats. Aust. Vet. J. 1998, 76, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.G. What Is New on Feline Lymphoma? J. Feline Med. Surg. 2001, 3, 171–176. [Google Scholar] [CrossRef]
- Cristo, T.G.; Biezus, G.; Noronha, L.F.; Pereira, L.H.H.S.; Withoeft, J.A.; Furlan, L.; Costa, L.S.; Traverso, S.D.; Casagrande, R.A. Feline Lymphoma and a High Correlation with Feline Leukaemia Virus Infection in Brazil. J. Comp. Pathol. 2019, 166, 20–28. [Google Scholar] [CrossRef]
- Williams, A.G.; Hohenhaus, A.; Lamb, K. Incidence and Treatment of Feline Renal Lymphoma: 27 Cases. J. Feline Med. Surg. 2021, 23, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.C.; Mariano, A.D.; Holmes, E.S.; Aronson, L. Characterization of Post-Transplantation Lymphoma in Feline Renal Transplant Recipients. J. Comp. Pathol. 2014, 150, 162–168. [Google Scholar] [CrossRef]
- Guimarães-Okamoto, P.T.C.; da Silva, M.C.L.; Sequeira, J.L.; Hataka, A.; Chacar, F.C. Primary Diffuse Large B-Cell Lymphoma in Kidney with Involvement of Central Nervous System and Heart in a Siamese Cat. Acta Sci. Vet. 2016, 44 (Suppl. S1), 167. [Google Scholar] [CrossRef]
- Wolfesberger, B.; Skor, O.; Hammer, S.E.; Flickinger, I.; Kleiter, M.; Rütgen, B.C.; Schwendenwein, I.; Tichy, A.; Hittmair, K.M.; Degasperi, B.; et al. Does Categorisation of Lymphoma Subtypes According to the World Health Organization Classification Predict Clinical Outcome in Cats? J. Feline Med. Surg. 2017, 19, 897–906. [Google Scholar] [CrossRef]
- Meichner, K.; Kruse, D.B.; Hirschberger, J.; Hartmann, K. Changes in Prevalence of Progressive Feline Leukaemia Virus Infection in Cats with Lymphoma in Germany. Vet. Rec. 2012, 171, 348. [Google Scholar] [CrossRef]
- Leiet-Filho, R.V.; Panziera, W.; Bandinelli, M.B.; Henker, L.C.; da Conceição Monteiro, K.; Corbellini, L.G.; Driemeier, D.; Sonne, L.; Pavarini, S.P. Epidemiological, pathological and immunohistochemical aspects of 125 cases of feline lymphoma in Southern Brazil. Vet. Comp. Oncol. 2020, 18, 224–230. [Google Scholar] [CrossRef]
- Ludwig, L.; Dobromylskyj, M.; Wood, G.A.; van der Weyden, L. Feline Oncogenomics: What Do We Know about the Genetics of Cancer in Domestic Cats? Vet. Sci. 2022, 9, 547. [Google Scholar] [CrossRef]
- Teske, E.; van Straten, G.; van Noort, R.; Rutteman, G.R. Chemotherapy with Cyclophosphamide, Vincristine, and Prednisolone (COP) in Cats with Malignant Lymphoma: New Results with an Old Protocol. J. Vet. Intern. Med. 2002, 16, 179–186. [Google Scholar] [CrossRef]
- Economu, L.; Stell, A.; O’Neill, D.G.; Schofield, I.; Stevens, K.; Brodbelt, D. Incidence and Risk Factors for Feline Lymphoma in UK Primary-Care Practice. J. Small Anim. Pract. 2021, 62, 97–106. [Google Scholar] [CrossRef]
- Jeglum, K.A.; Whereat, A.; Young, K.M. Chemotherapy of Lymphoma in 75 Cats. J. Am. Vet. Med. Assoc. 1987, 190, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Barrs, V.R.; Beatty, J.A. Feline Alimentary Lymphoma: 1. Classification, Risk Factors, Clinical Signs and Non-Invasive Diagnostics. J. Feline Med. Surg. 2012, 14, 182–190. [Google Scholar] [CrossRef]
- Oriekhova, K.V.; Shchebentovska, O.O. Pathomorphology of the Renal Form of Lymphoma in Cats. Regul. Mech. Biosyst. 2023, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Taneja, A.; Kumar, V.; Chandra, A.B. Primary Renal Lymphoma: A Population-Based Analysis Using the SEER Program (1973–2015). Eur. J. Haematol. 2020, 104, 390–399. [Google Scholar] [CrossRef]
- Chen, X.; Hu, D.; Fang, L.; Chen, Y.; Che, X.; Tao, J.; Weng, G.; Ye, X. Primary Renal Lymphoma: A Case Report and Literature Review. Oncol. Lett. 2016, 12, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- Yunus, S.A.; Usmani, S.Z.; Ahmad, S.; Shahid, Z. Renal Involvement in Non-Hodgkin’s Lymphoma: The Shaukat Khanum Experience. Asian Pac. J. Cancer Prev. 2007, 8, 249–252. [Google Scholar]
- Weiss, A.T.A.; Klopfleisch, R.; Gruber, A.D. Prevalence of Feline Leukaemia Provirus DNA in Feline Lymphomas. J. Feline Med. Surg. 2010, 12, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, F.; Calam, A.E.; Dobson, J.M.; Middleton, S.A.; Murphy, S.; Taylor, S.S.; Schwartz, A.; Stell, A.J. Feline Mediastinal Lymphoma: A Retrospective Study of Signalment, Retroviral Status, Response to Chemotherapy and Prognostic Indicators. J. Feline Med. Surg. 2014, 16, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Studer, N.; Lutz, H.; Saegerman, C.; Gönczi, E.; Meli, M.L.; Boo, G.; Hartmann, K.; Hosie, M.J.; Moestl, K.; Tasker, S.; et al. Pan-European Study on the Prevalence of the Feline Leukaemia Virus Infection—Reported by the European Advisory Board on Cat Diseases (ABCD Europe). Viruses 2019, 11, 993. [Google Scholar] [CrossRef]
- Raukar, J. Prevalence of Feline Coronavirus, Feline Leukemia Virus, and Feline Immunodeficiency Virus in Client-Owned Cats in Croatia. J. Adv. Nat. Sci. 2021, 8, 24–38. [Google Scholar] [CrossRef]
- Chino, J.; Fujino, Y.; Kobayashi, T.; Kariya, K.; Goto-Koshino, Y.; Ohno, K.; Nakayama, H.; Tsujimoto, H. Cytomorphological and Immunological Classification of Feline Lymphomas: Clinicopathological Features of 76 Cases. J. Vet. Med. Sci. 2013, 75, 701–707. [Google Scholar] [CrossRef]
- Collette, S.A.; Allstadt, S.D.; Chon, E.; Vernau, W.; Smith, A.N.; Garrett, L.D.; Choy, K.; Rebhun, R.B.; Rodriguez, C.O., Jr.; Skorupski, K.A. Treatment of Feline Intermediate- to High-Grade Lymphoma with a Modified University of Wisconsin–Madison Protocol: 119 Cases (2004–2012). Vet. Comp. Oncol. 2016, 14, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Zwahlen, C.H.; Lucroy, M.D.; Kraegel, S.A.; Madewell, B.R. Results of Chemotherapy for Cats with Alimentary Malignant Lymphoma: 21 Cases (1993–1997). J. Am. Vet. Med. Assoc. 1998, 213, 1144–1149. [Google Scholar] [CrossRef]
- Graf, R.; Grüntzig, K.; Boo, G.; Hässig, M.; Axhausen, K.W.; Fabrikant, S.; Welle, M.; Meier, D.; Guscetti, F.; Folkers, G.; et al. Swiss Feline Cancer Registry 1965–2008: The Influence of Sex, Breed and Age on Tumour Types and Tumour Locations. J. Comp. Pathol. 2016, 154, 195–210. [Google Scholar] [CrossRef]
- Fontaine, J.; Heimann, M.; Day, M.J. Cutaneous Epitheliotropic T-Cell Lymphoma in the Cat: A Review of the Literature and Five New Cases. Vet. Dermatol. 2011, 22, 454–461. [Google Scholar] [CrossRef]
- Plant, J.D. Would You Have Diagnosed Cutaneous Epitheliotropic Lymphoma in These Two Cats? Vet. Med. 1991, 86, 801–806. [Google Scholar]
- Komori, S.; Nakamura, S.; Takahashi, K.; Tagawa, M. Use of Lomustine to Treat Cutaneous Nonepitheliotropic Lymphoma in a Cat. J. Am. Vet. Med. Assoc. 2005, 226, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Eraghi, V.; Medven Zagradišnik, L.; Matasović, M.; Vlahović, D.; Huber, D.; Gudan Kurilj, A.; Šoštarić-Zuckermann, I.C.; Artuković, B.; Mihoković Buhin, I.; Ciprić, I.; et al. Canine Lymphoma in Croatia: A Fourteen-Year Retrospective Study. BMC Vet. Res. 2025, 21, 172. [Google Scholar] [CrossRef] [PubMed]
| Breed | Total Referrals (n, %) | Lymphoma Cases (n) | % Lymphoma (95% CI) | Odds Ratio (vs. Domestic) | p-Value |
|---|---|---|---|---|---|
| Domestic cats | 2795 (65.7%) | 93 | 3.3% (2.7–4.0) | 1.00 (ref) | – |
| Crossbreed | 929 (21.9%) | 24 | 2.6% (1.7–3.8) | 0.79 (0.51–1.24) | 0.32 |
| European Shorthair | 731 (17.2%) | 38 | 5.2% (3.8–7.0) | 1.61 (1.10–2.34) | 0.01 |
| Persian | 129 (3.0%) | 3 | 2.3% (0.8–6.5) | 0.69 (0.21–2.27) | 0.58 |
| Maine Coon | 114 (2.7%) | 2 | 1.8% (0.5–6.4) | 0.54 (0.13–2.24) | 0.39 |
| British Shorthair | 67 (1.6%) | 4 | 6.0% (2.4–14.4) | 1.88 (0.66–5.34) | 0.24 |
| Siamese | 76 (1.8%) | 2 | 2.6% (0.7–9.0) | 0.80 (0.19–3.35) | 1 |
| Ragdoll | 2 (0.05%) | 1 | 50.0% (9.5–90.5) | 28.18 (1.62–490.9) | 0.03 |
| Russian Blue | 20 (0.5%) | 1 | 5.0% (0.9–23.6) | 1.56 (0.20–12.26) | 0.47 |
| Oriental Shorthair | 11 (0.3%) | 1 | 9.1% (1.6–37.7) | 2.97 (0.36–24.6) | 0.31 |
| Sacred Cat of Burma | 9 (0.2%) | 1 | 11.1% (2.0–43.5) | 3.73 (0.45–30.7) | 0.24 |
| Burmese | 41 (1.0%) | 1 | 2.4% (0.4–12.5) | 0.72 (0.10–5.35) | 1 |
| Carthusian | 11 (0.3%) | 3 | 27.3% (9.7–57.3) | 10.36 (2.73–39.3) | 0.0006 |
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| Age (per year) | 0.88 (0.79–0.98) | 0.02 |
| Male sex | 0.74 (0.34–1.62) | 0.45 |
| FeLV positive | 2.41 (0.90–6.47) | 0.08 |
| Breed: Crossbreed | 0.90 (0.28–2.93) | 0.86 |
| Breed: European Shorthair | 1.49 (0.61–3.64) | 0.39 |
| Breed: Other | 0.81 (0.30–2.21) | 0.68 |
| Anatomical Form | n | Median Age (IQR, Range) | FeLV+ n/N (%) | Common Breeds (%) | Immunophenotype (B/T, %) |
|---|---|---|---|---|---|
| Alimentary | 34 | 10.0 (6.0–12.0, 1–17) | 2/34 (5.9%) | European Shorthair 39.4%; | B-cell 56.5%; T-cell 43.5% |
| Domestic cats 39.4%; | |||||
| Crossbreed 15.2% | |||||
| Extranodal | 27 | 8.0 (4.5–11.5, 1–18) | 1/27 (3.7%) | Domestic cats 64.0%; | B-cell 71.4%; T-cell 28.6% |
| European Shorthair 20.0%; | |||||
| Crossbreed 12.0% | |||||
| Mediastinal | 32 | 2.0 (1.8–6.0, 1–16) | 12/32 (37.5%) | Domestic cats 58.6%; | T-cell 100.0% |
| European Shorthair 24.1%; | |||||
| Crossbreed 10.3% | |||||
| Multicentric | 35 | 6.0 (3.0–12.0, 1–20) | 8/35 (22.9%) | Domestic cats 66.7%; | B-cell 60.0%; T-cell 40.0% |
| Crossbreed 18.2%; | |||||
| European Shorthair 9.1% | |||||
| Unknown | 60 | 5.0 (3.0–11.0, 1–16) | 7/60 (11.7%) | Domestic cats 42.6%; | T-cell 52.9%; B-cell 47.1% |
| Crossbreed 22.2%; | |||||
| European Shorthair 14.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eraghi, V.; Ciprić, I.; Serdar, N.; Jonker, A.; Zagradišnik, L.M.; Vlahović, D.; Mihoković Buhin, I.; Šoštarić-Zuckermann, I.-C.; Artuković, B.; Huber, D.; et al. Feline Lymphoma in Focus: Examining the Patterns and Types in Croatia’s Pathological Records. Vet. Sci. 2025, 12, 986. https://doi.org/10.3390/vetsci12100986
Eraghi V, Ciprić I, Serdar N, Jonker A, Zagradišnik LM, Vlahović D, Mihoković Buhin I, Šoštarić-Zuckermann I-C, Artuković B, Huber D, et al. Feline Lymphoma in Focus: Examining the Patterns and Types in Croatia’s Pathological Records. Veterinary Sciences. 2025; 12(10):986. https://doi.org/10.3390/vetsci12100986
Chicago/Turabian StyleEraghi, Vida, Iva Ciprić, Nikola Serdar, Anouk Jonker, Lidija Medven Zagradišnik, Dunja Vlahović, Ivana Mihoković Buhin, Ivan-Conrado Šoštarić-Zuckermann, Branka Artuković, Doroteja Huber, and et al. 2025. "Feline Lymphoma in Focus: Examining the Patterns and Types in Croatia’s Pathological Records" Veterinary Sciences 12, no. 10: 986. https://doi.org/10.3390/vetsci12100986
APA StyleEraghi, V., Ciprić, I., Serdar, N., Jonker, A., Zagradišnik, L. M., Vlahović, D., Mihoković Buhin, I., Šoštarić-Zuckermann, I.-C., Artuković, B., Huber, D., Matasović, M., Hohšteter, M., & Gudan Kurilj, A. (2025). Feline Lymphoma in Focus: Examining the Patterns and Types in Croatia’s Pathological Records. Veterinary Sciences, 12(10), 986. https://doi.org/10.3390/vetsci12100986






