Computed Tomography Volumetric Measurements of Adrenal Glands in 26 Dogs Under One Year of Age: A Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Population
2.2. Definition of the Groups
2.3. CT Imaging Protocols
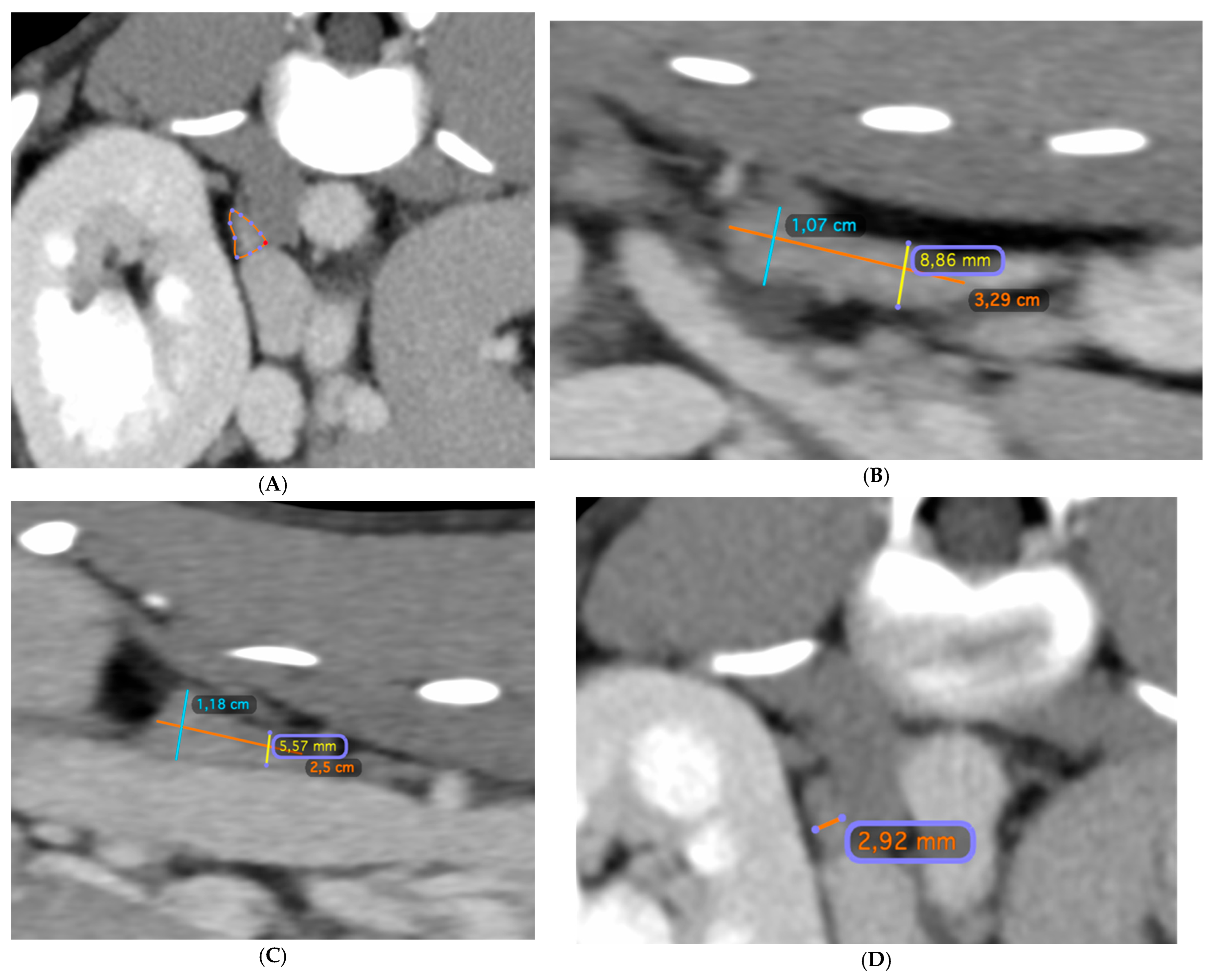
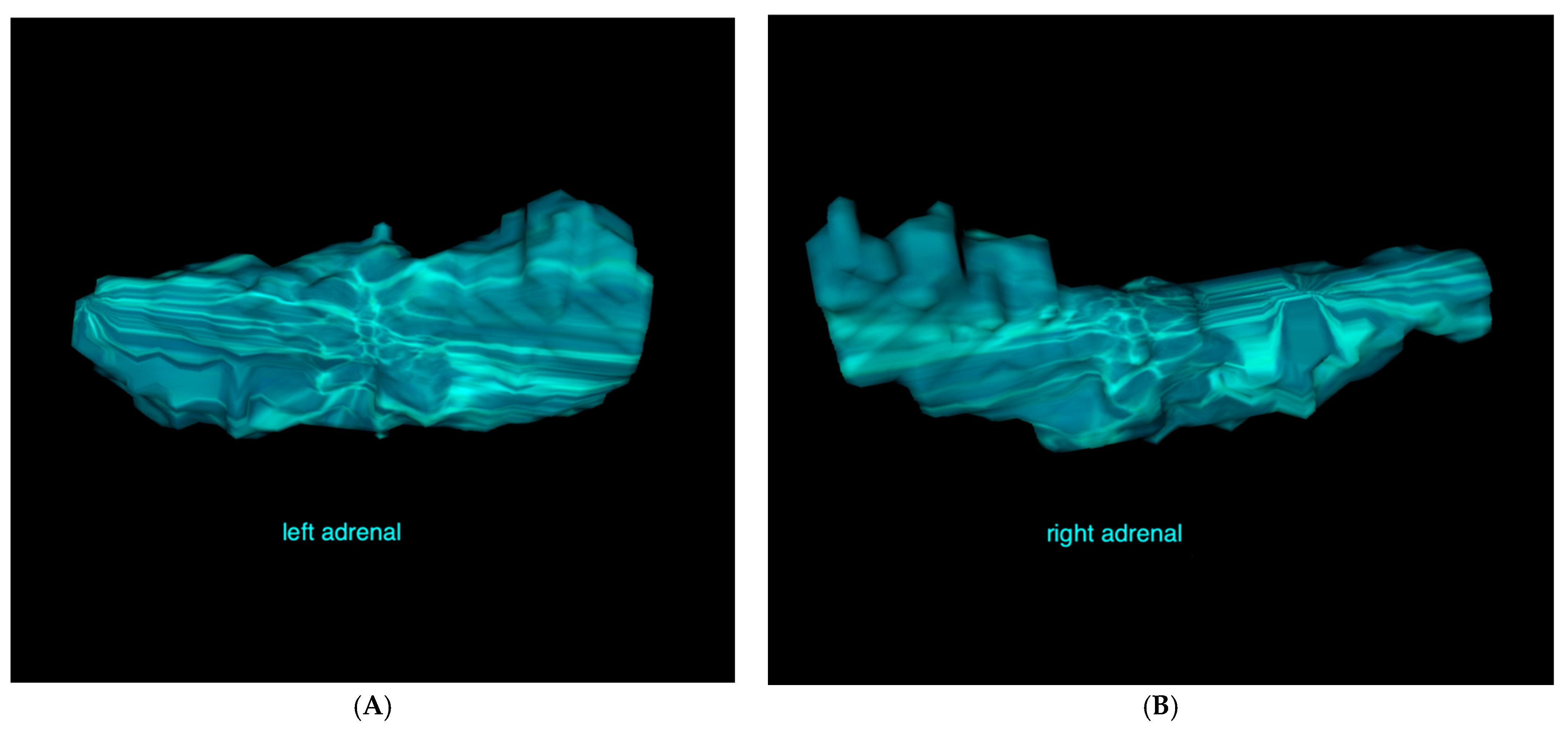
2.4. CT Measurements
2.5. Statistical Methods
3. Results
3.1. Sample Population
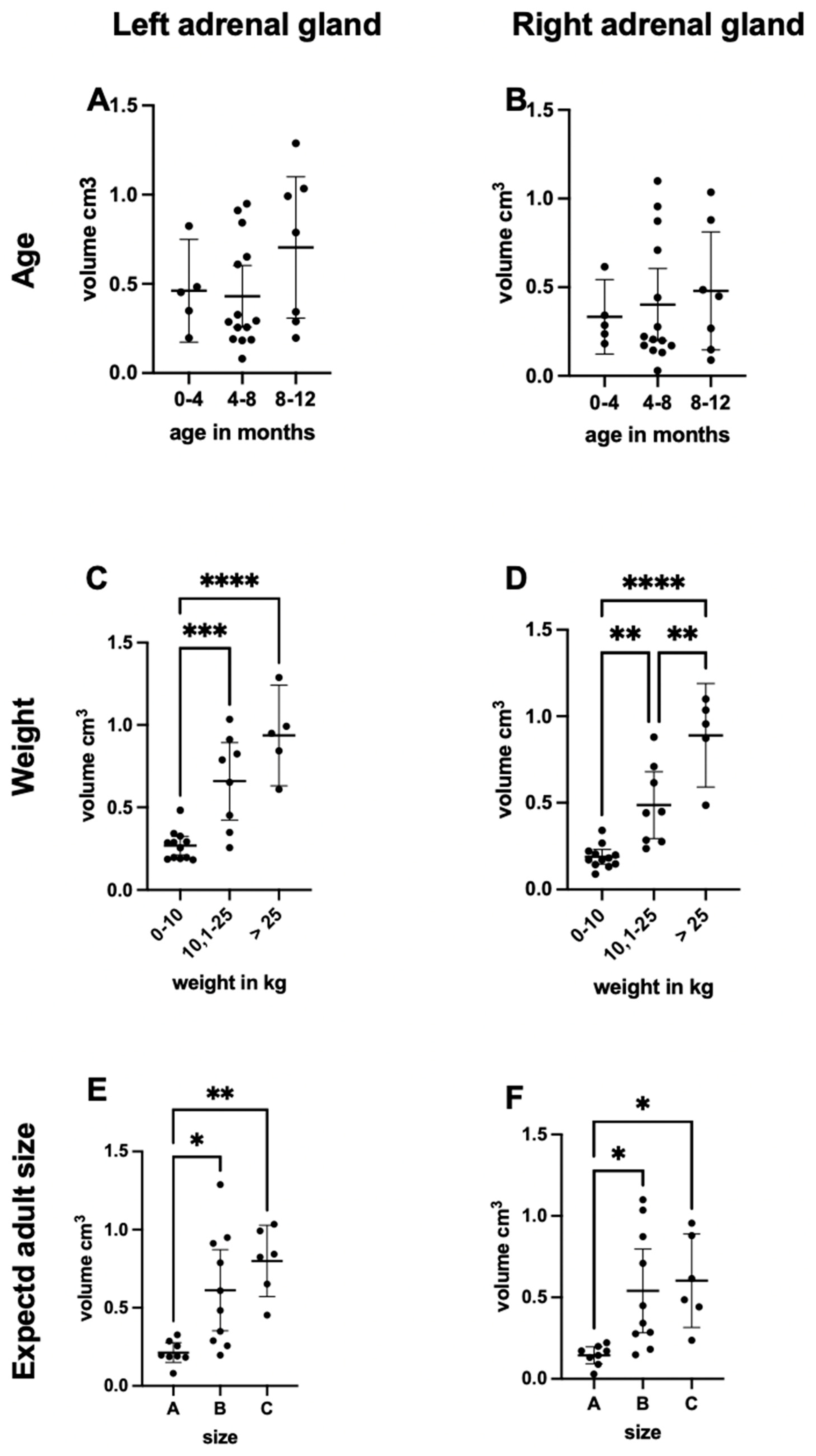
3.2. Groups Based on Age and Current and Adult Weight
3.3. Traditional Two-Dimensional Measurements of Length, Height and Width
3.4. Adrenal Gland Volume Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| abbrevation | meaning |
| ACTH | Adrenocorticotropic Hormone |
| BCS | Body Condition Score |
| CT | Computed Tomography |
| DD | Differential Diagnosis |
| DICOM | Digital Imaging and Communications in Medicine |
| F | Female |
| FCI | Fédération Cynologique Internationale |
| HU | Hounsfield Units |
| i.m. | Intramuscular |
| i.v. | Intravenous |
| KM | Kristina Merhof |
| L1 | left adrenal gland length |
| L2 | left adrenal gland height cranial pole |
| L3 | left adrenal gland height caudal pole |
| L4 | left adrenal gland width cranial pole |
| L5 | left adrenal gland width caudal pole |
| R1 | right adrenal gland length |
| R2 | right adrenal gland height cranial pole |
| R3 | right adrenal gland height caudal pole |
| R4 | right adrenal gland width cranial pole |
| R5 | right adrenal gland width caudal pole |
| M | Male |
| MRI | Magnetic Resonance Imaging |
| PRAA | Persistent Right Aortic Arch |
| p.o. | Per os (oral administration) |
| ROI | Region of Interest |
| SD | Standard Deviation |
| SPEC | Spectral Computed Tomography |
| Th | Thoracic vertebra |
| UKC | United Kennel Club |
| WC | Weight Category |
| OsiriX®MD | medical image processing software developed by Pixmeo SARL |
References
- Cook, A. Canine Hyperadrenocorticism. Todays Veterinary Practice, 7 December 2018. [Google Scholar]
- Guzmán Ramos, P.J.; Bennaim, M.; Shiel, R.E.; Mooney, C.T. Diagnosis of canine spontaneous hypoadrenocorticism. Canine Med. Genet. 2022, 9, 6. [Google Scholar] [CrossRef]
- Schofield, I.; Brodbelt, D.C.; Wilson, A.R.L.; Niessen, S.; Church, D.; O’Neill, D. Survival analysis of 219 dogs with hyperadrenocorticism attending primary care practice in England. Vet. Rec. 2020, 186, 348. [Google Scholar] [CrossRef]
- Pagani, E.; Tursi, M.; Lorenzi, C.; Tarducci, A.; Bruno, B.; Mondino, E.C.B.; Zanatta, R. Ultrasonographic features of adrenal gland lesions in dogs can aid in diagnosis. BMC Vet. Res. 2016, 12, 267. [Google Scholar] [CrossRef]
- Montoya Navarrete, A.L.; Tristán, T.Q.; Santillán, S.L.; Martínez, R.O.; Flores, A.G.V.; Martínez, L.M.; López, M.C.D.L. Effect of age, sex, and body size on the blood biochemistry and physiological constants of dogs from 4 wk. to >52 wk. of age. BMC Vet. Res. 2021, 17, 265. [Google Scholar] [CrossRef]
- Lund, C.; Kuhl, S.; Mischke, R.; Günzel-Apel, A.R. Reference values of the red blood profile in beagle, German shepard and golden retriever puppies. Berl. Munch. Tierarztl. Wochenschr. 2000, 113, 447–453. [Google Scholar]
- Mischke, R.; Lund, C.; Kuhl, S.; Beyerbach, M.; Günzel-Apel, A.R. Referenzen der Leukozytenzahl und des Differentialblutbildes bei Hundesaugwelpen verschiedenen Rassen. Prakt. Tierarzt. 2003, 84, 14–20. [Google Scholar]
- Lathan, P.; Thompson, A. Management of hypoadrenocorticism (Addison’s disease) in dogs. Vet. Med. Res. Rep. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Hupfeld, J.; Dölle, M.; Volk, H.; Rieder, J. A qualitative analysis of the impact of canine hypoadrenocorticism on the quality of life of owners. Vet. Med. 2023, 19, 152. [Google Scholar] [CrossRef] [PubMed]
- Emming, C.; Geks, A.K.; Sajadihezaveh, S.; Rieker, T.; Brutsche, J.; Volk, H.A.; Rieder, J. Comparison of prednisolone and alternative glucocorticoid dosing protocols for canine hypoadrenocorticism: Insights from a survey-based study. Front. Vet. Sci. 2025, 12, 1544750. [Google Scholar] [CrossRef] [PubMed]
- Neelis, D.A.; Mattoon, J.S.; Sellon, R.K.; Clifford, R.B. Adrenal Glands. In Small Animal Diagnostic Ultrasound, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Imbery, C.A.; Dieterle, F.; Ottka, C.; Weber, C.; Schlotterbeck, G.; Müller, E.; Lohi, H.; Giger, U. Metabolomic Abnormalities in Serum from Untreated and Treated Dogs with Hyper-and Hypoadrenocorticism. Metabolites 2022, 12, 339. [Google Scholar] [CrossRef]
- Gold, A.J.; Langlois, D.K.; Refsal, K.R. Evaluation of Basal Serum or Plasma Cortisol Concentrations for the Diagnosis of Hypoadrenocorticism in Dogs. J. Vet. Intern. Med. 2016, 30, 1798–1805. [Google Scholar] [CrossRef]
- Melian, C.; Peterson, M.E. Diagnosis and treatment of naturally occurring hypoadrenocorticism in 42 dogs. J. Small Anim. Pract. 1996, 37, 268–275. [Google Scholar] [CrossRef]
- Topmöller, J.; Merhof, K.; Packeiser, E.; Schmicke, M.; Volk, H.A.; Rieder, J. Prospective Ultrasonographic Evaluation of Adrenal Glands in a Population of Beagle Puppies and Functional Analysis of Basal Cortisol Levels in Blood. Vet. Sci. 2025, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, H.; Yoon, J. Ultrasonographic Adrenal Gland Measurements in Clinically Normal Small Breed Dogs and Comparison with Pituitary-Dependent Hyperadrenocorticism. J. Vet. Med. Sci. 2011, 73, 985–989. [Google Scholar] [CrossRef]
- Bento, P.L.; Center, S.A.; Yeager, A.E.; Bicalho, R. Associations between sex, body weight, age, and Ultrasonographically determined adrenal gland thickness in dogs with non-adrenal gland illness. AVMA J. Am. Vet. Med. Assoc. 2016, 248, 652–660. [Google Scholar] [CrossRef]
- Bertolini, G.; Furlanello, T.; De Lorenzi, D.; Caldin, M. Computed tomographic quantification of canine adrenal gland volume and attenuation. Vet. Radiol. Ultrasound 2006, 47, 444–448. [Google Scholar] [CrossRef]
- Büttelmann, G.; Harder, L.K.; Nolte, I.; Wefstaedt, P. Impact of body weight and sex in selected dog breeds on the canine adrenal gland dimensions measured by computed tomographic imaging. BMC Vet. Res. 2023, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Salonen, H.M.; Åhlberg, T.M.; Laitinen-Vapaavuori, O.M.; Mölsä, S.H. CT measurement of prostate volume using OsiriX® viewer is reliable, repeatable, and not dependent on observer, CT protocol, or contrast enhancement in dogs. Vet. Radiol. Ultrasound 2022, 63, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Hullinger, R.L. Adrenal Cortex of the Dog (Canis familiaris): I. Histomorphologic Changes during Growth, Maturity, and Aging. Anat. Histol. Embryol. 1978, 7, 1–27. [Google Scholar] [CrossRef]
- Soulsby, S.N.; Holland, M.; Hudson, J.A.; Behrend, E.N. Ultrasonographic evaluation of adrenal gland size compared to body weight in normal dogs. Vet. Radiol. Ultrasound 2015, 56, 317–326. [Google Scholar] [CrossRef]
- Beach, F.A. Hormonal Effects on Socio-Sexual Behavior in Dogs. In Mammalian Reproduction; Gibian, H., Plotz, E.J., Eds.; Springer: Heidelberg/Berlin, Germany, 1970. [Google Scholar]
- Melián, C.; Pérez-López, L.; Saavedra, P.; Ravelo-García, A.G.; Santos, Y.; Jaber, J.R. Ultrasound evaluation of adrenal gland size in clinically healthy dogs and in dogs with hyperadrenocorticism. Vet. Rec. 2021, 188, e80. [Google Scholar] [CrossRef]
- Hart, B.L.; Eckstein, R.A. The role of gonadal hormones in the occurrence of objectionable behaviours in dogs and cats. Appl. Anim. Behav. Sci. 1997, 52, 331–344. [Google Scholar] [CrossRef]
- Gallelli, M.F.; Lombardo, D.; Vissio, P.; Quiroga, A.; Caggiano, N.; Soler, E.; Meikle, A.; Castillo, V. Immunohistochemical analysis of the hypothalamic-pituitary-adrenal axis in dogs: Sex-linked and seasonal variation. Res. Vet. Sci. 2016, 104, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wenger, M.; Mueller, C.; Kook, P.H.; Reusch, C.E. Ultrasonographic evaluation of adrenal glands in dogs with primary hypoadrenocorticism or mimicking diseases. Vet. Rec. 2010, 167, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Barthez, P.Y.; Nyland, T.G.; Feldman, E.C. Ultrasonographic evaluation of the adrenal glands in dogs. J. Am. Vet. Med. Assoc. 1995, 207, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, P.; Quadri, S.K. Postnatal differentiation of adrenal cortex in the dog. Anat. Histol. Embryol. 1987, 16, 122–130. [Google Scholar]
- Ilias, I.; Sahdev, A.; Reznek, R.H.; Grossman, A.B.; Pacak, K. The optimal imaging of adrenal tumours: A comparison of different methods. Endocr.-Relat. Cancer 2007, 14, 587–599. [Google Scholar] [CrossRef]
- Askani, E.; Rospleszcz, S.; Lorbeer, R.; Wintergerst, C.; Müller-Peltzer, K.; Kiefer, L.S.; Kellner, E.; Reisert, M.; Rathmann, W.; Peters, A.; et al. Associations between adrenal gland volume and adipose tissue compartments—A whole body MRI study. Nutr. Metab. 2024, 21, 45. [Google Scholar] [CrossRef]
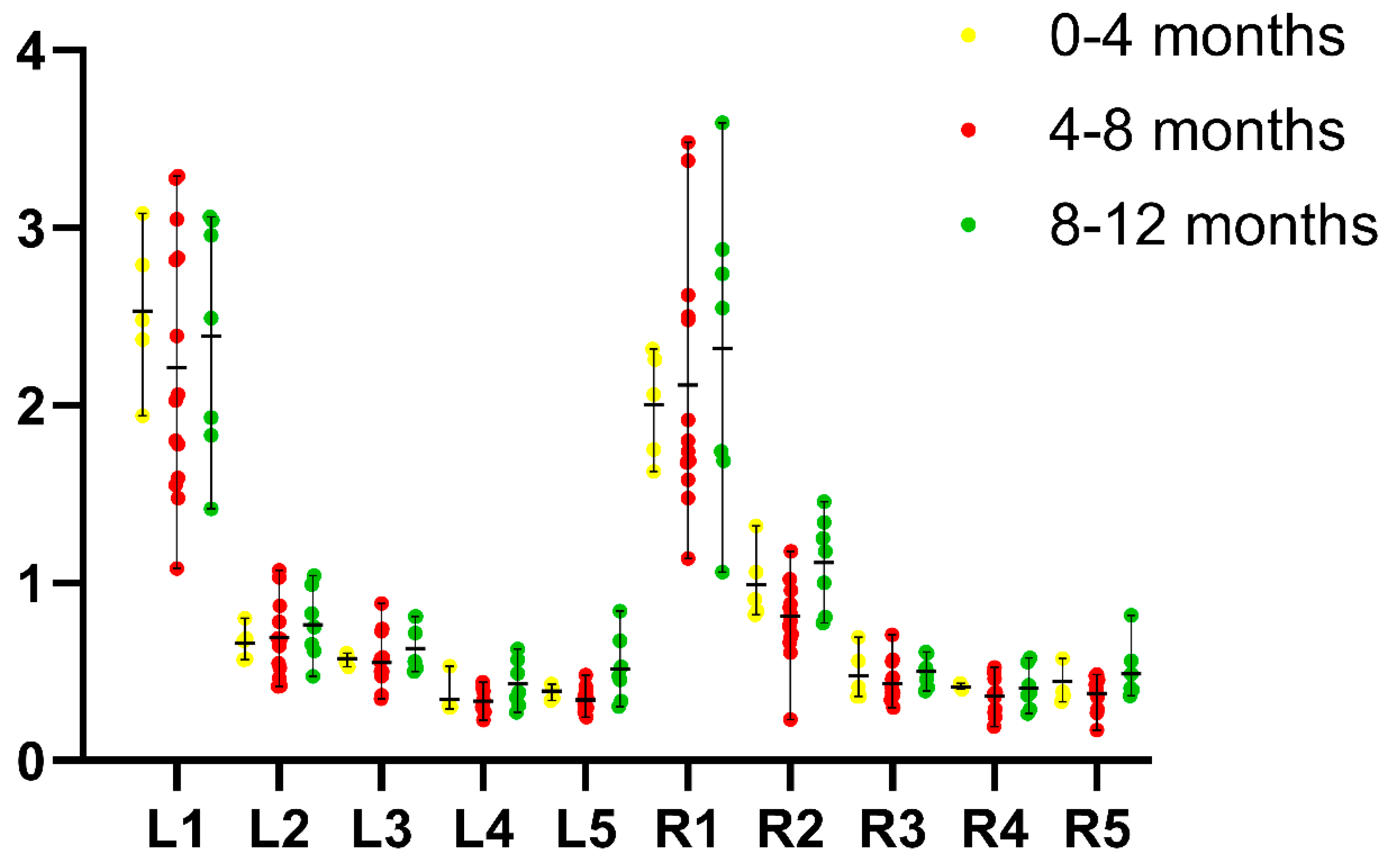
| Measurements | Left (Mean ± SD, cm) | Right (Mean ± SD, cm) |
|---|---|---|
| length | 2.32 (±0.64) | 2.15 (±0.68) |
| Cranial pole height | 0.71 (±0.19) | 0.93 (±0.26) |
| Caudal pole height | 0.58 (±0.13) | 0.49 (±0.11) |
| Cranial pole width | 0.36 (±0.10) | 0.39 (±0.10) |
| Caudal pole width | 0.40 (±0.13) | 0.42 (±0.12) |
| Age (Days) | Breed | Sex | Body Weight (kg) | Adult Weight Category | Left Adrenal Gland Volume (cm3) | Right Adrenal Gland Volume (cm3) |
|---|---|---|---|---|---|---|
| 57 | German Longhaired Pointer | f | 5.0 | B | 0.20 | 0.18 |
| 87 | Rhodesian Ridgeback | m | 13.4 | C | 0.45 | 0.24 |
| 110 | Husky | f | 10.2 | B | 0.35 | 0.29 |
| 118 | Boerboel | m | 24.0 | C | 0.82 | 0.62 |
| 122 | Golden Retriever | m | 9.4 | B | 0.48 | 0.34 |
| 136 | Yorkshire Terrier | f | 2.0 | A | 0.19 | 0.13 |
| 152 | Boxer | m | 16.2 | B | 0.26 | 0.28 |
| 154 | Berger Blanc Suisse | m | 24.4 | C | 0.65 | 0.44 |
| 169 | Chihuahua | m | 1.04 | A | 0.08 | 0.03 |
| 171 | Yorkshire Terrier | m | 2.2 | A | 0.19 | 0.17 |
| 173 | Welsh Corgi | m | 5.8 | A | 0.29 | 0.22 |
| 190 | Irish Setter | m | 15.6 | B | 0.91 | 0.71 |
| 194 | Havanese | f | 2.4 | A | 0.18 | 0.14 |
| 209 | Bordeaux Mastiff | m | 43.0 | C | 0.84 | 0.96 |
| 220 | Miniature Dachshund | m | 4.2 | A | 0.26 | 0.17 |
| 230 | Golden Retriever | m | 31.6 | B | 0.61 | 0.87 |
| 230 | American Bulldog | m | 32.1 | B | 0.95 | 1.10 |
| 246 | German Hunting Terrier | f | 5.8 | A | 0.33 | 0.20 |
| 253 | Galgo | m | 21.0 | C | 1.03 | 0.88 |
| 255 | Old English Bulldog | m | 20.0 | B | 0.79 | 0.45 |
| 272 | Bernese Mountain Dog | m | 37.0 | C | 0.65 | 0.44 |
| 274 | Poodle | m | 6.4 | B | 0.29 | 0.15 |
| 331 | German Wirehaired Pointer | m | 33.6 | B | 1.29 | 1.04 |
| 362 | Chihuahua | f | 1.8 | A | 0.20 | 0.09 |
| 155 | Mixed Breed | f | 6.8 | Not categorized | 0.29 | 0.21 |
| 289 | Mixed Breed | f | 5.2 | Not categorized | 0.34 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topmöller, J.; Rieder, J.; Meller, S.; von Pückler, K.; Volk, H.; Merhof, K. Computed Tomography Volumetric Measurements of Adrenal Glands in 26 Dogs Under One Year of Age: A Retrospective Study. Vet. Sci. 2025, 12, 974. https://doi.org/10.3390/vetsci12100974
Topmöller J, Rieder J, Meller S, von Pückler K, Volk H, Merhof K. Computed Tomography Volumetric Measurements of Adrenal Glands in 26 Dogs Under One Year of Age: A Retrospective Study. Veterinary Sciences. 2025; 12(10):974. https://doi.org/10.3390/vetsci12100974
Chicago/Turabian StyleTopmöller, Julia, Johanna Rieder, Sebastian Meller, Kerstin von Pückler, Holger Volk, and Kristina Merhof. 2025. "Computed Tomography Volumetric Measurements of Adrenal Glands in 26 Dogs Under One Year of Age: A Retrospective Study" Veterinary Sciences 12, no. 10: 974. https://doi.org/10.3390/vetsci12100974
APA StyleTopmöller, J., Rieder, J., Meller, S., von Pückler, K., Volk, H., & Merhof, K. (2025). Computed Tomography Volumetric Measurements of Adrenal Glands in 26 Dogs Under One Year of Age: A Retrospective Study. Veterinary Sciences, 12(10), 974. https://doi.org/10.3390/vetsci12100974






