In Vitro Screening of Antibacterial Efficacy of Moringa oleifera and Thymus vulgaris Methanolic Extracts Against Different Escherichia coli Strains and Their In Vivo Effects Against E. coli-Induced Infection in Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of M. oleifera and T. vulgaris Methanolic Extracts
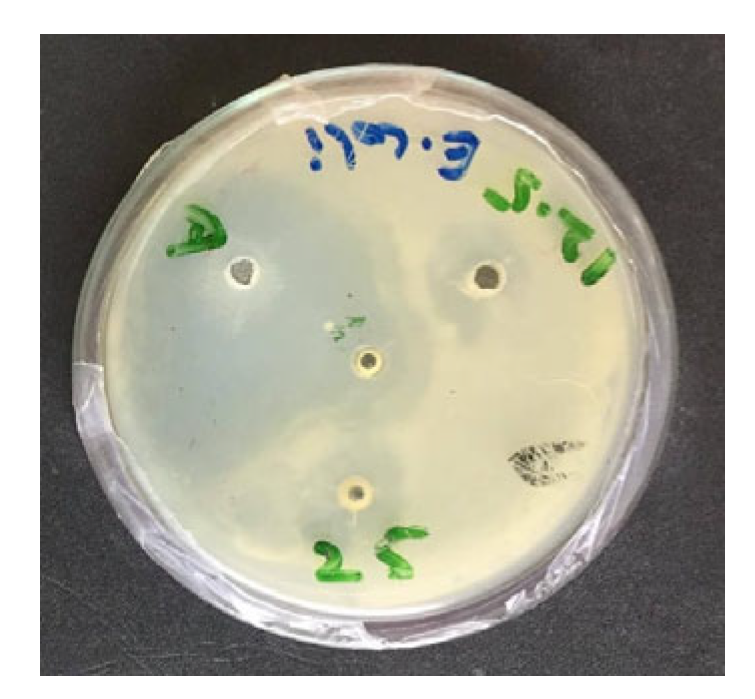
2.2. Antibacterial Screening
2.3. Preparation of E. coli Inoculum and Challenge to Broiler Chickens
2.4. Experimental Design and Treatments Protocol
2.5. Growth Performance and Carcass Characteristics
2.6. Gut pH
2.7. Ileal Microbial Count
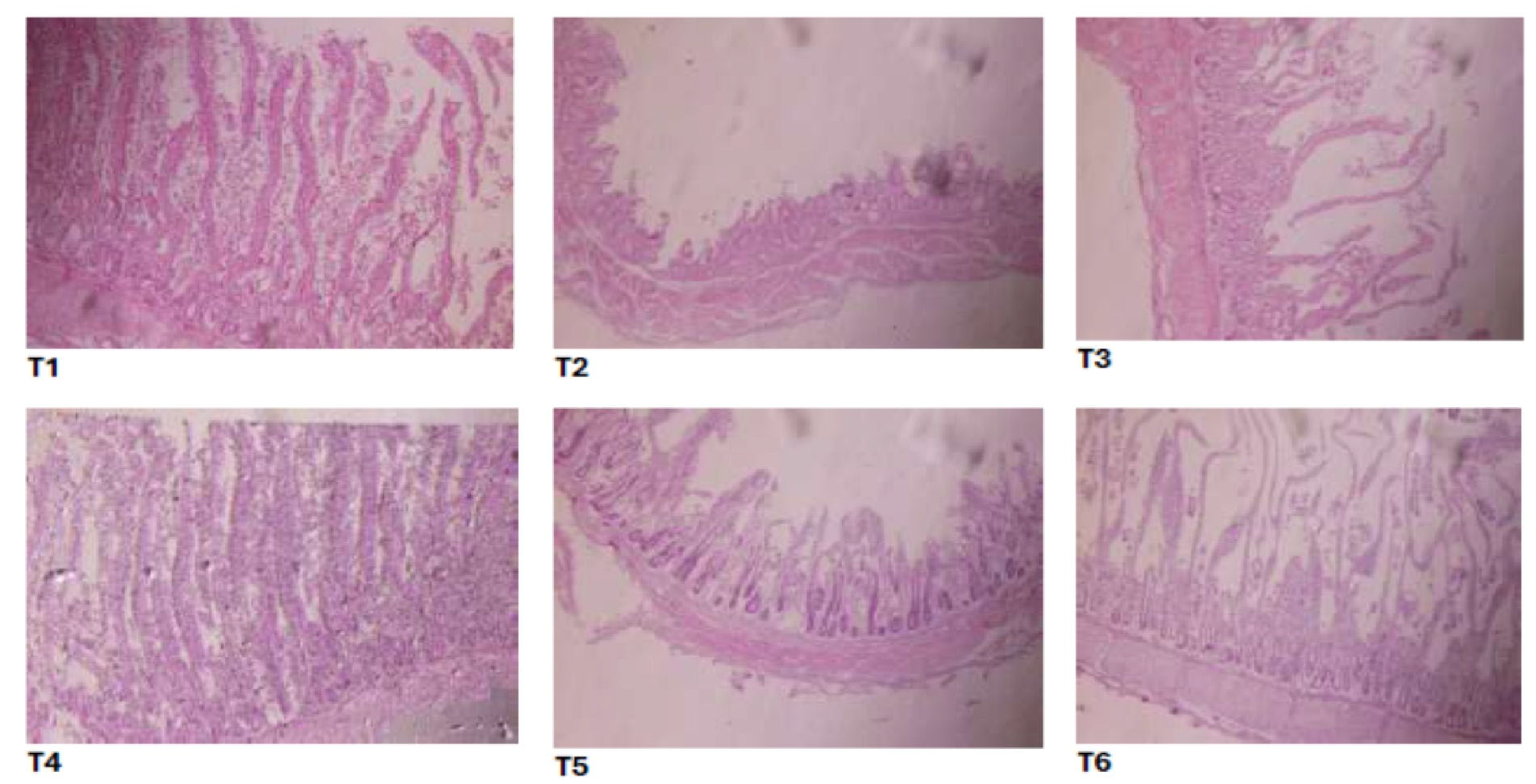
2.8. Intestinal Histomorphology
2.9. Statistical Analysis
3. Results
3.1. In Vitro Antibacterial Activity
3.2. Growth Performance (0–42 Days)
3.3. Carcass Characteristics
3.4. Gastrointestinal pH
3.5. Ileal Microbial Counts
3.6. Intestinal Histomorphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasir, J.; Nasir, A.; Khan, R.U.; Laudadio, V. In vitro screening of antibacterial efficacy of stem waste of oyster mushrooms (Pleurotus ostreatus) against different Escherichia coli strains and in vivo effects against E. coli-induced infection in Japanese quails. Cogent Food Agric. 2024, 10, 2335810. [Google Scholar] [CrossRef]
- Kausar, S.; Chand, N.; Naz, S.; Alhidary, I.A.; Dai, S.; Ayasan, T.; Khan, R.U. In vitro and in vivo effects of methanolic extract of dietary ginger (Zingiber officinale) and onion (Allium cepa) addition on growth performance and fecal microbiota in Escherichia coli-infected broiler chickens. Livest. Sci. 2024, 281, 105416. [Google Scholar] [CrossRef]
- Habib, M.A.; Salma, U.; Amin, M.N.; Rahman, M.G.; Haque, M.A. The effect of halquinol (HAL) on growth performance, carcass traits, and blood-lipid profile in broiler chickens. Int. J. Agric. Biosci. 2024, 13, 39–45. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Parada, J.; Luna María, J.; Corti, M.; Escobar, F.M.; Fernández, C.; Coniglio, M.V.; Ortiz, M.E.; Wittouck, P.; Watson, S.; et al. Impact of probiotic Saccharomyces cerevisiae var. boulardii RC009 alone and in combination with a phytase in broiler chickens fed with antibiotic-free diets. Agrobiol. Rec. 2024, 16, 1–10. [Google Scholar] [CrossRef]
- Yu, H.; Rahman, A.; Sadique, M.A.; Batool, T.; Imtiaz, B.; Zaman, M.A.; Riaz, T.; Anwar, M.Z.; Waqas, M. Impact of Bacillus subtilis probiotic on growth performance, bone health, intestinal morphology, and cecal microbiota in Cobb broiler chicks. Pak. Vet. J. 2024, 44, 1243–1248. [Google Scholar] [CrossRef]
- Ajmal, A.S.; Hussain, Z.; Jalees, M.M.; Shafi, J.; Manzoor, S.; Haq, A.U. Performance of broiler birds on feeding natural anti-stressors in summer during heat stress. Asian J. Agric. Biol. 2023, 2023, 2022024. [Google Scholar] [CrossRef]
- Dinasarki, D.; Tenrisanna, V.; Amrawaty, A.A. Broiler product quality: The global scientific research landscape and implications for marketing performance. Int. J. Agric. Biosci. 2024, 13, 306–312. [Google Scholar] [CrossRef]
- Fathanah, S.Y.E.; Rahardja, D.P.; Purwanti, S. Impacts of probiotic and dietary Lamtoro leaf meal on the growth performance, digestibility and small intestinal morphometry of Kampung chicken. Int. J. Agric. Biosci. 2024, 13, 333–339. [Google Scholar] [CrossRef]
- Ali, M.; Chand, N.; Khan, R.U.; Naz, S.; Gul, S. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 2019, 47, 79–84. [Google Scholar] [CrossRef]
- Dabool, A.S.; Atwah, B.; Alghamdi, S.; Momenah, M.A.; Saleh, O.; Alhazmi, N.; Mostafa, Y.S.; Alamri, S.A.; Alyoubi, W.A.A.; Alshammari, N.M.; et al. Could Paenibacillus xylanexedens MS58 be an eco-friendly antibiotic in poultry production? Impacts on performance, blood biochemistry, gut microbiota and meat quality. Pak. Vet. J. 2024, 44, 352–360. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Ta, N.A.T.; Van, H.G.; Chu, D.T.; Nguyen, T.S.; Can, V.M.; Nguyen, Q.U.; Vinh, H.V. Effects of the multi-strain probiotic preparation LabMix on some immune indices and intestinal microbiota in an antibiotic-associated diarrhea rat model. Asian J. Agric. Biol. 2024, 2024, 2023241. [Google Scholar] [CrossRef]
- Othman, M.F.; Chung, A.Y.K.; Halim, R.M.; Kalil, M.S.; Bakar, N.A.; Aziz, A.A. Valorization and optimization of protein in fermented palm kernel cake: Influence on broiler chicks growth. Int. J. Agric. Biosci. 2024, 13, 744–752. [Google Scholar] [CrossRef]
- Aljohani, A.S.M. Phenolics of botanical origin for the control of coccidiosis in poultry. Pak. Vet. J. 2024, 44, 222–228. [Google Scholar] [CrossRef]
- Malik, S.; Sial, N.; Shahzad, M.I.; Anjum, S.; Javid, A.; Rivera, G. Characterization and identification of bioactive natural products in the ethanol extracts of Acacia nilotica, Melia azedarach, and Euphorbia hirta from Cholistan desert, Pakistan. Asian J. Agric. Biol. 2024, 2024, 2023180. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Hassan, H.M.A. Phytogenic substances as safe growth promoters in poultry nutrition. Int. J. Vet. Sci. 2023, 12, 89–100. [Google Scholar] [CrossRef]
- Nouri, H.S.A.; Ali, S.A.M.; Abdalla, H.O.; Ahmed, H.B. Performance and carcass characteristics of broiler chickens kept on heated soybean meal. Agrobiol. Rec. 2024, 17, 75–82. [Google Scholar] [CrossRef]
- Utari, A.; Warly, L.; Hermon; Suyitman, E. Metabolic response and meat quality of goats fed Artocarpus heterophyllus and Moringa oleifera. Int. J. Vet. Sci. 2023, 12, 498–503. [Google Scholar] [CrossRef]
- Liang-liang, L.; Kuan-hui, L.; Shi-hui, X.; Ting, L.; Guang-shuang, Z.; Almutairi, M.H.; Zahid, M.; Gondal, M.A.; Qudratullah Mehmood, K.; Wu, Y. Immune enhancing activity of garlic polysaccharide, selenizing Codonopsis pilosula compounds in chickens. Asian J. Agric. Biol. 2023, 2023, 2023149. [Google Scholar] [CrossRef]
- Aljohani, A.S.M.; Zaman, M.A. Evaluation of anticoccidial activity of ethanolic extract of clove in broiler chicken. Pak. Vet. J. 2024, 44, 757–762. [Google Scholar] [CrossRef]
- Mohamed, R.G.; Tony, M.A.; Abdelatty, A.M.; Hady, M.M.; Ismail, E.Y. Sweet orange (Citrus sinensis) peel powder with xylanase addition improved growth performance, antioxidant status, and immunity of broiler chickens. Int. J. Vet. Sci. 2023, 12, 175–181. [Google Scholar] [CrossRef]
- Shaukat, N.; Farooq, U.; Akram, K.; Shafi, A.; Hayat, Z.; Naz, A.; Hakim, A.; Hayat, K.; Naseem, S.; Khan, M.Z. Antimicrobial potential of banana peel: A natural preservative to improve food safety. Asian J. Agric. Biol. 2023, 2023, 202003188. [Google Scholar] [CrossRef]
- Ali, M.; Chand, N.; Khan, S.; Ahmad, S.; Tahir, M. Effect of different combinations of methanolic extract of Moringa oleifera and Moringa oleifera on production performance, gut morphology, hematology and nutrient digestibility in broilers. Pak. J. Zool. 2023, 56, 1477–1484. [Google Scholar]
- Bebas, W.; Gorda, I.W.; Agustina, K.K. Spermatozoa quality of Kintamani dogs in coconut water-egg yolk diluent with addition of Moringa leaves and carrot extract. Int. J. Vet. Sci. 2023, 12, 333–340. [Google Scholar] [CrossRef]
- Al-Suwailem, N.K.; Kamel, N.N.; Abbas, A.O.; Nassar, F.S.; Mohamed, H.S.; Gouda, G.F.; Safaa, H.M. The impact of dietary Moringa oleifera leaf addition on stress markers, immune responses, and productivity in heat-stressed broilers. Int. J. Vet. Sci. 2024, 13, 980–987. [Google Scholar] [CrossRef]
- Khan, R.U.; Khan, A.; Naz, S.; Ullah, Q.; Laudadio, V.; Tufarelli, V.; Ragni, M. Potential applications of Moringa oleifera in poultry health and production as alternative to antibiotics: A review. Antibiotics 2021, 10, 1540. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Tahir, M.; Alhidary, I.; Abdelrahman, M.; Swelum, A.A.; Khan, R.U. Role of dietary Moringa oleifera leaf extract on productive parameters, humoral immunity and lipid peroxidation in broiler chicks. Anim. Biotechnol. 2022, 33, 1353–1358. [Google Scholar] [CrossRef]
- Yu, H.; Rahman, A.; Umer, F.; Waqas, M.; Mahmood, M.; Berberoğlu, T.M.; Riaz, T.; Sherzada, S.; Khan, M.; Raza, A.; et al. Effect of supplementing a blend of essential oils on the growth performance, carcass characteristics, meat quality, serological parameters and gut health in broiler chickens. Pak. Vet. J. 2024, 44, 1329–1337. [Google Scholar] [CrossRef]
- Franciosini, M.P.; Casagrande-Proietti, P.; Forte, C.; Beghelli, D.; Acuti, G.; Zanichelli, D.; Moscati, L.; Battistacci, L.; Castellini, C. Effects of oregano (Origanum vulgare L.) and rosemary (Rosmarinus officinalis L.) aqueous extracts on broiler performance, immune function and meat quality traits. Ital. J. Anim. Sci. 2016, 15, 212–219. [Google Scholar]
- Rahimi, S.; Teymouri, Z.Z.; Karimi, T.M.A.; Omidbaigi, R.; Rokni, H. Effect of three herbal extracts on growth performance, immune system, blood factors and intestinal selected bacterial population in broiler chickens. J. Agric. Sci. Technol. 2011, 13, 527–539. [Google Scholar]
- Olayaki, L.A.; Irekpita, J.E.; Yakubu, M.T.; Ojo, O.O. Methanolic extract of Moringa oleifera leaves improves glucose tolerance, glycogen synthesis and lipid metabolism in alloxan-induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 585–593. [Google Scholar] [CrossRef]
- Bennour, N.; Mighri, H.; Eljani, H.; Zammouri, T.; Akrout, A. Effect of solvent evaporation method on phenolic compounds and the antioxidant activity of Moringa oleifera cultivated in Southern Tunisia. S. Afr. J. Bot. 2020, 129, 181–190. [Google Scholar] [CrossRef]
- Mona, M.H.; Rizk, E.S.T.; Nassef, M.; Salama, W.; Younis, M.L. In vitro evaluation of antibacterial activity of total protein extracts of some invertebrates against human pathogenic bacteria. Egypt. J. Exp. Biol. 2018, 14, 137–143. [Google Scholar]
- Jorgensen, J.H.; Hindler, J.F.; Reller, L.B.; Weinstein, M.P. New consensus guidelines from the Clinical and Laboratory Standards Institute for antimicrobial susceptibility testing of infrequently isolated or fastidious bacteria. Clin. Infect. Dis. 2007, 44, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Hindler, J.F.; Stelling, J. Analysis and presentation of cumulative antibiograms: A new consensus guideline from the Clinical and Laboratory Standards Institute. Clin. Infect. Dis. 2007, 44, 867–873. [Google Scholar] [CrossRef]
- Mabelebele, M.; Norris, D.; Brown, D.; Ginindza, M.M.; Ngambi, J.W. Breed and sex differences in the gross anatomy, digesta pH and histomorphology of the gastrointestinal tract of Gallus gallus domesticus. Braz. J. Poult. Sci. 2017, 19, 339–346. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Al-Atiyat, R.M.; Stanley, D.; Aljassim, R.; Albatshan, H.A. The effect of corn distiller’s dried grains with solubles (DDGS) fortified with enzyme on growth performance of broiler. Environ. Sci. Pollut. Res. 2017, 24, 21412–21420. [Google Scholar] [CrossRef]
- Sultana, S.; Riaz, A.; Qureshi, A.S.; Khan, R.U. Effect of dietary addition of Moringa oleifera leaves on growth performance, immune response and antioxidant status in broiler chickens. S. Afr. J. Anim. Sci. 2015, 45, 387–393. [Google Scholar]
- Brenes, A.; Roura, E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed. Sci. Technol. 2010, 158, 1–14. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- McDougald, L.R.; Fitz-Coy, S.H. Coccidiosis. In Diseases of Poultry, 12th ed.; Saif, Y.M., Ed.; Iowa State Press: Ames, IA, USA, 2008; pp. 1068–1085. [Google Scholar]
- Lee, K.W.; Everts, H.; Kappert, H.J.; Frehner, M.; Losa, R.; Beynen, A.C. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poult. Sci. 2003, 44, 450–457. [Google Scholar] [CrossRef]
- Nkukwana, T.T.; Muchenje, V.; Masika, P.J.; Hoffman, L.C.; Dzama, K.; Descalzo, A.M. Fatty acid composition and oxidative stability of breast meat from broilers supplemented with Moringa oleifera leaf meal over a period of refrigeration. Food Chem. 2014, 142, 255–261. [Google Scholar] [CrossRef]
- Gakuya, D.W.; Mbugua, P.N.; Mwaniki, S.M.; Kiama, S.G.; Muchemi, G.M.; Njuguna, A.; Wachira, T. Effect of addition of Moringa oleifera leaf meal in broiler chicken feed. Int. J. Poult. Sci. 2014, 13, 379–384. [Google Scholar] [CrossRef]
- Hernandez, F.; Madrid, J.; Garcia, V.; Orengo, J.; Megias, M.D. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004, 83, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Catala-Gregori, P.; Hernandez, F.; Megias, M.D.; Madrid, J. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J. Appl. Poult. Res. 2008, 16, 555–562. [Google Scholar] [CrossRef]
- Cross, D.E.; Acamovic, T.; Deans, S.G.; MacKie, C.A. The effects of dietary inclusion of herbs and their volatile oils on the performance of chickens. Br. Poult. Sci. 2007, 48, 496–506. [Google Scholar] [CrossRef]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Jamroz, D.; Wiliczkiewicz, A.; Wertelecki, T.; Orda, J.; Skorupinska, J. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 2005, 46, 485–493. [Google Scholar] [CrossRef]
- Saki, A.A.; Harcini, R.N.; Rahmatnejad, E.; Salary, J. Herbal additives and organic acids as antibiotic alternatives in broiler chickens diet for organic production. Afr. J. Biotechnol. 2014, 11, 2139–2145. [Google Scholar]
- Abu Hafsa, S.H.; Ibrahim, S.A.; Hassan, A.A. Effect of dietary addition with Moringa oleifera leaves and/or probiotics on performance, carcass traits and blood chemistry of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2016, 100, 908–916. [Google Scholar]
- Kalantar, M.; Ghasemi, H.A.; Taherpour, K.; Jafarnejad, S. Effects of thyme and cinnamon essential oils on growth performance, carcass traits and blood parameters of broilers. Ann. Biol. Res. 2011, 2, 379–385. [Google Scholar]
- Pournazari, M.; Qotbi, A.A.A.; Seidavi, A.; Corazzin, M.; Sadeghi, A.A.; Laudadio, V. Effects of different levels of thyme (Moringa oleifera) powder on carcass traits, meat quality and blood parameters of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1065–1074. [Google Scholar]
| Ingredients (%) | Starter Phase (1–21 Day) | Finisher Phase (22–42) Day |
|---|---|---|
| Corn gluten meal | 1.98 | 7.14 |
| Soybean meal | 37.6 | 24.0 |
| Corn | 54.1 | 60.2 |
| Corn oil | 2.18 | 2.70 |
| Dicalcium phosphate | 2.35 | 2.10 |
| Limestone | 0.81 | 0.67 |
| Dicalcium phosphate | 2.35 | 2.10 |
| VM mix a | 0.50 | 0.50 |
| Salt | 0.50 | 0.55 |
| Lysine HCL | 0.21 | 0.40 |
| Threonine | 0.12 | 0.10 |
| DL-Methionine | 0.22 | 0.12 |
| Choline chloride | 0.06 | 0.06 |
| Chemical composition | ||
| Crude protein (%) | 23.0 | 21.0 |
| ME (Kcal/kg) | 3000 | 3150 |
| Methionine (%) | 0.54 | 0.44 |
| Sulphur amino acid (%) | 0.9 | 0.78 |
| Lysine (%) | 1.40 | 1.22 |
| Calcium (%) | 1.06 | 0.90 |
| Phosphorus (%) | 0.50 | 0.46 |
| Threonine (%) | 0.95 | 0.88 |
| Group | Combination/Concentration | E. coli O78 Mean Zone of Inhibition (mm) ± SEM | E. coli O126 Mean Zone of Inhibition (mm) ± SEM | E. coli O157 Mean Zone of Inhibition (mm) ± SEM | Means (Combination) |
|---|---|---|---|---|---|
| Standard antibiotics | Enrofloxacin (20 µg/disc) | 17.47 ± 0.12 b | 14.39 ± 0.34 b | 16.65 ± 0.11 b | 16.17 |
| Ciprofloxacin (20 µg/disc) | 22.51 ± 0.34 a | 20.36 ± 0.88 a | 22.05 ± 0.35 a | 21.64 | |
| Tetracycline (20 µg/disc) | 18.45 ± 0.65 b | 17.93 ± 0.91 b | 19.79 ± 0.66 b | 18.72 | |
| p-values | 0.98 | 0.11 | 0.43 | 0.44 | |
| M100 | M. oleifera (200 mg/mL) | 9.40 ± 0.41 c | 1.44 ± 0.14 e | 4.15 ± 0.05 b | 5.00 ± 0.35 c |
| T100 | T. vulgaris (300 mg/mL) | 10.15 ± 0.18 b | 6.70 ± 0.04 b | 3.50 ± 0.11 c | 6.78 ± 0.29 b |
| M50T50 | M. oleifera (100 mg/mL) + T. vulgaris (150 mg/mL) | 13.70 ± 0.10 a | 8.50 ± 0.16 a | 5.15 ± 0.03 a | 9.12 ± 0.37 a |
| M75T25 | M. oleifera (150 mg/mL) + T. vulgaris (75 mg/mL) | 9.55 ± 0.07 c | 2.30 ± 0.04 d | 2.45 ± 0.05 d | 4.77 ± 0.35 d |
| M25T75 | M. oleifera (50 mg/mL) + T. vulgaris (225 mg/mL) | 7.50 ± 0.04 d | 2.75 ± 0.09 c | 1.75 ± 0.05 e | 4.00 ± 0.27 e |
| Mean (E. coli) | – | 10.06 ± 0.21 a | 4.34 ± 0.29 b | 3.40 ± 0.12 c | – |
| p-values | – | 0.025 | 0.003 | 0.015 | – |
| Groups | Feed Intake (g) | Weight Gain (g) | Feed Conversion Ratio | Livability (%) | Broiler Performance Efficiency Factor | Broiler Farm Economy Index |
|---|---|---|---|---|---|---|
| −Ve cont. (uninfected, untreated negative control) | 3613.37 a ± 1.7 | 2174.94 a ± 5.56 | 1.66 ± 0.02 c | 100 ± 0.00 | 131 ± 4.26 a | 3.11 ± 0.10 a |
| +Ve cont. (infected, untreated positive control) | 3270.96 d ± 4.2 | 1623.61 d ± 3.7 | 2.01 ± 0.04 a | 73.99 ± 10.18 | 80.77 ± 4.01 c | 1.23 ± 0.19 d |
| Standard | 3549.36 b ± 1.41 | 2025.01 b ± 3.6 | 1.75 ± 0.05 bc | 88.88 ± 2.22 | 115.71 ± 0.57 b | 2.44 ± 0.08 b |
| M100T150 | 3515.37 b ± 2.38 | 2053.64 b ± 12.9 | 1.69 ± 0.02 c | 88.88 ± 5.87 | 121.51 ± 2.69 a | 2.57 ± 0.18 b |
| M50T75 | 3450.36 c ± 2.5 | 1968.64 bc ± 7.2 | 1.74 ± 2.90 bc | 75.55 ± 11.75 | 113.14 ± 0.24 b | 2.03 ± 0.32 c |
| M150T225 | 3420.99 c ± 1.9 | 1887.34 c ± 1.5 | 1.81 ± 0.01 b | 82.22 ± 11.75 | 104.27 b ± 2.02 | 2.04 c ± 0.49 |
| p value | 0.001 | 0.001 | 0.001 | 0.0740 | 0.001 | 0.0025 |
| Groups | Dressing (%) | Thigh (%) | Breast (%) | Wing (%) |
|---|---|---|---|---|
| Negative control | 68.94 a ± 0.14 | 19.17 ± 0.46 a | 32.38 ± 0.23 a | 10.28 ± 0.95 |
| Positive control | 61.39 c ± 0.26 | 14.58 ± 0.21 d | 27.17 ± 0.10 c | 7.76 ± 1.41 |
| Standard | 65.15 b ± 0.13 | 16.96 ± 0.01 bc | 30.11 ± 0.37 b | 8.37 ± 0.57 |
| M100T150 | 65.52 b ± 0.72 | 17.51 ± 0.05 b | 30.50 ± 0.29 b | 8.95 ± 1.32 |
| M50T75 | 65.88 b ± 0.32 | 17.48 ± 0.58 b | 30.34 ± 0.45 b | 8.30 ± 0.89 |
| M150T225 | 63.81 bc ± 0.42 | 15.67 ± 0.18 c | 28.18 ± 0.18 bc | 8.43 ± 0.77 |
| p value | 0.003 | 0.0015 | 0.0045 | 0.72 |
| Groups | Duodenum | Jejunum | Ileum | Crop | Gizzard |
|---|---|---|---|---|---|
| Negative control | 6.21 a ± 0.13 | 7.12 ± 0.22 a | 7.47 ± 0.04 a | 5.63 ± 0.20 | 3.65 ± 0.20 |
| Positive control | 6.17 a ± 0.08 | 7.01 ± 0.15 a | 7.43 ± 0.04 a | 5.49 ± 0.26 | 3.61 ± 0.23 |
| Standard | 5.96 b ± 0.08 | 6.42 ± 0.29 b | 7.17 ± 0.35 bc | 5.69 ± 0.41 | 3.90 ± 0.14 |
| M100T150 | 5.01 c ± 0.19 | 5.09 ± 0.08 c | 7.05 ± 0.08 c | 5.34 ± 0.37 | 3.95 ± 0.10 |
| M50T75 | 5.79 b ± 0.24 | 6.41 ± 0.21 b | 7.31 ± 0.06 b | 5.79 ± 0.43 | 4.06 ± 0.40 |
| M150T225 | 5.86 b ± 0.14 | 6.08 b ± 0.31 | 7.27 b ± 0.04 | 5.67 ± 0.17 | 3.91 ± 0.04 |
| p value | 0.001 | 0.001 | 0.001 | 0.93 | 0.66 |
| Groups | E. coli (log cfu/g) | Lactobacillus (log cfu/g) |
|---|---|---|
| Negative control | 3.20 ± 0.02 d | 4.84 ± 0.06 b |
| Positive control | 8.67 ± 0.24 a | 4.28 ± 0.14 c |
| Standard | 5.17 ± 0.11 c | 4.74 ± 0.05 b |
| M100T150 | 5.57 ± 0.23 c | 5.72 ± 0.14 a |
| M50T75 | 6.21 ± 0.30 b | 5.48 ± 0.11 a |
| M150T225 | 6.65 ± 0.17 b | 4.88 ± 0.06 b |
| p value | 0.003 | 0.0004 |
| Group | Villus Height (µm) | Villus Width (µm) | Crypt Depth (µm) | Villus Height to Crypt Depth Ratio | Villus Surface Area (µm2) |
|---|---|---|---|---|---|
| Negative control | 830 ± 4.08 a | 81 ± 0.30 a | 71 ± 0.53 c | 11.69 ± 0.34 | 211 ± 0.2 a |
| Positive control | 182 ± 1.21 d | 31 ± 0.32 d | 176 ± 1.23 a | 1.03 ± 0.18 | 17.71 ± 0.34 d |
| Standard | 728 ± 2.66 b | 67 ± 0.17 b | 91 ± 2.22 bc | 8.00 ± 0.26 | 153.15 ± 2.32 b |
| M100T150 | 713 ± 1.31 b | 61 ± 0.11 b | 103 ± 2.78 b | 6.92 ± 0.43 | 136.56 ± 1.40 b |
| M50T75 | 607 ± 0.41 c | 43 ± 0.22 c | 151 ± 2.22 ab | 4.01 ± 0.08 | 85.39 ± 0.12 c |
| M150T225 | 612 ± 1.50 c | 49 ± 0.33 c | 147 ± 2.78 ab | 4.16 ± 0.17 | 94.16 ± 0.56 c |
| p value | 0.0040 | 0.0455 | 0.0035 | 0.318 | 0.0225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.; Chand, N.; Khan, S.; Khan, R.U.; Maqbool, B.; Naz, S.; Abudabos, A.; Hafeez, A.; Alhidary, I.A. In Vitro Screening of Antibacterial Efficacy of Moringa oleifera and Thymus vulgaris Methanolic Extracts Against Different Escherichia coli Strains and Their In Vivo Effects Against E. coli-Induced Infection in Broiler Chickens. Vet. Sci. 2025, 12, 957. https://doi.org/10.3390/vetsci12100957
Ali M, Chand N, Khan S, Khan RU, Maqbool B, Naz S, Abudabos A, Hafeez A, Alhidary IA. In Vitro Screening of Antibacterial Efficacy of Moringa oleifera and Thymus vulgaris Methanolic Extracts Against Different Escherichia coli Strains and Their In Vivo Effects Against E. coli-Induced Infection in Broiler Chickens. Veterinary Sciences. 2025; 12(10):957. https://doi.org/10.3390/vetsci12100957
Chicago/Turabian StyleAli, Majid, Naila Chand, Sarzamin Khan, Rifat Ullah Khan, Babar Maqbool, Shabana Naz, Ala Abudabos, Abdul Hafeez, and Ibrahim A. Alhidary. 2025. "In Vitro Screening of Antibacterial Efficacy of Moringa oleifera and Thymus vulgaris Methanolic Extracts Against Different Escherichia coli Strains and Their In Vivo Effects Against E. coli-Induced Infection in Broiler Chickens" Veterinary Sciences 12, no. 10: 957. https://doi.org/10.3390/vetsci12100957
APA StyleAli, M., Chand, N., Khan, S., Khan, R. U., Maqbool, B., Naz, S., Abudabos, A., Hafeez, A., & Alhidary, I. A. (2025). In Vitro Screening of Antibacterial Efficacy of Moringa oleifera and Thymus vulgaris Methanolic Extracts Against Different Escherichia coli Strains and Their In Vivo Effects Against E. coli-Induced Infection in Broiler Chickens. Veterinary Sciences, 12(10), 957. https://doi.org/10.3390/vetsci12100957











