Simple Summary
The Tibetan pig is a unique breed native to the Qinghai–Tibet Plateau, which has adapted to the grazing system of high-altitude pastures and exhibits remarkable tolerance to crude fiber. However, with the transition to large-scale intensive farming, their diets have been optimized for short-term growth efficiency. This nutritional composition conflicts with the Tibetan pig’s evolved adaptation to high-fiber intake. Despite possessing high fiber tolerance, the mechanisms by which enzymes and bacteria synergistically enable efficient utilization of coarse fiber in the Tibetan pig gut remain unclear. This study examined the effect of high-fiber diets on the proliferation of fecal fiber-degrading bacteria, activity of fiber-digesting enzymes, and production of short-chain fatty acids in Tibetan pigs during their growth phase.
Abstract
The systematic analysis of the synergistic mechanism between microbial fiber-degrading enzymes and short-chain fatty acids under high-fiber diet conditions is limited. In this study, we evaluated the effects of a high-fiber diet on the growth performance, nutrient digestibility, blood and serum metrics, cellulase/hemicellulase activity, and fecal microbial composition of growing Tibetan pigs. Forty Tibetan pigs were allocated to a control group (CON, the diet contains 5% crude fiber) or a high-fiber group (HF, the diet contains 10% crude fiber) based on crude fiber levels as a blocking factor. The pre-trial period was 7 d, and the formal trial lasted 28 d. CON group and HF group showed no effect on growth performance and nutrient apparent digestibility (p > 0.05). The HF group showed significantly higher fecal cellulase and hemicellulase activities than those of the CON group (p < 0.05). Additionally, the HF group showed significantly elevated levels of acetic, propionic, and butyric acids, as well as increased relative abundances of Fibrobacter and p-75-a5 in the feces (p < 0.05). The correlation analysis revealed that Fibrobacter exhibited significant positive correlations with acetic acid, butyric acid, cellulase, and hemicellulase, whereas p-75-a5 was significantly positively correlated with hemicellulase (p < 0.05). In conclusion, this study provides strong evidence that the efficient utilization of dietary fiber by Tibetan pigs results from highly specialized microbial mechanisms in their large intestine, as reflected by their fecal microbiota composition. Fibrobacter and p-75-a5 play a crucial role in enabling these pigs to utilize fiber effectively. Certain specific microbiota secrete a greater quantity of enzymes to facilitate the decomposition of dietary fiber, and this process ultimately leads to the generation of more metabolites.
1. Introduction
Tibetan pigs have undergone millennia of natural selection across various terrains of the Tibetan Plateau, leading to their exceptional adaptation to harsh environmental conditions [1]. As a distinct genetic resource in this region, they exhibit specialized physiological mechanisms that enable them to thrive in high-altitude, low-oxygen environments [2]. Tibetan pigs exhibit robust fiber digestion capabilities [3], providing a crucial physiological foundation for their adaptation to various diets and the effective utilization of crude fiber [4,5]. The focus of animal husbandry research has recently shifted towards improving nutrient utilization efficiency in stall-feeding systems [6]. An important consideration in this domain is the optimization of crude fiber levels to ensure the healthy growth of Tibetan pigs [7]. With concentrated feed prices, raw materials with high crude fiber are relatively inexpensive, and increasing the proportion of crude fiber in diets can effectively reduce breeding costs [8]. Thus, optimizing the dietary fiber levels is essential for balancing the healthy growth of Tibetan pigs with cost control.
Fiber, a prolific and globally distributed renewable resource, forms the primary structure of plant cell walls and is mainly composed of non-starch polysaccharides and lignin [9,10]. It is an effective alternative to growth promoters and positively influences the growth and health of monogastric animals [11]. Fermented fiber feed enhances growth by increasing appetite and feed intake, thereby contributing to the widespread use of fiber-rich materials in pig diets [12,13]. Adding dietary fiber to Tibetan pig feed not only matches the high-fiber dietary characteristics of their original habitat, but also improves feed conversion efficiency and average daily gain by shaping a unique intestinal microbiota to help Tibetan pigs enhance their buffering adaptability to high-fiber diets, and it also increases the satiety of Tibetan pigs while enabling the efficient and stable degradation of fiber by their microorganisms to maintain a certain fiber digestibility [14,15]. Additionally, dietary fiber regulates appetite, improves blood glucose and lipid responses, and modulates plasma cholesterol by restricting bile salt absorption, enhancing digestive function, and optimizing nutrient digestion and absorption [16,17]. Thus, dietary fiber is vital for Tibetan pig nutrition.
The gastrointestinal tract of animals hosts trillions of microorganisms which are crucial for host health and perform essential metabolic, immune, and protective functions [18]. Gut health can be promoted by dietary fiber through modulating the composition and metabolism of the gut microbiota, stimulating mucus production, enhancing gut motility, and maintaining gut barrier integrity [19]. Intestinal microbiota are crucial for the digestive system of Tibetan pigs because their intestinal tract contains a diverse community of microorganisms, including Fibrobacter, Alloprevotella, and Succinivibrio. These bacteria efficiently degrade cellulose, hemicellulose, and other intricate polysaccharides, thereby enhancing the digestion and assimilation of high-fiber diets in Tibetan pigs [20]. Intestinal microorganisms are involved in the synthesis of short-chain fatty acids (SCFAs), multivitamins, and amino acids, thereby providing supplementary energy sources for Tibetan pigs and enhancing their resilience to the harsh plateau conditions [21]. The gut microbiota produce SCFAs through the fermentation of dietary fiber, which are an energy source for the colonic epithelium and also lower luminal pH, thereby inhibiting the overgrowth of pathogenic bacteria [22,23]. SCFAs are important regulators of proliferation, immunity, and metabolism in pigs [24,25,26,27]. Consequently, a fiber-rich diet positively affects the gut microbiota, as well as the host’s metabolic, immune, and gastrointestinal functions [28,29]. However, current studies have two main limitations. First, there has been a predominant focus on describing the microbial bacteria’s composition, with limited systematic analysis of the synergistic mechanism between microbial fiber-degrading enzymes and SCFAs under high-fiber diet conditions. Additionally, the pathways through which core functional bacteria, such as Fibrobacter, regulate metabolites via enzyme activity remain insufficiently understood. Second, existing studies on the adaptation of Tibetan pigs to high-fiber diets during the growth stage have primarily concentrated on individual indices, such as microbial composition or digestibility, without establishing a comprehensive regulatory chain comprising dietary fiber levels, changes in microbial communities, enzyme activities, and SCFA production. These limitations affect the elucidation of efficient adaptation mechanisms of Tibetan pigs to high-fiber diets in plateau environments. Addressing these gaps provides opportunities for further exploration.
We hypothesized that increasing the crude fiber content in the diet of maturing Tibetan pigs could enhance the production of short-chain fatty acids by facilitating the proliferation of intestinal fiber-degrading bacterial genera and increasing the activity of fiber-digesting enzymes. This process would establish a synergistic relationship among bacteria, enzymes, and metabolites, facilitating the adaptation of Tibetan pigs to a high-fiber diet. Two diet groups were established: one with the diet contains 5% crude fiber (CON) and the other with the diet contains 10% crude fiber (HF). This study investigated the effects of feeding high fiber diet on growth performance, nutrient digestibility, blood parameters, fecal microbiota, enzyme activity and metabolites in Tibetan pigs.
2. Materials and Methods
The procedures used in this study were approved by the Institutional Animal Care and Use Committee of Xizang Agricultural and Animal Husbandry University (Approval No.: XZA-2023-003). The corn, lignocellulose, and soybean hulls in the dietary fiber were sourced from Linzhi Bajian Tibetan Pig Industry Feed Processing and Production Co., Ltd., Xizang Autonomous Region, China.
2.1. Experimental Design, Diet, and Animal Housing
Forty Tibetan pigs (average body weight 21.52 ± 3.45 kg) at 120 d of age were randomly divided into two groups, each set comprised 20 repetitions, with an equal number of males and females, and each repetition contained one pig: (1) a control group (CON, the diet contains 5% crude fiber) and (2) a high-fiber group (HF, the diet contains 10% crude fiber). The experimental period lasted for 28 days following a pre-experimental period of 7 days. According to the nutritional requirements of GB/T39235-2020 standard and with reference to the “Chinese Fat-Type Growth-Finishing Pig Feeding Standards” [30,31]. Two diets were fed with equal energy and nitrogen but with different fiber levels (Table 1). The test pigs were kept in separate pens (1.5 m × 2.0 m). Each pen was equipped with one stainless steel trough feeder placed on the left side and one automatic nipple drinker on the right side, feed and water were provided ad libitum. The pen floor was made of reinforced concrete, and covered with a 5 cm thick layer of clean rice husk bedding (replaced every 3 days) to maintain dryness and comfort. The rearing environment was controlled at a temperature of 16–21 °C and humidity of 55–65%. During the experimental period, the pens were cleaned daily to remove feces and leftover feed. Breeders conducted daily health monitoring, recording indicators such as feed intake, fecal consistency, and mental state; any pigs with abnormal symptoms were immediately isolated and examined.

Table 1.
Dietary compositions and chemical component contents (air dry matter basis, %).
2.2. Growth Performance Evaluation
Individual body weight (BW) and feed intake of pigs were monitored every 2 weeks, and average daily gain (ADG), average daily feed intake (ADFI), and feed-to-weight ratio (F:G) were calculated. The average daily feed intake (g/d) was calculated as follows: (total feed weight during the trial period–remaining feed weight during the trial period)/number of days in the trial. The average daily weight gain (g/d) was calculated as follows: (final body weight–initial body weight)/number of days in the trial. The feed-to-weight ratio was determined by dividing the average daily feed intake by the average daily weight gain [32].
2.3. Sample Collection
After the 28-day experiment, following a 12 h fast, 20 pigs (10 female and 10 male per treatment) were selected per group, and blood samples were collected from jugular veins. The blood was divided into two portions: One portion was collected in a procoagulant vacuum tube and centrifuged at 3000 rpm for 15 min to collect the serum, which was then transferred to 1.5 mL centrifuge tubes for multiple serum indicator testing. The remaining portion was collected directly into ethylenediaminetetraacetic acid dipotassium salt (EDTA-K2) anticoagulant tubes. Both samples were stored in –20 °C freezers. Fecal samples were collected between days 21 and 24, with a total of four collections. When the pig was defecating or about to defecate, rectal stimulation was performed. One person promptly collected the feces using a self-sealing plastic bag to prevent contact with the ground, while another person marked the pig with spray lacquer. This fecal collection method allowed for clear tracing of fecal samples to their corresponding pig IDs and ensured proper organization of each pig’s fecal samples. Prior to sampling, tweezers, 2 mL cryopreservation tubes, and gloves used for microbial sample collection were autoclaved and dried to ensure sterility. Uncontaminated fecal samples from the central portion were collected into sterile tubes: portions were stored at −80 °C for 16S rRNA sequencing, fiber-degrading enzyme activity determination, and SCFA analysis, while the remaining feces were stored at −20 °C for chemical analysis.
2.4. Apparent Total Tract Nutrient Digestibility Analysis
The feed and fecal samples used for nutrient digestibility analysis in this section were collected and processed in chapter 2.3, and were subsequently dried, ground, and sieved through a 1 mm sieve. Dry matter (DM) was analyzed using Method 934.01, crude protein (CP) using Method 976.05, ether extract (EE) using Method 922.06, and crude fiber (CF) was determined, all following the guidelines of the Association of Official Analytical Chemists (AOAC, 2007). Acid-insoluble ash (AIA) was used as an marker (indigestible and unabsorbed in the gastrointestinal tract) to calculate digestibility. Its content in both feed and fecal samples was determined with reference to AOAC Official Method 942.05 [33]. Digestibility was determined using the following formula: apparent total tract digestibility (ATTD) of nutrients = [1 − (acid-insoluble ash content in feed/acid-insoluble ash content in feces) × (nutrient content in feces/nutrient content in feed)] × 100%.
2.5. Blood Parameter Analysis
Blood samples for hematological examination were collected into tubes containing EDTA-K2 to prevent coagulation. After gentle inversion to ensure homogeneity, the samples were analyzed immediately following the operational procedures outlined in the automated hematology analyzer’s instruction manual to avoid cellular degradation. All hematological parameters were measured using an automated hematology analyzer (Dymind-DH71CRP, Shenzhen, China). The parameters assessed included white blood cells (WBC), red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT), platelets (PLT), and procalcitonin (PCT) levels; calculated parameters, including mean corpuscular volume (MCV), mean corpuscular hemoglobin content (MCH), mean corpuscular hemoglobin concentration (MCHC), mean platelet volume (MPV), platelet distribution width (PDW), and red blood cell distribution width (RDW), were all derived from the above directly measured values or cell volume distribution data detected by the analyzer. All hematological analyses were performed within two hours of sample collection to prevent artifacts and ensure data accuracy.
2.6. Serum Parameter Analysis
Serum parameters were analyzed using a biochemical analyzer (Toshiba-BS120, Beijing, China) with kits produced by Suzhou Keming Biotechnology Co., Ltd. (Suzhou, China); the specific operating procedures were carried out with reference to the kit instructions, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), globulin (GLO), serum alkaline phosphatase (ALP), urea (UREA), creatinine (CREA), total cholesterol (TC), and blood glucose (GLU); The AST/ALT ratio and albumin/globulin ratio (A/G) were calculated from the measured values.
2.7. Enzyme Activity Analysis
Cellulase and hemicellulase activities in fecal supernatants of experimental animals were measured using porcine. Cellulase and hemicellulase ELISA kits (Jiangsu Enzyme Exemption Industry Co., Ltd., Nanjing, China) following manufacturer’s instructions. Cellulase standards were diluted to 32, 16, 8, 4, 2 IU/L by adding 15 μL original standard to 150 μL diluent. Blank, standard, and sample wells were set: 50 μL standard per standard well; 40 μL diluent + 10 μL sample (5-fold dilution) per sample well. Samples were added to well bottoms and mixed gently. Plates were sealed and incubated at 37 °C for 30 min. After 5 washes (30-fold diluted washing solution, 30 s each), 50 μL enzyme reagent (except blanks) was added, followed by incubation and washing. Then 50 μL chromogens A and B were added, incubated at 37 °C in dark for 10 min. Reaction was terminated with 50 μL stop solution (blue to yellow). OD values were measured using a microplate reader (Thermo VARIOSKAN LUX, Shanghai Yetuo Technology Co., Ltd., Shanghai, China) at 450 nm within 15 min, using blank well as reference.
2.8. Analysis of Fecal Short-Chain Fatty Acids
Each fecal sample (0.2 g) was mixed with 0.8 mL of 0.1 mol/L hydrochloric acid and thoroughly vortexed for 1 min to ensure complete homogenization, then incubated on ice for 30 min. The mixture was centrifuged at 12,000 rpm for 15 min in a Centrifuge 5804 (Eppendorf, Hamburg, Germany). The supernatant was filtered through a 0.22 μm membrane and analyzed for SCFA content using a gas chromatograph (SCION 456i, Shanghai, China).
2.9. Fecal Microbiota Diversity Analysis
Fecal samples were collected from groups consisting of ten repetitions (one pig per repetition), including 5 males and 5 females, to balance gender and avoid gender-related biases. were analyzed for fecal microbiota diversity at Shanghai Personalbio Biotechnology Co., Ltd. The primer sequences were as follows: forward primer, F: ACTCCTACGGGAGGCAGCA; reverse primer, R: GGACTACHVGGGTWTCTAAT. Total microbial DNA was extracted from fecal samples using a QIAamp Fast DNA Stool Mini Kit. Paired-end sequencing of the V3–V4 region of the 16S rRNA gene of fecal bacteria was conducted on the Illumina platform, and QIIME2 (2019.7) software was used to eliminate erroneous and low-quality sequences. Vsearch software (2.0.5) was employed to cluster the sequences obtained. The average read length after pruning was 419, with a minimum read length of 404. Amplicon Sequence Variables (ASVs) were categorized based on a 97% sequence similarity. Representative sequences of Operational Taxonomic Units (OTUs) were compared with the GenBank database to acquire taxonomic information. Fecal microbiota diversity was assessed using four α-diversity indices (Shannon, Simpson, Chao1, and Faith_pd), and non-metric multidimensional scaling (NMDS) analysis was conducted in Origin software. The default ASV/OTU table or the absolute abundance table of taxa at various hierarchical levels (phylum, class, order, family, and genus) was utilized. The analysis involved employing the “classify_samples_ncv” function in q2-sample-classifier to conduct random forest analysis and nested stratified cross-validation. A random forest analysis was performed to identify the top 15 marker genes at the genus level. Subsequently, a Venn diagram was used to illustrate the overlap between the top 20 genes based on their relative abundance and the top 15 genes based on their importance at the genus level. By analyzing the composition of fecal microorganisms, a correlation analysis was conducted based on Pearson’s correlation coefficient with Tibetan pig fiber indicators.
2.10. Statistical Analysis
Microsoft Excel 2019 was used for data entry and preliminary organization, before conducting formal statistical analysis, performed using SPSS software (version 21.0) the assumptions of normality and homogeneity of variance for continuous variables were verified: normality was tested using the Shapiro–Wilk test, and homogeneity of variance was assessed using Levene’s test. For continuous variables conforming to both the normality and homogeneity of variance assumptions, independent sample t-tests to compare differences between groups (each group consisted of twenty repetitions, with one pig per repetition). All significance was based on a probability threshold of p < 0.05. Significance levels are denoted as * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Results
3.1. Influence of High-Fiber Diet on Growth Performance of Tibetan Pigs
No significant difference was observed in the initial body weight of Tibetan pigs between the HF and CON groups (p > 0.05; Table 2), confirming the homogeneity of the two groups at the start of the experiment. Consistent with this, compared to the CON group, the HF group also showed no significant effects on the final body weight, average daily gain, average daily feed intake, or feed-to-gain ratio of Tibetan pigs at each stage (p > 0.05).

Table 2.
Effects of a high-fiber diet on the growth performance of Tibetan pigs.
3.2. Influence of High-Fiber Diet on Apparent Nutrient Digestibility of Tibetan Pigs
No significant differences were observed in the digestibility of crude protein, ether extract, and crude fiber between the HF and CON groups (p > 0.05; Table 3), the amount of crude fiber digested in HF group was twice that of CON group (Formula = CF digestibility × crude fiber content; calculated as CON: 63.28% × 5% = 3.16%, HF: 63.40% × 10% = 6.34%, nearly doubling that of the CON group).

Table 3.
Effects of a high-fiber diet on the apparent nutrient digestibility of Tibetan pigs.
3.3. Influence of High-Fiber Diet on Blood Parameters of Tibetan Pigs
No significant differences were observed in the WBC, RBC, HGB, HCT, MCV, MCH, MCHC, RDW, PLT, MPV, PDW, and PCT levels between the HF and CON groups (p > 0.05; Table 4).

Table 4.
Effects of a high-fiber diet on the blood parameters of Tibetan pigs.
3.4. Influence of High-Fiber Diet on Serum Parameters of Tibetan Pigs
No significant differences were observed in the ALT, AST, TP, ALB, GLO, A/G, ALP, UREA, CREA, TC, and GlU levels between the HF and CON groups (p > 0.05; Table 5).

Table 5.
Effects of a high-fiber diet on the serum parameters of Tibetan pigs.
3.5. Influence of High-Fiber Diet on Enzyme Activity of Tibetan Pigs
Compared to the CON group, the HF group had significantly higher levels of cellulase and hemicellulase activities in Tibetan pigs (p < 0.05; Table 6)

Table 6.
Effects of a high-fiber diet on the enzyme activity of Tibetan pigs.
3.6. Influence of High-Fiber Diet on SCFAs of Tibetan Pigs
The levels of acetic, propionic, and butyric acids were significantly higher in the HF group than in the CON group (p < 0.05). However, changes in the crude fiber levels in the Tibetan pig diet did not significantly affect the levels of formic, isobutyric, isovaleric acid, and valeric acid between the two groups (p > 0.05; Table 7).

Table 7.
Effects of a high-fiber diet on SCFAs of Tibetan pigs.
3.7. Influence of High-Fiber Diet on Fecal Microbiota of Tibetan Pigs
The Chao1 index in the HF group was significantly higher than that in the CON group (p < 0.05; Figure 1A). No significant differences were observed in the Simpson, Faith_pd, and Shannon indices between the two groups (p > 0.05). Non-metric multidimensional scaling (NMDS) was used to analyze the β-diversity of the microbiota, and the stress value of 0.214 indicated that the ordination results were reliable. Microbiota structures in the CON and HF groups were not significantly different (Figure 1B).
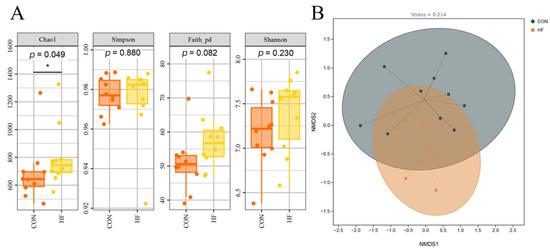
Figure 1.
Effects of different dietary fiber levels on the α-diversity index and β-diversity of fecal microbiota in confined Tibetan pigs: (A) α-diversity indices between groups; (B) non-metric multidimensional scaling (NMDS) plot between groups, with stress value = 0.214. CON, the diet contains 5% crude fiber; HF, the diet contains 10% crude fiber; Values are the means ± merged SEM (n = 20). In (A), orange boxes represent the CON group and yellow boxes represent the HF group; in (B), black dots represent the CON group and orange dots represent the HF group. “*” indicates statistically significant differences in the two groups (p < 0.05).
The dominant microbiota at the phylum level in the CON and HF groups were Firmicutes, Bacteroidetes, Spirochaetes, Tenericutes, Proteobacteria, Fibrobacteres, Actinobacteria, TM7, WPS-2, and Verrucomicrobia (relative abundance, p > 1%; Figure 2A). Figure 2B shows the top 20 genera in relative abundance at the genus level in the CON and HF groups. Figure 2C shows the top 15 genera ranked by importance, calculated using the random forest algorithm at the genus level, for both groups. Figure 2D shows a Venn diagram of the intersection between the top 20 genera in relative abundance and the top 15 genera in importance at the genus level. As shown in Figure 2D, the overlapping genera were p-75-a5, Lachnospira, Roseburia, Fibrobacter, Phascolarctobacterium, Coprococcus, and Prevotella.
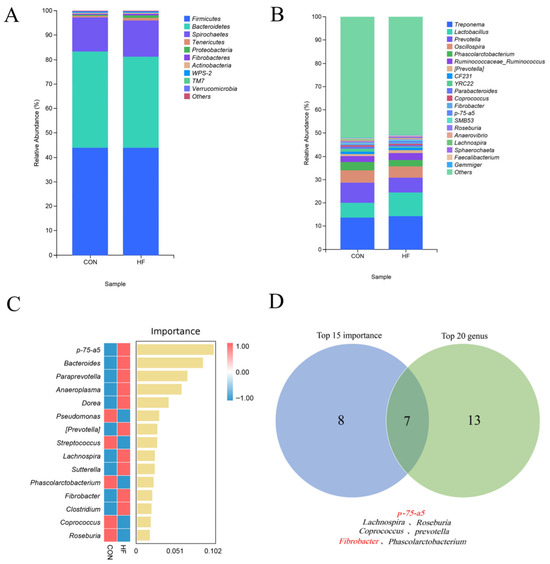
Figure 2.
Effects of different dietary fiber levels on the relative abundance of fecal microbiota in confined Tibetan pigs: (A) the top 10 phyla with the highest relative abundance at the phylum level; (B) the top 20 genera with the highest relative abundance at the genus level; (C) the top 15 genera based on importance according to a random forest analysis; (D) a Venn diagram showing seven genera intersecting between importance and relative abundance.
The relative abundances of p-75-a5 (phylum Firmicutes) and Fibrobacter were significantly higher in the HF group than in the CON group (p < 0.05). No significant differences were observed in the relative abundance of Lachnospira, Roseburia, Phascolarctobacterium, Coprococcus, and Prevotella between the two groups (p > 0.05; Table 8).

Table 8.
Differences in the relative abundances of the cross-genera between the two groups (%).
Fibrobacter showed a highly significant positive correlation with acetic acid (p< 0.01) and was significantly associated with butyric acid, cellulase, and hemicellulase (p < 0.05). p-75-a5 exhibited a significant positive correlation with cellulose (p < 0.05; Figure 3).
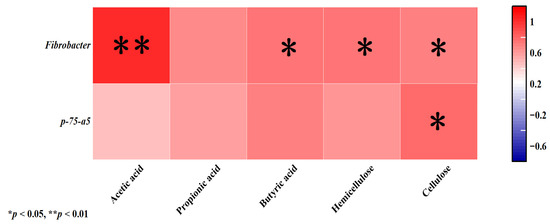
Figure 3.
Correlation analysis between the relative abundance of the bacterial community in horizontal feces and short-chain fatty acids, cellulase, and hemicellulase. Red indicates positive correlation; purple indicates negative correlation. “*” indicates a statistically significant correlation between corresponding short-chain fatty acids, enzymes, and bacterial communities (p < 0.05). “**” indicates that the correlation between the corresponding short-chain fatty acids, enzymes, and bacterial communities reached a statistically significant level (p < 0.01).
4. Discussion
4.1. Effects of High-Fiber Diets on the Growth Performance and Health Status of Tibetan Pigs
Growth performance is a fundamental metric for assessing the advantages of animal husbandry and is intricately linked to economic returns. Optimal fiber levels are crucial in enhancing the growth performance and microbial bacterial maturation of pigs [34]. Incorporating wheat bran fiber into the diet of growing pigs within a suitable range does not significantly affect final body weight, average daily gain, or feed efficiency [35]. In one study, it was found that the ADFI and ADG of pigs remained unaffected by the inclusion of wheat straw powder [36]. Similarly, in a different study, altering the levels of wheat bran fiber within the optimal fiber range did not significantly affect the ADFI and ADG of pigs [37]. This indicates that maintaining fiber levels within an appropriate range sustains stable growth performance in pigs without the adverse effects of a high-fiber diet. These findings align with the outcomes of this research, indicating that 10% crude fiber concentration is a suitable range for fiber supplementation in Tibetan pigs under the specific conditions of this study. It should be particularly noted that the addition of 4.9% soybean oil in this study will not affect the conclusions of related research. At this addition level, there is no significant fluctuation in the daily weight gain and feed intake of Tibetan pigs, and studies have shown that an addition of 4% soybean oil can improve the efficiency of pig feed intake without affecting their early growth, neither interfering with development nor masking the effects of fiber [38]. Secondly, studies have confirmed that adding 6% olive oil, sunflower oil, and other vegetable oils to high-concentration diets does not disrupt the overall structural stability of microbial communities, and indicators directly related to bacterial metabolic functions such as substrate degradation efficiency and total volatile fatty acid production show no significant changes [39,40].
Nutrient transformation and utilization within the digestive system of pigs are interconnected processes rather than operating in isolation; they collectively constitute a cohesive system [41]. Apparent digestibility is an indicator of dietary utilization in pigs, as well as their digestive and metabolic conditions [42]. Replacing corn starch with various high-fiber additives in pig feed does not significantly affect the coefficient of total tract apparent digestibility (CTTAD) of nutrients such as dry matter and organic matter across different levels of supplementation [43]. This finding aligns with the results of the present study, which revealed no significant differences in the digestibility of crude protein and ether extract in Tibetan pigs fed diets with varying crude fiber proportions. This indicates that fibers from different sources, due to their varying physicochemical properties, affect the transport speed of digesta in the pig’s gastrointestinal tract and the sites of nutrient digestion [44]. Often, the total fiber digestibility is limited by the indigestible components in the diet, setting an upper limit for digestibility [45]. Therefore, increasing the level of fiber within the digestible range will stimulate the growth of microorganisms capable of utilizing these components and the production of corresponding enzymes. In terms of digestive volume, high-fiber groups have a higher total amount of dietary fiber, but the actual absolute amount of fiber digested differs from the control group. However, due to the presence of indigestible parts, the apparent overall digestibility per unit weight of feed may not show a linear increase. Consequently, substituting low-fiber diets with high-fiber alternatives is feasible.
The blood physiology of Tibetan pigs at high altitudes is intricately linked to their adaptation to hypoxia and disease resistance. Conversely, serum biochemical parameters are associated with animal growth and metabolism and are indicators of nutrient utilization, overall health, and growth performance [46]. The administration of high-fiber diets to pigs does not lead to significant changes in their white and red blood cell counts, liver and kidney functions, glucose and lipid metabolism, and other physiological indicators [47]. This suggests that, provided the fiber content remains within the animal’s tolerance threshold, high-fiber diets do not have any pathological effects on blood and serum parameters [48]. In a separate investigation on Duroc-Landrace-Yorkshire (DLY) pigs, appropriate levels of dietary fiber maintained blood homeostasis without inducing metabolic stress [49,50]. These findings indicate that high-fiber diets maintain overall health and basal metabolism, while concurrently reducing breeding expenses and preserving the normal growth performance of Tibetan pigs. Consequently, this provides a solid foundation for the large-scale breeding of Tibetan pigs.
4.2. Response Mechanisms of Bacterial Flora, Enzyme Activities, and Metabolites Under High Fiber Regulation
Cellulose, a product of photosynthesis following the accumulation of significant plant biomass, functions as the primary structural element in plant cell walls [51]. Dietary fiber, in which cellulose plays a crucial role, is a valuable carbohydrate source for the intestinal microbiota. This substrate is an energy reservoir for gut microbiota and a carbon reservoir for the host [52,53]. Animals lack the cellulases necessary to directly digest cellulose [54]. Therefore, cellulose relies on the gut microbiota for breakdown during anaerobic fermentation. Cellulose, a photosynthetic product, is degraded by cellulolytic microorganisms. These microorganisms employ two primary mechanisms: the free cellulase mechanism, in which various secreted enzymes work synergistically [55], and the use of cellulolytic enzyme complexes attached to the outer wall (cellulosomes) for cellulose digestion, resulting in the production of short-chain fatty acids [56]. Short-chain fatty acids, the main microbial metabolites derived from fiber fermentation, play a central role in maintaining intestinal integrity and energy metabolism in Tibetan pigs. Fibrobacter, the sole anaerobic Gram-negative bacterium within the Fibrobacteraceae family of the Fibrobacter order, is characterized by rod-shaped or polymorphic oval cells measuring (3–5 μm × 0.8–1.6 μm). It is recognized as the primary cellulose-degrading bacterium in the gastrointestinal tract of animals [57,58]. In our study, a significant positive correlation was observed between Fibrobacter and acetic acid, cellulase, and hemicellulase levels following the administration of high-fiber diets to Tibetan pigs. This research only shows statistical correlations between bacterial genera, SCFAs, and enzymes, and additional experiments are required to verify potential regulatory directions. This correlation can be attributed to Fibrobacter’s reliance on carbon dioxide (CO2), short-chain fatty acids, ammonia (NH3), and multivitamins for growth. Fibrobacter exhibits a specific ability to ferment cellulose and cellobiose, while showing a limited capacity to metabolize other sugars. Through the pentose phosphate pathway (PPP) and glycolytic pathway (EMP), Fibrobacter converts its substrates into pyruvate when exposed to high-fiber diets, leading to the production of acetic acid and succinate, with occasional minor byproduct formation. These distinctive metabolic characteristics enable Fibrobacter to occupy a specialized niche within the intestinal microecology [59,60,61,62,63,64,65]. Acetic acid, a metabolic byproduct, is crucial in regulating intestinal health by providing essential energy to intestinal epithelial cells and facilitating their physiological functions [66]. Additionally, it modulates the intestinal microbiota balance by promoting the growth of beneficial bacteria and inhibiting harmful bacteria proliferation [67]. Fibrobacter produces various cellulose-degrading enzymes, including endoglucanases and exoglucanases. The cellulosome structure on the cell wall efficiently anchors the cellulose microfibrils, facilitating the breakdown of cellulose into cellobiose and glucose via the synergistic action of multiple enzymes [68]. The β-1,4 glucanase enzyme of Fibrobacter demonstrates optimal activity at a pH range of 5.5–6.5, aligning well with the acidic conditions found in the posterior segment of the porcine intestine. This pH-dependent regulation of enzyme function confers a significant functional advantage for the degradation of dietary fiber [69]. This degradation process provides fermentation substrates for p-75-a5, establishing a “substrate relay” mechanism. A diet rich in fiber enhances the synthesis of B vitamins, thereby improving the host’s intestinal immunity [70], and stimulates the growth of Fibrobacter through a positive feedback loop.
The genus p-75-a5, belonging to the family Erysipelotrichaceae, is a Gram-positive, obligate anaerobic, non-spore-forming, and non-motile bacterium [71]. Its abundance is positively associated with carbohydrate digestion in high-fiber diets [72,73]. As a member of the Erysipelotrichaceae family, p-75-a5 exhibits functional characteristics that align with the overall metabolic potential of the family. It is a core functional bacterium in animal intestines and is a key contributor to butyric acid production [74]. Butyric acid promotes Interleukin-18 (IL-18) transcription by activating the G Protein-Coupled Receptor 109A (GPR109A) and G Protein-Coupled Receptor 43 (GPR43) receptors and induces the NOD-Like Receptor Pyrin Domain-Containing Protein 3 (NLRP3) inflammasome, thereby reducing mucosal inflammation, enhancing intestinal barrier integrity, and preventing bacterial invasion [75,76,77,78]. Erysipelotrichaceae, a thick-walled phylum, is rich in carbohydrate-active enzyme (CAZyme) genes, particularly cellulase and xylanase, which degrade complex carbohydrates into oligosaccharides and monosaccharides and facilitate subsequent acid production during fermentation [79,80,81,82,83,84]. The Erysipelotrichaceae CAZyme genes in Dansoniaceae are significantly correlated with carbohydrate metabolic pathways, such as glycolysis and amino sugar metabolism [85,86]. As obligate anaerobes, members of the family Erysipelotrichaceae convert carbohydrates into pyruvate via glycolysis, ultimately producing acetic and butyric acid [87,88,89].
In this experiment, a high abundance of Fibrobacter, p-75-a5, and related bacteria was detected in Tibetan pigs. These bacterial groups were positively correlated with the production of dietary cellulase, hemicellulase, and short-chain fatty acids. This finding confirms that Tibetan pigs can efficiently degrade dietary fiber, which is closely linked to the colonization by these fecal microbiota. However, it is necessary to acknowledge certain limitations in this study. First, regarding the effect of animal age, the experiment used 120-day-old growing Tibetan pigs, with a trial duration of 28 days, which only covered their early growth phase, whereas Tibetan pigs typically reach market weight and are slaughtered at 240 to 300 days. It remains to be verified whether the observed synergistic action of “bacteria-enzymes-metabolites” in fiber degradation is stable during their later growth phase. Second, regarding the long-term adaptation mechanism, this study focused on adaptive changes within 28 days to a high-fiber diet, but it cannot fully reflect the inherent long-term adaptive regulatory mechanisms of Tibetan pigs that have evolved on the Tibetan Plateau through natural selection. Finally, it should be noted that the SCFAs and fiber-degrading enzyme activities analyzed in this study were measured in fecal samples, which may not fully represent their production, absorption, or concentration within the intestinal tract. Nevertheless, a 10% crude fiber level complies with animal welfare principles, allowing Tibetan pigs to fully access fibrous feed sources and maintain their inherent characteristics of fiber utilization, laying the foundation for subsequent long-term trials. The practical application of this scheme in live pig production awaits further verification. This finding fills the theoretical gap for high-fiber standards in large-scale confined Tibetan pigs, providing a theoretical basis for developing specialized feed, reducing costs through low-cost high-fiber raw materials, and ensuring the healthy growth of large-scale farmed Tibetan pigs.
5. Conclusions
Our findings provide strong evidence that the effective utilization of dietary fiber by Tibetan pigs results from highly specialized microbial mechanisms in the large intestine, as reflected by their fecal microbiota composition. Fibrobacter and p-75-a5 are key to effective fiber utilization in these pigs. Specific microorganisms secreted more enzymes to promote dietary fiber breakdown, resulting in more metabolites. This study may inspire new ways to deepen the understanding of physiological traits in regional species, improve dietary fiber, and fully exploit fiber adaptation in Tibetan pigs.
Author Contributions
Z.T. and Y.L. conceived and designed the experiments. Z.L. and S.H. performed the experiments and wrote the manuscript. B.L. and S.Y. conducted the data collection. Z.S. and P.S. and S.L. provided constructive comments on the results and discussion. Z.L. and S.H. contributed equally to this study. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Joint Fund of Xizang Agricultural and Animal Husbandry University and Nanjing Agricultural University (KYYZ2023003), the National Natural Science Foundation of China (U22A20519), and the Xizang Agricultural and Animal Husbandry University Graduate Innovation Project (YJS2024-17).
Institutional Review Board Statement
This study was conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of Xizang Agricultural and Animal Husbandry University (XZA-2023-003), date: 17 March 2023.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included on FigShare at. DOI: 10.6084/m9.figshare.30225790.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, Y.; Yuan, H.; Yang, Q.; Cai, Y.; Ren, Y.; Li, Y.; Gao, C.; Zhao, S. Post-Transcriptional Regulation through Alternative Splicing in the Lungs of Tibetan Pigs under Hypoxia. Gene 2022, 819, 146268. [Google Scholar] [CrossRef]
- Zhou, S.; Luo, R.; Gong, G.; Wang, Y.; Gesang, Z.; Wang, K.; Xu, Z.; Suolang, S. Characterization of Metagenome-Assembled Genomes and Carbohydrate-Degrading Genes in the Gut Microbiota of Tibetan Pig. Front. Microbiol. 2020, 11, 595066. [Google Scholar] [CrossRef]
- Tan, Z.K.; Chi, F.M.; Shang, Z.D.; Shang, P.; Liu, S.Z.; QiangBa, Y. Fungal Community in the Feces of Grazing Tibetan Pigs, Captive Tibetan Pigs, and Commercial Pigs and Its Interaction with Dietary Fiber Digestion. Acta Microbiol. Sin. 2022, 62, 259–274. [Google Scholar] [CrossRef]
- Pu, G.; Hou, L.; Du, T.; Wang, B.; Liu, H.; Li, K.; Niu, P.; Zhou, W.; Huang, R.; Li, P. Effects of Short-Term Feeding with High Fiber Diets on Growth, Utilization of Dietary Fiber, and Microbiota in Pigs. Front. Microbiol. 2022, 13, 963917. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.; Kong, X.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; Yu, J.; Luo, J.; Yan, H.; et al. Effects of Dietary Fiber on Growth Performance, Nutrient Digestibility and Intestinal Health in Different Pig Breeds. Animals 2022, 12, 3298. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Azzoni, A.R.; Freitas, S. On the Production Cost of Lignocellulose-degrading Enzymes. Biofuels Bioprod. Biorefining 2021, 15, 85–99. [Google Scholar] [CrossRef]
- Lee, G.I.; Nielsen, T.S.; Lærke, H.N.; Bach Knudsen, K.E. The Ileal and Total Tract Digestibility Fibre and Nutrients in Pigs Fed High-Fibre Cereal-Based Diets Provided without and with a Carbohydrase Complex. Animal 2023, 17, 100872. [Google Scholar] [CrossRef]
- Li, B.; Ren, S.; Fu, Y.; Tu, F.; Fang, X.; Wang, X. Effects of Different Dietary Crude Fiber Levels on the Growth Performance of Finishing Su-Shan Pigs. Int. J. Anim. Vet. Sci. 2018, 12, 145–148. [Google Scholar]
- Khan, J.; Gul, P.; Rashid, M.T.; Li, Q.; Liu, K. Composition of Whole Grain Dietary Fiber and Phenolics and Their Impact on Markers of Inflammation. Nutrients 2024, 16, 1047. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing Fibrillated Cellulose as a Sustainable Technological Material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Verstegen, M.W.A.; Williams, B.A. Alternatives to the Use of Antibiotics as Growth Promoters for Monogastric Animals. Anim. Biotechnol. 2002, 13, 113–127. [Google Scholar] [CrossRef]
- Tan, Z.K.; Liu, Y.; Liu, S.Z.; Shang, P.; Qiang Ba, Y.Z. Study on the Correlation between Intestinal Factors and Apparent Digestibility of Dietary Crude Fiber in Tibetan Pigs. J. Plateau Agric. 2021, 5, 465–471. [Google Scholar] [CrossRef]
- Yang, L.; Yao, B.; Zhang, S.; Yang, Y.; Wang, G.; Pan, H.; Zeng, X.; Qiao, S. Division Mechanism of Labor in Diqing Tibetan Pigs Gut Microbiota for Dietary Fiber Efficiently Utilization. Microbiol. Res. 2025, 290, 127977. [Google Scholar] [CrossRef]
- Yang, L.; Yao, B.; Zhang, S.; Yang, Y.; Pan, H.; Zeng, X.; Qiao, S. Study on the Difference of Gut Microbiota in DLY and Diqing Tibetan Pigs Induce by High Fiber Diet. J. Anim. Physiol. Anim. Nutr. 2025, 109, 233–242. [Google Scholar] [CrossRef]
- Che, L.; Feng, D.; Wu, D.; Fang, Z.; Lin, Y.; Yan, T. Effect of Dietary Fibre on Reproductive Performance of Sows during the First Two Parities. Reprod. Domest. Anim. 2011, 46, 1061–1066. [Google Scholar] [CrossRef]
- Loisel, F.; Farmer, C.; Ramaekers, P.; Quesnel, H. Effects of High Fiber Intake during Late Pregnancy on Sow Physiology, Colostrum Production, and Piglet Performance. J. Anim. Sci. 2013, 91, 5269–5279. [Google Scholar] [CrossRef]
- Williams, B.A.; Verstegen, M.W.A.; Tamminga, S. Fermentation in the Large Intestine of Single-Stomached Animals and Its Relationship to Animal Health. Nutr. Res. Rev. 2001, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, J.; Tan, B.; Chen, J.; Zhang, H.; Li, Z.; Ma, X. Physiological Function and Application of Dietary Fiber in Pig Nutrition: A Review. Anim. Nutr. 2021, 7, 259–267. [Google Scholar] [CrossRef]
- Ye, S.; Shah, B.R.; Li, J.; Liang, H.; Zhan, F.; Geng, F.; Li, B. A Critical Review on Interplay between Dietary Fibers and Gut Microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Guo, Q.; Li, F.; Duan, Y.; Wen, C.; Wang, W.; Zhang, L.; Huang, R.; Yin, Y. Oxidative Stress, Nutritional Antioxidants and Beyond. Sci. China Life Sci. 2020, 63, 866–874. [Google Scholar] [CrossRef]
- Wei, X.; Dong, Z.; Cheng, F.; Shi, H.; Zhou, X.; Li, B.; Wang, L.; Wang, W.; Zhang, J. Seasonal Diets Supersede Host Species in Shaping the Distal Gut Microbiota of Yaks and Tibetan Sheep. Sci. Rep. 2021, 11, 22626. [Google Scholar] [CrossRef]
- Bai, X.; Gu, Y.; Li, D.; Li, M. Gut Metagenome Reveals the Microbiome Signatures in Tibetan and Black Pigs. Animals 2025, 15, 753. [Google Scholar] [CrossRef]
- Shah, B.R.; Li, B.; Al Sabbah, H.; Xu, W.; Mráz, J. Effects of Prebiotic Dietary Fibers and Probiotics on Human Health: With Special Focus on Recent Advancement in Their Encapsulated Formulations. Trends Food Sci. Technol. 2020, 102, 178–192. [Google Scholar] [CrossRef]
- Chi, Z.; Zhang, M.; Fu, B.; Wang, X.; Yang, H.; Fang, X.; Li, Z.; Teng, T.; Shi, B. Branched Short-Chain Fatty Acid-Rich Fermented Protein Food Improves the Growth and Intestinal Health by Regulating Gut Microbiota and Metabolites in Young Pigs. J. Agric. Food Chem. 2024, 72, 21594–21609. [Google Scholar] [CrossRef]
- Erinle, T.J.; Babatunde, O.O.; Htoo, J.K.; Mendoza, M.; Columbus, D.A. PSII-27 Dietary Fiber Fractions Supplementation Modulates pro-Inflammatory Cytokines, Hindgut Ammonia-Nitrogen Concentration, and Diarrhea Severity of Nursery Pigs. J. Anim. Sci. 2024, 102, 700. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. Maintenance of Gut Microbiome Stability for Optimum Intestinal Health in Pigs—A Review. J. Anim. Sci. Biotechnol. 2022, 13, 140. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.; Ma, L.; Hou, Q.; Zhang, Y.; Kong, X.; Huang, X.; Tang, Z.; Wei, H.; Wang, X.; et al. Characterizing Core Microbiota and Regulatory Functions of the Pig Gut Microbiome. ISME J. 2024, 18, wrad037. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Song, X.; Yang, M.; Wang, H.; Wu, Y. Feeding Dietary Fermentable Fiber Improved Fecal Microbial Composition and Increased Acetic Acid Production in a Nursery Pig Model. J. Anim. Sci. 2023, 101, skad260. [Google Scholar] [CrossRef]
- Woyengo, T.A.; Beltranena, E.; Zijlstra, R.T. Nonruminant Nutrition Symposium: Controlling Feed Cost by Including Alternative Ingredients into Pig Diets: A Review. J. Anim. Sci. 2014, 92, 1293–1305. [Google Scholar] [CrossRef]
- Agyekum, A.K.; Nyachoti, C.M. Nutritional and Metabolic Consequences of Feeding High-Fiber Diets to Swine: A Review. Engineering 2017, 3, 716–725. [Google Scholar] [CrossRef]
- Pitts, G.C. Cellular Aspects of Growth and Catch-up Growth in the Rat: A Reevaluation. Growth 1986, 50, 419–436. [Google Scholar]
- Latimer, G.W.J. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016; ISBN 978-0-935584-87-5. [Google Scholar]
- Nogal, A.; Valdes, A.M.; Menni, C. The Role of Short-Chain Fatty Acids in the Interplay between Gut Microbiota and Diet in Cardio-Metabolic Health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Bai, K.; Jiang, L.; Li, Q.; Zhang, J.; Zhang, L.; Wang, T. Dietary Dimethylglycine Sodium Salt Supplementation Alleviates Redox Status Imbalance and Intestinal Dysfunction in Weaned Piglets with Intrauterine Growth Restriction. Anim. Nutr. 2022, 10, 188–197. [Google Scholar] [CrossRef]
- GB/T 39235-2020; Nutrient Requirements of Swine. Standards Press of China: Beijing, China, 2020.
- Tokach, M.D.; Pettigrew, J.E.; Johnston, L.J.; Overland, M.; Rust, J.W.; Cornelius, S.G. Effect of Adding Fat and(or) Milk Products to the Weanling Pig Diet on Performance in the Nursery and Subsequent Grow-Finish Stages. J. Anim. Sci. 1995, 73, 3358–3368. [Google Scholar] [CrossRef]
- Vargas, J.E.; Andrés, S.; López-Ferreras, L.; Snelling, T.J.; Yáñez-Ruíz, D.R.; García-Estrada, C.; López, S. Dietary Supplemental Plant Oils Reduce Methanogenesis from Anaerobic Microbial Fermentation in the Rumen. Sci. Rep. 2020, 10, 1613. [Google Scholar] [CrossRef]
- Moore, W.-E.; Moore, L.-V.; Cato, E.-P.; Wilkins, T.-D.; Kornegay, E.-T. Effect of High-Fiber and High-Oil Diets on the Fecal Flora of Swine. Appl. Environ. Microbiol. 1987, 53, 1638–1644. [Google Scholar] [CrossRef]
- Hong, J.; Halbur, J.; Petry, A.-L.; Doung, T.; Llamas-Moya, S.; Kitt, S.; Bertram, M.; Weaver, E. Effects of a Fiber-Degrading Enzyme on Ileal Digestibility of Amino Acids and Fiber and Total Tract Digestibility of Energy and Fiber in Growing Pigs Fed Diets with High Level of Corn Distillers Grains with Solubles. J. Anim. Sci. 2025, 103, skaf076. [Google Scholar] [CrossRef]
- Park, S.; Choe, J.; Cho, J.H.; Jang, K.B.; Kyoung, H.; Park, K.I.; Kim, Y.; Ahn, J.; Kim, H.B.; Song, M. Determination of Optimal Energy System and Level for Growing Pigs. J. Anim. Sci. Technol. 2024, 66, 514–522. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, Z.; Wang, F.; Guo, J.; Zhao, X.; Zhao, J. Effects of Dietary Fiber Type on Growth Performance, Serum Parameters and Fecal Microbiota Composition in Weaned and Growing-Finishing Pigs. Animals 2022, 12, 1579. [Google Scholar] [CrossRef]
- Pu, G.; Hou, L.; Du, T.; Zhou, W.; Liu, C.; Niu, P.; Wu, C.; Bao, W.; Huang, R.; Li, P. Increased Proportion of Fiber-Degrading microbiota and Enhanced Cecum Development Jointly Promote Host to Digest Appropriate High-Fiber Diets. mSystems 2023, 8, e00937-22. [Google Scholar] [CrossRef]
- Wang, J.; Qin, C.; He, T.; Qiu, K.; Sun, W.; Zhang, X.; Jiao, N.; Zhu, W.; Yin, J. Alfalfa-Containing Diets Alter Luminal Microbiota Structure and Short Chain Fatty Acid Sensing in the Caecal Mucosa of Pigs. J. Anim. Sci. Biotechnol. 2018, 9, 11. [Google Scholar] [CrossRef]
- Du, T.; Li, P.; Niu, Q.; Pu, G.; Wang, B.; Liu, G.; Li, P.; Niu, P.; Zhang, Z.; Wu, C.; et al. Effects of Varying Levels of Wheat Bran Dietary Fiber on Growth Performance, Fiber Digestibility and Gut Microbiota in Erhualian and Large White Pigs. Microorganisms 2023, 11, 2474. [Google Scholar] [CrossRef]
- Patil, Y.; Gooneratne, R.; Ju, X.-H. Interactions between Host and Gut Microbiota in Domestic Pigs: A Review. Gut Microbes 2020, 11, 310–334. [Google Scholar] [CrossRef]
- Huang, C.; Li, P.; Ma, X.; Jaworski, N.W.; Stein, H.-H.; Lai, C.; Zhao, J.; Zhang, S. Methodology Effects on Determining the Energy Concentration and the Apparent Total Tract Digestibility of Components in Diets Fed to Growing Pigs. Asian-Australas. J. Anim. Sci. 2018, 31, 1315–1324. [Google Scholar] [CrossRef]
- Navarro, D.M.D.L.; Bruininx, E.M.A.M.; De Jong, L.; Stein, H.H. Effects of Inclusion Rate of High Fiber Dietary Ingredients on Apparent Ileal, Hindgut, and Total Tract Digestibility of Dry Matter and Nutrients in Ingredients Fed to Growing Pigs. Anim. Feed Sci. Technol. 2019, 248, 1–9. [Google Scholar] [CrossRef]
- Graugnard, D.E.; Bionaz, M.; Trevisi, E.; Moyes, K.M.; Salak-Johnson, J.L.; Wallace, R.L.; Drackley, J.K.; Bertoni, G.; Loor, J.J. Blood Immunometabolic Indices and Polymorphonuclear Neutrophil Function in Peripartum Dairy Cows Are Altered by Level of Dietary Energy Prepartum. J. Dairy Sci. 2012, 95, 1749–1758. [Google Scholar] [CrossRef]
- Lynd, L.R.; Wyman, C.E.; Gerngross, T.U. Biocommodity Engineering. Biotechnol. Prog. 1999, 15, 777–793. [Google Scholar] [CrossRef]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of Gut Microbiota and Nutrients in Amyloid Formation and Pathogenesis of Alzheimer Disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving Our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- O’Grady, J.; O’Connor, E.M.; Shanahan, F. Review Article: Dietary Fibre in the Era of Microbiome Science. Aliment. Pharmacol. Ther. 2019, 49, 506–515. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Riesco, R.; Benito, P.; Carro, L. Endophytic Actinobacteria and the Interaction of Micromonospora and Nitrogen Fixing Plants. Front. Microbiol. 2015, 6, 1341. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A.; Belaich, J.-P.; Shoham, Y.; Lamed, R. The Cellulosomes: Multienzyme Machines for Degradation of Plant Cell Wall Polysaccharides. Annu. Rev. Microbiol. 2004, 58, 521–554. [Google Scholar] [CrossRef] [PubMed]
- Anguita, M.; Gasa, J.; Nofrarias, M.; Martín-Orúe, S.M.; Pérez, J.F. Effect of Coarse Ground Corn, Sugar Beet Pulp and Wheat Bran on the Voluntary Intake and Physicochemical Characteristics of Digesta of Growing Pigs. Livest. Sci. 2007, 107, 182–191. [Google Scholar] [CrossRef]
- Qiang Ba, Y.Z.; Zhang, H.; Bai Ma, Y.Z.; Liu, J.F.; Shang, P.; Dan Zeng, W.J. Determination of Blood Physiological Parameters in Tibet Pig at High Altitude. Southwest China J. Agric. Sci. 2011, 24, 2382–2384. [Google Scholar] [CrossRef]
- Weng, R.-C. Dietary Supplementation with Different Types of Fiber in Gestation and Lactation: Effects on Sow Serum Biochemical Values and Performance. Asian-Australas. J. Anim. Sci. 2020, 33, 1323–1331. [Google Scholar] [CrossRef]
- Feng, L.; Luo, Z.; Wang, J.; Wu, K.; Wang, W.; Li, J.; Ma, X.; Tan, B.E. Fermentation Characteristics of Different Sources of Dietary Fiber in Vitro and Impacts on Growth Performance, Nutrient Digestibility and Blood Parameters of Piglets. J. Funct. Foods 2023, 108, 105761. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Niu, J.; Cui, Z.; Zhao, X.; Li, W.; Zhang, Y.; Yang, Y.; Gao, P.; Guo, X.; et al. Impacts of Dietary Fiber Level on Growth Performance, Apparent Digestibility, Intestinal Development, and Colonic Microbiota and Metabolome of Pigs. J. Anim. Sci. 2023, 101, skad174. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, G.; Cidan, Y.; Shi, B.; Tan, Z.; Zhang, J.; Basang, W. Comprehensive Multi-Omic Evaluation of the Microbiota and Metabolites in the Colons of Diverse Swine Breeds. Animals 2024, 14, 1221. [Google Scholar] [CrossRef]
- Kalala, G.; Kambashi, B.; Everaert, N.; Beckers, Y.; Richel, A.; Pachikian, B.; Neyrinck, A.M.; Delzenne, N.M.; Bindelle, J. Characterization of Fructans and Dietary Fibre Profiles in Raw and Steamed Vegetables. Int. J. Food Sci. Nutr. 2018, 69, 682–689. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Abdul Rahman, N.; Parks, D.H.; Vanwonterghem, I.; Morrison, M.; Tyson, G.W.; Hugenholtz, P. A Phylogenomic Analysis of the Bacterial Phylum Fibrobacteres. Front. Microbiol. 2016, 6, 1469. [Google Scholar] [CrossRef]
- Ransom-Jones, E.; Jones, D.L.; McCarthy, A.J.; McDonald, J.E. The Fibrobacteres: An Important Phylum of Cellulose-Degrading Bacteria. Microb. Ecol. 2012, 63, 267–281. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Tazoe, H.; Otomo, Y.; Kaji, I.; Tanaka, R.; Karaki, S.-I.; Kuwahara, A. Roles of Short-Chain Fatty Acids Receptors, GPR41 and GPR43 on Colonic Functions. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59, 251–262. [Google Scholar]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, E.N.; Maclean, P.; Thomas, D.G.; Cave, N.J.; Young, W. Key Bacterial Families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) Are Related to the Digestion of Protein and Energy in Dogs. PeerJ 2017, 5, e3019. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered Gut Microbiota and Short Chain Fatty Acids in Chinese Children with Autism Spectrum Disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Singh, R.P. Glycan Utilisation System in Bacteroides and Bifidobacteria and Their Roles in Gut Stability and Health. Appl. Microbiol. Biotechnol. 2019, 103, 7287–7315. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human Nutrition, the Gut Microbiome and the Immune System. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Shi, Z.; Wei, X.; Wei, Y.; Zhang, Z.; Wan, S.; Gao, H.; Qin, Z. Biochemical Properties and Application of a Multi-Domain β-1,3-1,4-GlUcanase from Fibrobacter sp. Int. J. Biol. Macromol. 2024, 273, 133026. [Google Scholar] [CrossRef]
- Shinkai, T.; Ueki, T.; Kobayashi, Y. Detection and Identification of Rumen Bacteria Constituting a Fibrolytic Consortium Dominated by Fibrobacter succinogenes. Anim. Sci. J. 2010, 81, 72–79. [Google Scholar] [CrossRef]
- Song, X.; Sun, J.; Yue, Y.; Li, D.; Chen, F. Microbiota-Derived Succinic Acid Mediates Attenuating Effect of Dietary Tomato Juice Supplementation on Steatohepatitis through Enhancing Intestinal Barrier. Food Res. Int. 2024, 196, 115123. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.T.; Parrish, A.; Boudaud, M.; Hunewald, O.; Hirayama, A.; Ollert, M.; Fukuda, S.; Desai, M.S. Dietary Fibers Boost Gut Microbiota-Produced B Vitamin Pool and Alter Host Immune Landscape. Microbiome 2024, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Umar, M.; Hassan, F.; Alagawany, M.; Arif, M.; Taha, A.E.; Elnesr, S.S.; El-Tarabily, K.A.; Abd El-Hack, M.E. Applications of Butyric Acid in Poultry Production: The Dynamics of Gut Health, Performance, Nutrient Utilization, Egg Quality, and Osteoporosis. Anim. Health Res. Rev. 2022, 23, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-Y.; Li, Y.; Xue, C.-X.; Lidbury, I.D.E.A.; Todd, J.D.; Lea-Smith, D.J.; Tian, J.; Zhang, X.-H.; Liu, J. Deep-Sea Bacteroidetes from the Mariana Trench Specialize in Hemicellulose and Pectin Degradation Typically Associated with Terrestrial Systems. Microbiome 2023, 11, 175. [Google Scholar] [CrossRef]
- Ke, S.; Fang, S.; He, M.; Huang, X.; Yang, H.; Yang, B.; Chen, C.; Huang, L. Age-Based Dynamic Changes of Phylogenetic Composition and Interaction Networks of Health Pig Gut Microbiome Feeding in a Uniformed Condition. BMC Vet. Res. 2019, 15, 172. [Google Scholar] [CrossRef]
- McGovern, E.; McGee, M.; Byrne, C.J.; Kenny, D.A.; Kelly, A.K.; Waters, S.M. Investigation into the Effect of Divergent Feed Efficiency Phenotype on the Bovine Rumen Microbiota across Diet and Breed. Sci. Rep. 2020, 10, 15317. [Google Scholar] [CrossRef]
- Jiao, J.; Wu, J.; Zhou, C.; Tang, S.; Wang, M.; Tan, Z. Composition of Ileal Bacterial Community in Grazing Goats Varies across Non-Rumination, Transition and Rumination Stages of Life. Front. Microbiol. 2016, 7, 1469. [Google Scholar] [CrossRef]
- Zhang, T.; Mu, Y.; Zhang, D.; Lin, X.; Wang, Z.; Hou, Q.; Wang, Y.; Hu, Z. Determination of Microbiological Characteristics in the Digestive Tract of Different Ruminant Species. MicrobiologyOpen 2019, 8, e00769. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The Epigenetic Effects of Butyrate: Potential Therapeutic Implications for Clinical Practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef]
- Li, R.; Li, L.; Hong, P.; Lang, W.; Hui, J.; Yang, Y.; Zheng, X. β-Carotene Prevents Weaning-Induced Intestinal Inflammation by Modulating Gut Microbiota in Piglets. Anim. Biosci. 2021, 34, 1221–1234. [Google Scholar] [CrossRef]
- Van Den Abbeele, P.; Moens, F.; Pignataro, G.; Schnurr, J.; Ribecco, C.; Gramenzi, A.; Marzorati, M. Yeast-Derived Formulations Are Differentially Fermented by the Canine and Feline Microbiome as Assessed in a Novel in Vitro Colonic Fermentation Model. J. Agric. Food Chem. 2020, 68, 13102–13110. [Google Scholar] [CrossRef]
- Fan, M.; Cheng, L.E.; Wang, M.; Chen, J.; Fan, W.; Jashari, F.; Wang, W. Monomodular and Multifunctional Processive Endocellulases: Implications for Swine Nutrition and Gut Microbiome. Animal Microbiome 2024, 6, 4. [Google Scholar] [CrossRef]
- Cox, L.M.; Cho, I.; Young, S.A.; Anderson, W.H.K.; Waters, B.J.; Hung, S.; Gao, Z.; Mahana, D.; Bihan, M.; Alekseyenko, A.V.; et al. The Nonfermentable Dietary Fiber Hydroxypropyl Methylcellulose Modulates Intestinal Microbiota. FASEB J. 2013, 27, 692–702. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).