Standardized Artemisia annua Exhibits Dual Antileishmanial Activity and Immunomodulatory Potential In Vitro
Simple Summary
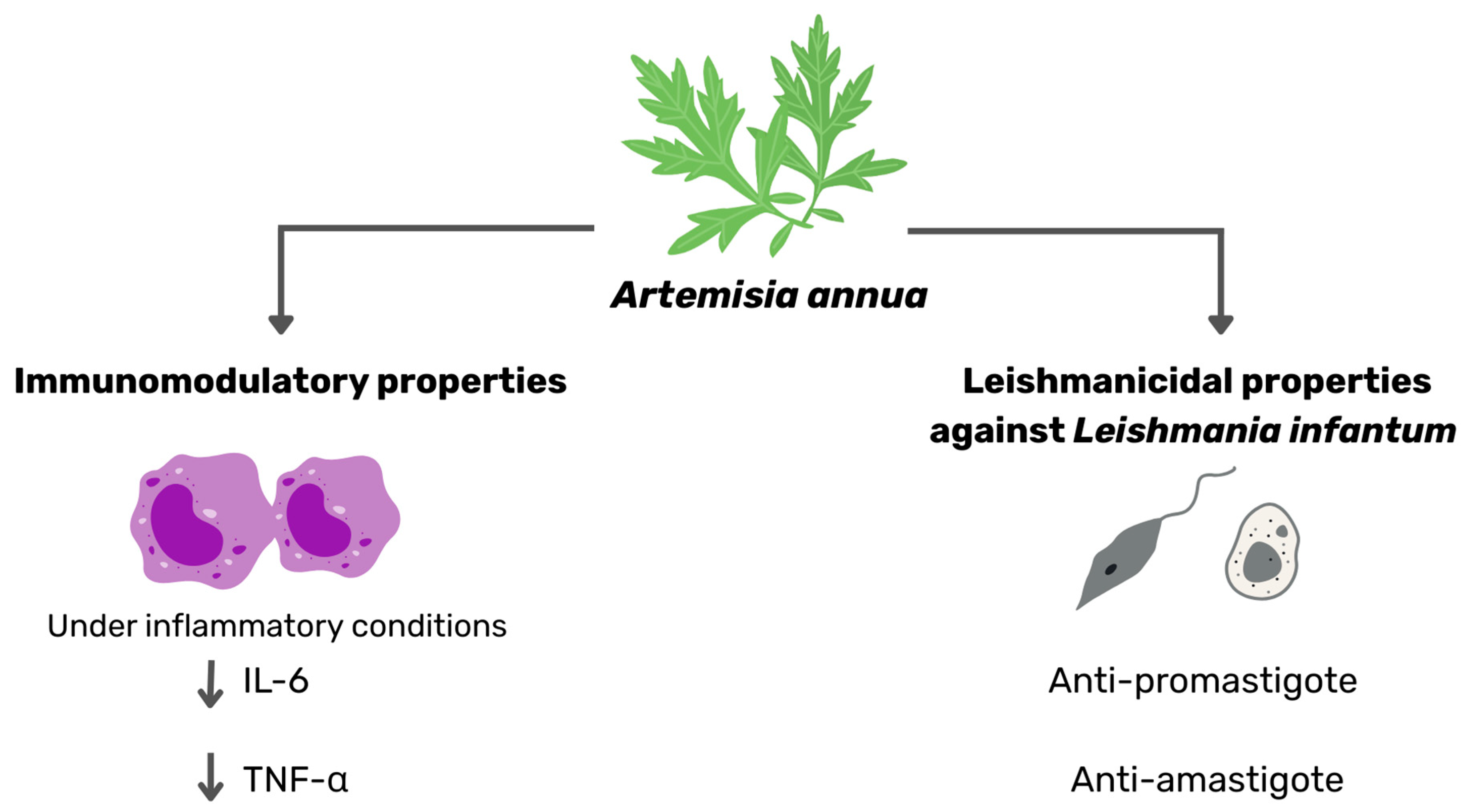
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Quantification of Artemisinin Content
2.3. In Vitro Activity Assays Against the Promastigote Stage of Leishmania infantum
2.4. Activity Assays Against Intracellular Leishmania infantum Amastigotes
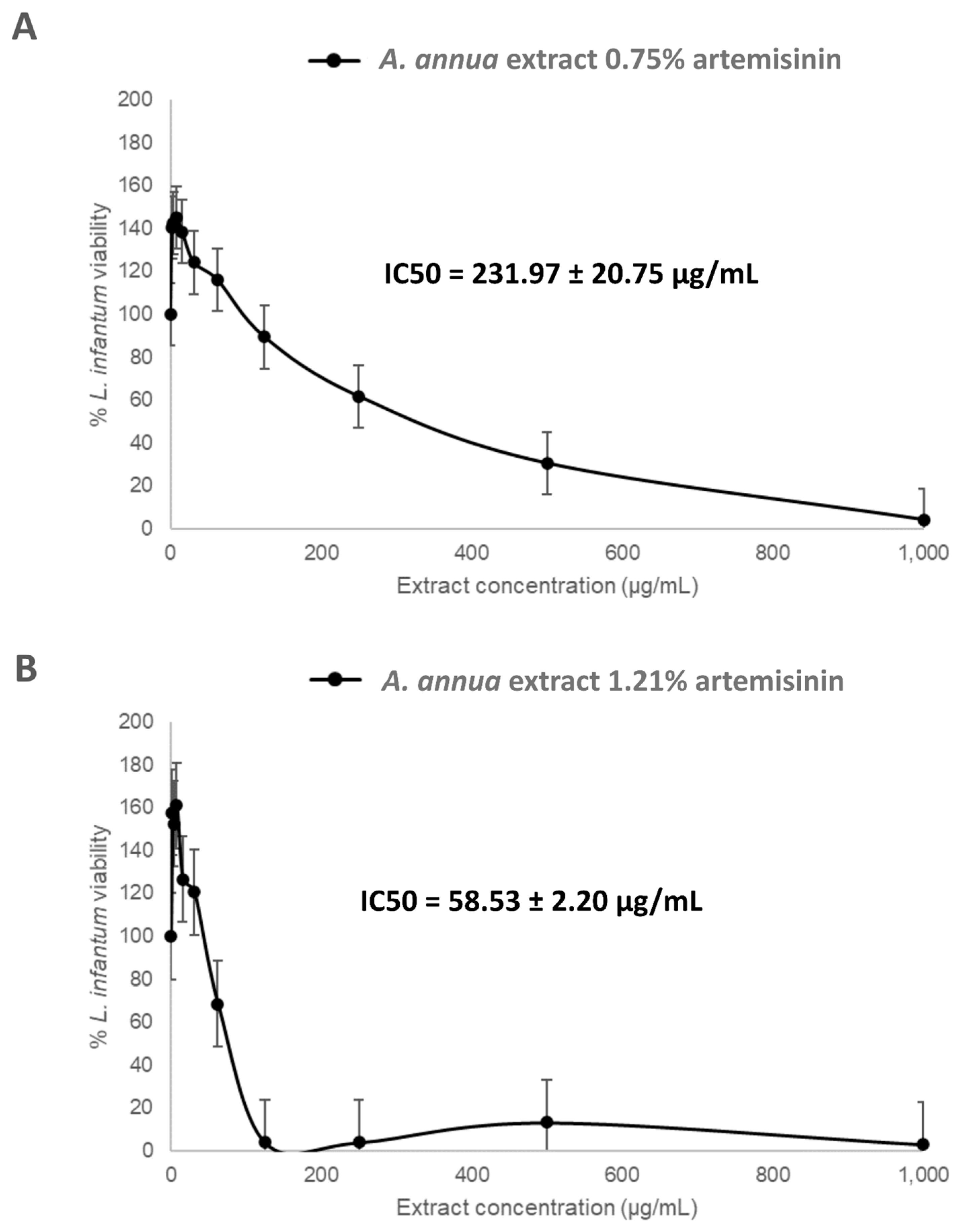
2.5. Determination of IC50
2.6. Citotoxicity Assay in Macrophages
2.7. Cell Culture, Maintenance and Cytotoxicity Assessment by MTT Assay
2.8. Evaluation of the Extract on the Immune Response
2.9. Statistical Analysis
3. Results
3.1. In Vitro Activity of Artemisia annua Extracts Against Leishmania infantum Promastigotes
3.2. Activity of Artemisia annua Extracts Against Leishmania infantum Amastigotes
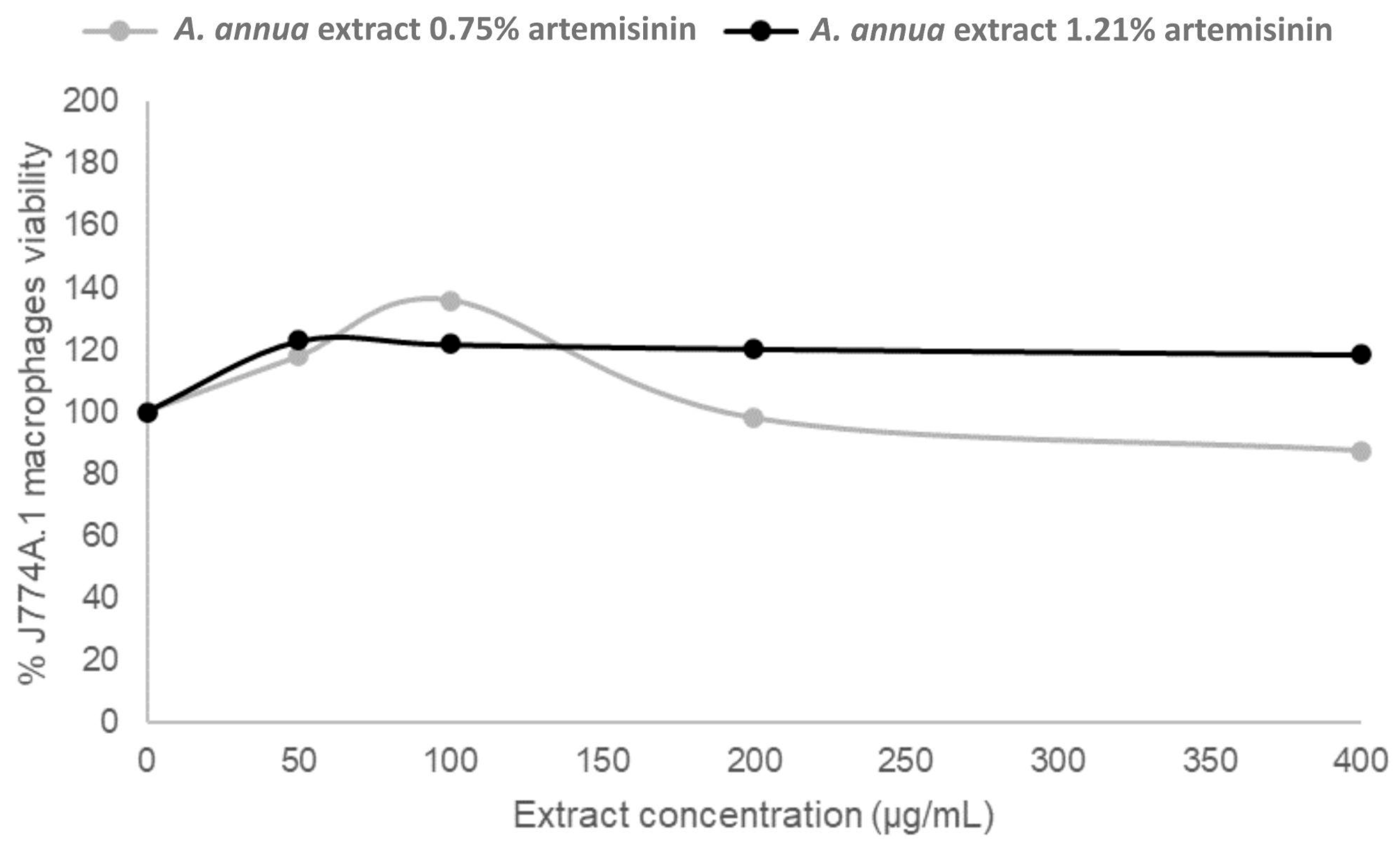
3.3. Cytotoxicity of Artemisia annua Extracts in Murine Macrophages
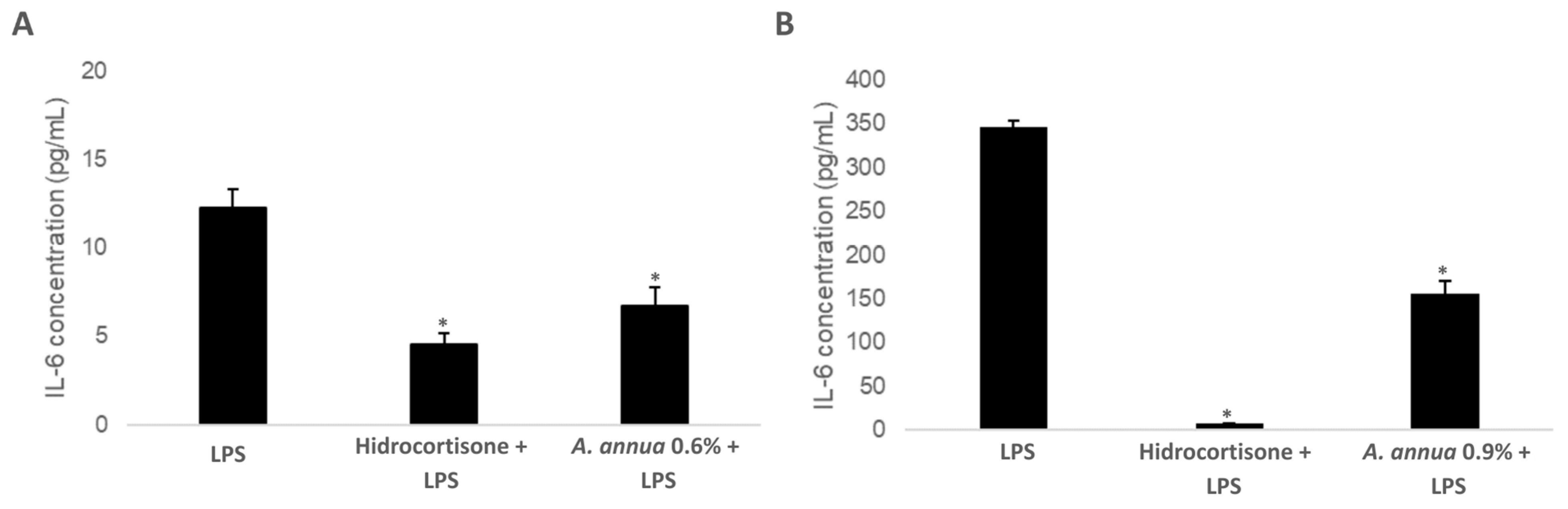
3.4. Evaluation of the Immunomodulatory Effect of Artemisia annua Extracts: Impact on IL-6 Production
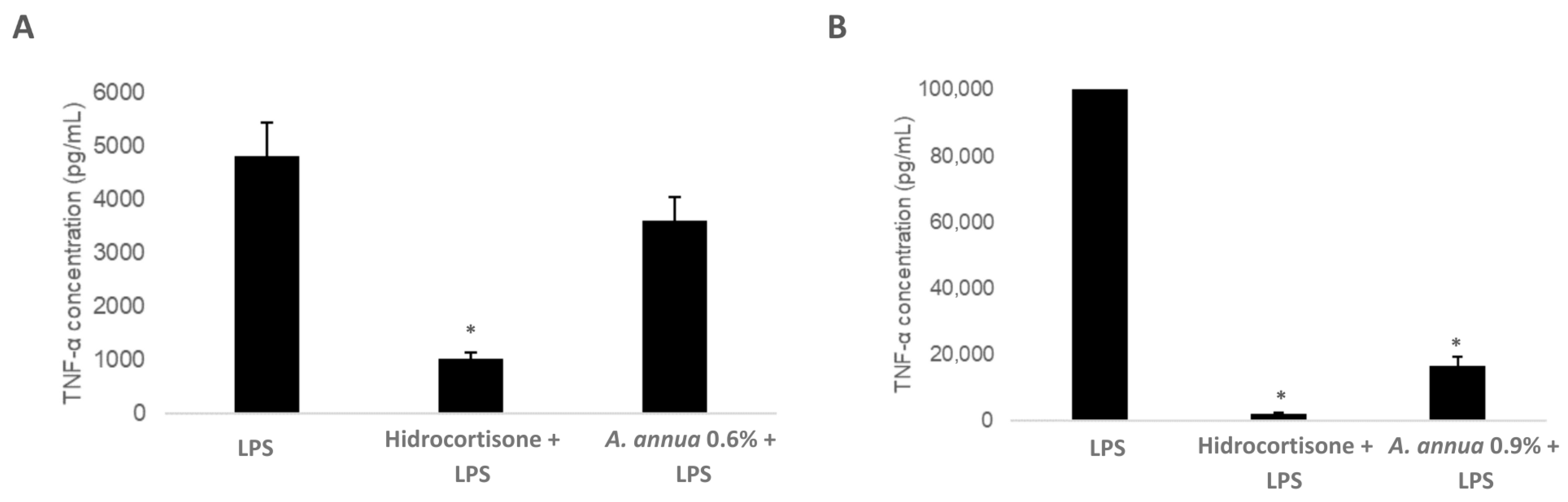
3.5. Evaluation of the Immunomodulatory Effect of Artemisia annua Extracts: Impact on TNF-α Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vilas-Boas, D.F.; Nakasone, E.K.N.; Gonçalves, A.A.M.; Lair, D.F.; Oliveira, D.S.D.; Pereira, D.F.S.; Silva, G.G.; Conrado, I.D.S.S.; Resende, L.A.; Zaldívar, M.F.; et al. Global Distribution of Canine Visceral Leishmaniasis and the Role of the Dog in the Epidemiology of the Disease. Pathogens 2024, 13, 455. [Google Scholar] [CrossRef]
- Priolo, V.; Ippolito, D.; Rivas-Estanga, K.; De Waure, C.; Martínez-Orellana, P. Canine Leishmaniosis Global Prevalence over the Last Three Decades: A Meta-Analysis and Systematic Review. Comp. Immunol. Microbiol. Infect. Dis. 2024, 112, 102211. [Google Scholar] [CrossRef]
- Saridomichelakis, M.N.; Baneth, G.; Colombo, S.; Dantas-Torres, F.; Ferrer, L.; Fondati, A.; Miró, G.; Ordeix, L.; Otranto, D.; Noli, C. World Association for Veterinary Dermatology Consensus Statement for Diagnosis, and Evidence-Based Clinical Practice Guidelines for Treatment and Prevention of Canine Leishmaniosis. Vet. Dermatol. 2025, vde.70006. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.R.; Michalick, M.S.M.; Da Silva, M.E.; Dos Santos, C.C.P.; Frézard, F.J.G.; Da Silva, S.M. Canine Leishmaniasis: An Overview of the Current Status and Strategies for Control. BioMed Res. Int. 2018, 29, 3296893. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, J.-C.; Campino, L.; Cañavate, C.; Dedet, J.-P.; Gradoni, L.; Soteriadou, K.; Mazeris, A.; Ozbel, Y.; Boelaert, M. Spread of Vector-Borne Diseases and Neglect of Leishmaniasis, Europe. Emerg. Infect. Dis. 2008, 14, 1013–1018. [Google Scholar] [CrossRef]
- Ready, P.D. Biology of Phlebotomine Sand Flies as Vectors of Disease Agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet Guidelines for the Practical Management of Canine Leishmaniosis. Parasites Vectors 2011, 4, 86. [Google Scholar] [CrossRef]
- Pennisi, M.-G.; Cardoso, L.; Baneth, G.; Bourdeau, P.; Koutinas, A.; Miró, G.; Oliva, G.; Solano-Gallego, L. LeishVet Update and Recommendations on Feline Leishmaniosis. Parasites Vectors 2015, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine Leishmaniasis: Update on Epidemiology, Diagnosis, Treatment, and Prevention. Vet. Sci. 2022, 9, 387. [Google Scholar] [CrossRef]
- Velez, R.; Gállego, M. Commercially Approved Vaccines for Canine Leishmaniosis: A Review of Available Data on Their Safety and Efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef]
- Guarga, J.L.; Moreno, J.; Lucientes, J.; Gracia, M.J.; Peribáñez, M.A.; Castillo, J.A. Evaluation of a Specific Immunochemotherapy for the Treatment of Canine Visceral Leishmaniasis. Vet. Immunol. Immunopathol. 2002, 88, 13–20. [Google Scholar] [CrossRef]
- Yasur-Landau, D.; Jaffe, C.L.; Doron-Faigenboim, A.; David, L.; Baneth, G. Induction of Allopurinol Resistance in Leishmania Infantum Isolated from Dogs. PLoS Negl. Trop. Dis. 2017, 11, e0005910. [Google Scholar] [CrossRef]
- Yasur-Landau, D.; Jaffe, C.L.; David, L.; Baneth, G. Allopurinol Resistance in Leishmania Infantum from Dogs with Disease Relapse. PLoS Negl. Trop. Dis. 2016, 10, e0004341. [Google Scholar] [CrossRef]
- Baneth, G.; Solano-Gallego, L. Leishmaniasis. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.H.; Su, X. Artemisinin: Discovery from the Chinese Herbal Garden. Cell 2011, 146, 855–858. [Google Scholar] [CrossRef]
- Krishna, S.; Uhlemann, A.; Haynes, R. Artemisinins: Mechanisms of Action and Potential for Resistance. Drug Resist. Updates 2004, 7, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R.; Yang, Y.Z.; Lima, V.; Kuypers, F.; Kamchonwongpaisan, S.; Yuthavong, Y. Iron-Dependent Free Radical Generation from the Antimalarial Agent Artemisinin (Qinghaosu). Antimicrob. Agents Chemother. 1993, 37, 1108–1114. [Google Scholar] [CrossRef]
- Krauth-Siegel, R.L.; Comini, M.A. Redox Control in Trypanosomatids, Parasitic Protozoa with Trypanothione-Based Thiol Metabolism. Biochim. Biophys. Acta BBA—Gen. Subj. 2008, 1780, 1236–1248. [Google Scholar] [CrossRef]
- De Sarkar, S.; Sarkar, D.; Sarkar, A.; Dighal, A.; Chakrabarti, S.; Staniek, K.; Gille, L.; Chatterjee, M. The Leishmanicidal Activity of Artemisinin Is Mediated by Cleavage of the Endoperoxide Bridge and Mitochondrial Dysfunction. Parasitology 2019, 146, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Bandyopadhyay, S.; Dutta, A.; Mandal, G.; Ganguly, S.; Saha, P.; Chatterjee, M. Artemisinin Triggers Induction of Cell-Cycle Arrest and Apoptosis in Leishmania donovani Promastigotes. J. Med. Microbiol. 2007, 56, 1213–1218. [Google Scholar] [CrossRef]
- Ghaffarifar, F.; Esavand Heydari, F.; Dalimi, A.; Hassan, Z.M.; Delavari, M.; Mikaeiloo, H. Evaluation of Apoptotic and Antileishmanial Activities of Artemisinin on Promastigotes and BALB/C Mice Infected with Leishmania Major. Iran. J. Parasitol. 2015, 10, 258–267. [Google Scholar] [PubMed]
- Sen, R.; Ganguly, S.; Saha, P.; Chatterjee, M. Efficacy of Artemisinin in Experimental Visceral Leishmaniasis. Int. J. Antimicrob. Agents 2010, 36, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.M.; Ali, H.Z. Ex Vivo Study of Anti-Leishmanial Activity of Artemisinin against Leishmania tropica Amastigote. Res. J. Pharm. Technol. 2020, 13, 3787. [Google Scholar] [CrossRef]
- Soares, D.C.; Portella, N.A.; Ramos, M.F.D.S.; Siani, A.C.; Saraiva, E.M. Trans—β-Caryophyllene: An Effective Antileishmanial Compound Found in Commercial Copaiba Oil (Copaifera Spp.). Evid. Based Complement. Altern. Med. 2013, 2013, 761323. [Google Scholar] [CrossRef]
- Da Silva, E.T.; De Andrade, G.F.; Araújo, A.D.S.; Almeida, A.D.C.; Coimbra, E.S.; De Souza, M.V.N. In Vitro Assessment of Camphor Hydrazone Derivatives as an Agent Against Leishmania amazonensis. Acta Parasitol. 2020, 65, 203–207. [Google Scholar] [CrossRef]
- Islamuddin, M.; Chouhan, G.; Farooque, A.; Dwarakanath, B.S.; Sahal, D.; Afrin, F. Th1-Biased Immunomodulation and Therapeutic Potential of Artemisia annua in Murine Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e3321. [Google Scholar] [CrossRef]
- Islamuddin, M.; Chouhan, G.; Tyagi, M.; Abdin, M.Z.; Sahal, D.; Afrin, F. Leishmanicidal Activities of Artemisia annua Leaf Essential Oil against Visceral Leishmaniasis. Front. Microbiol. 2014, 5, 626. [Google Scholar] [CrossRef]
- Hosein, S.; Blake, D.P.; Solano-Gallego, L. Insights on Adaptive and Innate Immunity in Canine Leishmaniosis. Parasitology 2017, 144, 95–115. [Google Scholar] [CrossRef]
- Toepp, A.J.; Petersen, C.A. The Balancing Act: Immunology of Leishmaniosis. Res. Vet. Sci. 2020, 130, 19–25. [Google Scholar] [CrossRef]
- Arens, K.; Filippis, C.; Kleinfelder, H.; Goetzee, A.; Reichmann, G.; Crauwels, P.; Waibler, Z.; Bagola, K.; Van Zandbergen, G. Anti-Tumor Necrosis Factor α Therapeutics Differentially Affect Leishmania Infection of Human Macrophages. Front. Immunol. 2018, 9, 1772. [Google Scholar] [CrossRef] [PubMed]
- Shakir, L.; Hussain, M.; Javeed, A.; Ashraf, M.; Riaz, A. Artemisinins and Immune System. Eur. J. Pharmacol. 2011, 668, 6–14. [Google Scholar] [CrossRef]
- Cui, Y.; Leng, X.; Zhao, Y.; Zhao, Y.; Wang, Q. Effects of Dietary Artemisia annua Supplementation on Growth Performance, Antioxidant Capacity, Immune Function, and Gut Microbiota of Geese. Poult. Sci. 2024, 103, 103594. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Ma, J.; Xing, Y.; Shi, L.; Zhang, L.; Xu, Y.; Jin, X.; Yan, S.; Shi, B. Artemisia Annua L. Aqueous Extract Promotes Intestine Immunity and Antioxidant Function in Broilers. Front. Vet. Sci. 2022, 9, 934021. [Google Scholar] [CrossRef]
- Shinyuy, L.M.; Loe, G.E.; Jansen, O.; Mamede, L.; Ledoux, A.; Noukimi, S.F.; Abenwie, S.N.; Ghogomu, S.M.; Souopgui, J.; Robert, A.; et al. Secondary Metabolites Isolated from Artemisia Afra and Artemisia annua and Their Anti-Malarial, Anti-Inflammatory and Immunomodulating Properties—Pharmacokinetics and Pharmacodynamics: A Review. Metabolites 2023, 13, 613. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, L.; Cui, C.; Zhao, H.; Zhang, Y.; Tian, Z.; Guan, W.; Chen, F. Maternal Supplementation with Artemisia annua L. Ameliorates Intestinal Inflammation via Inhibiting the TLR4/NF-κB and MAPK Pathways and Improves the Oxidative Stability of Offspring. Food Funct. 2022, 13, 9311–9323. [Google Scholar] [CrossRef]
- Neamah, S.D.; Ali, H.Z.; Al-Halbosiy, M.M.F. Detection of Artemisinin Effect on Macrophage Inducible Nitric Oxide Gene Expression in Macrophage Infected with Leishmania donovani. Ann. Parasitol. 2022, 68, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.M.; Liew, F.Y. Effects of Qinghaosu (Artemisinin) and Its Derivatives on Experimental Cutaneous Leishmaniasis. Parasitology 1993, 106, 7–11. [Google Scholar] [CrossRef]
- Want, M.Y.; Islamuddin, M.; Chouhan, G.; A. Ozbak, H.; A. Hemeg, H.; P. Chattopadhyay, A.; Afrin, F. Nanoliposomal Artemisinin for the Treatment of Murine Visceral Leishmaniasis. Int. J. Nanomed. 2017, 12, 2189–2204. [Google Scholar] [CrossRef]
- Dos Santos Meira, C.; Gedamu, L. Protective or Detrimental? Understanding the Role of Host Immunity in Leishmaniasis. Microorganisms 2019, 7, 695. [Google Scholar] [CrossRef] [PubMed]
- González-Tafoya, E.; Diupotex, M.; Zamora-Chimal, J.; Salaiza-Suazo, N.; Ruiz-Remigio, A.; Becker, I. TNF Contributes to T-Cell Exhaustion in Chronic L. Mexicana Infections of Mice through PD-L1 up-Regulation. Cell. Immunol. 2020, 358, 104196. [Google Scholar] [CrossRef]
- Bilia, A.R.; Melillo De Malgalhaes, P.; Bergonzi, M.C.; Vincieri, F.F. Simultaneous Analysis of Artemisinin and Flavonoids of Several Extracts of Artemisia annua L. Obtained from a Commercial Sample and a Selected Cultivar. Phytomedicine 2006, 13, 487–493. [Google Scholar] [CrossRef]
- Jain, S.K.; Sahu, R.; Walker, L.A.; Tekwani, B.L. A Parasite Rescue and Transformation Assay for Antileishmanial Screening Against Intracellular Leishmania donovani Amastigotes in THP1 Human Acute Monocytic Leukemia Cell Line. J. Vis. Exp. 2012, 4054. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, J.; Li, A.; Yang, X.; Sprangers, B.; Li, S. Prolongation of Allograft Survival by Artemisinin Treatment Is Associated with Blockade of OX40-OX40L. Immunopharmacol. Immunotoxicol. 2021, 43, 291–298. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, Q.; Jiang, N.; Zhang, Y.; Su, Z.; Lv, L.; Sang, X.; Chen, R.; Feng, Y.; Chen, Q. Dihydroartemisinin Regulates Immune Cell Heterogeneity by Triggering a Cascade Reaction of CDK and MAPK Phosphorylation. Signal Transduct. Target. Ther. 2022, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the Interface of Human Health and Disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef]
- Titus, R.G.; Sherry, B.; Cerami, A. The Involvement of TNF, IL-1 and IL-6 in the Immune Response to Protozoan Parasites. Immunol. Today 1991, 12, A13–A16. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M. Interleukin-6: From an Inflammatory Marker to a Target for Inflammatory Diseases. Trends Immunol. 2012, 33, 571–577. [Google Scholar] [CrossRef]
- Gurung, P.; Kanneganti, T.-D. Immune Responses against Protozoan Parasites: A Focus on the Emerging Role of Nod-like Receptors. Cell. Mol. Life Sci. 2016, 73, 3035–3051. [Google Scholar] [CrossRef]
- Silva, L.B.; Dos Santos Neto, A.P.; Maia, S.M.A.S.; Dos Santos Guimarães, C.; Quidute, I.L.; Carvalho, A.D.A.T.; Júnior, S.A.; Leão, J.C. The Role of TNF-α as a Proinflammatory Cytokine in Pathological Processes. Open Dent. J. 2019, 13, 332–338. [Google Scholar] [CrossRef]
- Bastos, L.L.; Mariano, D.; Lemos, R.P.; Bialves, T.S.; Oliveira, C.J.F.; De Melo-Minardi, R.C. The Role of Structural Bioinformatics in Understanding Tumor Necrosis Factor α-Interacting Protein Mechanisms in Chronic Inflammatory Diseases: A Review. Immuno 2024, 4, 14–42. [Google Scholar] [CrossRef]
- Alesaeidi, S.; Miraj, S. A Systematic Review of Anti-Malarial Properties, Immunosuppressive Properties, Anti-Inflammatory Properties, and Anti-Cancer Properties of Artemisia annua. Electron. Physician 2016, 8, 3150–3155. [Google Scholar] [CrossRef]
- Massiha, A.; Khoshkholgh-Pahlavian, M.M.; Issazadeh, K.; Bidarigh, S.; Zarrabi, S. Antibacterial Activity of Essential Oils and Plant Extracts of Artemisia (Artemisia annua L.) In Vitro. Zahedan J. Res. Med. Sci. 2012, 15, 14–18. [Google Scholar]
- Mesa, L.E.; Vasquez, D.; Lutgen, P.; Vélez, I.D.; Restrepo, A.M.; Ortiz, I.; Robledo, S.M. In Vitro and in Vivo Antileishmanial Activity of Artemisia annua L. Leaf Powder and Its Potential Usefulness in the Treatment of Uncomplicated Cutaneous Leishmaniasis in Humans. Rev. Soc. Bras. Med. Trop. 2017, 50, 52–60. [Google Scholar] [CrossRef][Green Version]
- Mesquita, J.T.; Tempone, A.G.; Reimão, J.Q. Combination Therapy with Nitazoxanide and Amphotericin B, Glucantime®, Miltefosine and Sitamaquine against Leishmania (Leishmania) Infantum Intracellular Amastigotes. Acta Trop. 2014, 130, 112–116. [Google Scholar] [CrossRef]
- Carrió, J.; Portús, M. In Vitro Susceptibility to Pentavalent Antimony in Leishmania Infantum Strains Is Not Modified During In Vitro or In Vivo Passages but Is Modified After Host Treatment with Meglumine Antimoniate. BMC Pharmacol. 2002, 2, 11. [Google Scholar] [CrossRef]
- Carrió, J.; De Colmenares, M.; Riera, C.; Gállego, M.; Arboix, M.; Portús, M. Leishmania Infantum: Stage-Specific Activity of Pentavalent Antimony Related with the Assay Conditions. Exp. Parasitol. 2000, 95, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Couto, C.G.; London, C.A.; Kisseberth, W.C.; Phelps, M.A.; Dalton, J.T. Comparison of High-Dose Intermittent and Low-Dose Continuous Oral Artemisinin in Dogs with Naturally Occurring Tumors. J. Am. Anim. Hosp. Assoc. 2014, 50, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Weathers, P.J. Artemisinin as a Therapeutic vs. Its More Complex Artemisia Source Material. Nat. Prod. Rep. 2023, 40, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, M.R.; Mittleman, A.; Weathers, P.J. Dried Leaf Artemisia Annua Improves Bioavailability of Artemisinin via Cytochrome P450 Inhibition and Enhances Artemisinin Efficacy Downstream. Biomolecules 2020, 10, 254. [Google Scholar] [CrossRef]
- Möller, B.; Villiger, P.M. Inhibition of IL-1, IL-6, and TNF-α in Immune-Mediated Inflammatory Diseases. Springer Semin. Immunopathol. 2006, 27, 391–408. [Google Scholar] [CrossRef]
- Álvarez, L.; Marín-García, P.-J.; Rentero-Garrido, P.; Martinez-Jimenez, C.P.; Llobat, L. Interleukin 6 and Interferon Gamma Haplotypes Are Related to Cytokine Serum Levels in Dogs in an Endemic Leishmania Infantum Region. Infect. Dis. Poverty 2023, 12, 9. [Google Scholar] [CrossRef]
- De Lima, V.M.F.; Peiro, J.R.; De Oliveira Vasconcelos, R. IL-6 and TNF-α Production during Active Canine Visceral Leishmaniasis. Vet. Immunol. Immunopathol. 2007, 115, 189–193. [Google Scholar] [CrossRef]
- Khan, A.W.; Farooq, M.; Hwang, M.-J.; Haseeb, M.; Choi, S. Autoimmune Neuroinflammatory Diseases: Role of Interleukins. Int. J. Mol. Sci. 2023, 24, 7960. [Google Scholar] [CrossRef]
- Forrester, J.S.; Bick-Forrester, J. Persistence of Inflammatory Cytokines Cause a Spectrum of Chronic Progressive Diseases: Implications for Therapy. Med. Hypotheses 2005, 65, 227–231. [Google Scholar] [CrossRef]
- Guan, Q.; Gao, X.; Wang, J.; Sun, Y.; Shekhar, S. Cytokines in Autoimmune Disease. Mediat. Inflamm. 2017, 2017, 5089815. [Google Scholar] [CrossRef]
- Moore, K.J.; Matlashewski, G. Intracellular Infection by Leishmania Donovani Inhibits Macrophage Apoptosis. J. Immunol. 1994, 152, 2930–2937. [Google Scholar] [CrossRef]
- Cianciulli, A.; Porro, C.; Calvello, R.; Trotta, T.; Panaro, M.A. Resistance to Apoptosis in Leishmania Infantum-Infected Human Macrophages: A Critical Role for Anti-Apoptotic Bcl-2 Protein and Cellular IAP1/2. Clin. Exp. Med. 2018, 18, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gálvez, S.-G.; Álvarez-Hernández, D.-A.; Gutiérrez-Kobeh, L.; Vázquez-López, R. Leishmania: Manipulation of Signaling Pathways to Inhibit Host Cell Apoptosis. Ther. Adv. Infect. Dis. 2021, 8, 20499361211014977. [Google Scholar] [CrossRef] [PubMed]
- Gregory, D.J.; Godbout, M.; Contreras, I.; Forget, G.; Olivier, M. A Novel Form of NF-kappaB Is Induced by Leishmania Infection: Involvement in Macrophage Gene Expression. Eur. J. Immunol. 2008, 38, 1071–1081. [Google Scholar] [CrossRef]
- Martínez-López, M.; Soto, M.; Iborra, S.; Sancho, D. Leishmania Hijacks Myeloid Cells for Immune Escape. Front. Microbiol. 2018, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Oghumu, S.; Satoskar, A.R. Mechanisms of Immune Evasion in Leishmaniasis. Adv. Appl. Microbiol. 2013, 82, 155–184. [Google Scholar] [CrossRef]
- Bogdan, C. Macrophages as Host, Effector and Immunoregulatory Cells in Leishmaniasis: Impact of Tissue Micro-Environment and Metabolism. Cytokine X 2020, 2, 100041. [Google Scholar] [CrossRef]
- Umamaheswari, D.; Muthuraja, R.; Kumar, M.; Venkateswarlu, B.S. Standardization of Herbal Drugs—A Overview. Int. J. Pharm. Sci. Rev. Res. 2021, 68, 47583. [Google Scholar] [CrossRef]
- Tandon, N.; Yadav, S.S. Contributions of Indian Council of Medical Research (ICMR) in the Area of Medicinal Plants/Traditional Medicine. J. Ethnopharmacol. 2017, 197, 39–45. [Google Scholar] [CrossRef]
- Balekundri, A.; Mannur, V. Quality Control of the Traditional Herbs and Herbal Products: A Review. Future J. Pharm. Sci. 2020, 6, 67. [Google Scholar] [CrossRef]
- Sachan, A.K.; Vishnoi, G.; Kumar, R. Need of Standardization of Herbal Medicines in Modern Era. Int. J. Phytomedicine 2016, 8, 300. [Google Scholar] [CrossRef]
- Sumbul, S.; Ahmad, M.; Asif, M.; Akhtar, M.; Saud, I. Physicochemical and Phytochemical Standardization of Berries of Myrtus Communis Linn. J. Pharm. Bioallied Sci. 2012, 4, 322. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E. A Broad Review of Commercially Important Southern African Medicinal Plants. J. Ethnopharmacol. 2008, 119, 342–355. [Google Scholar] [CrossRef]
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Kunle Standardization of Herbal Medicines—A Review. Int. J. Biodivers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Delabays, N.; Simonnet, X.; Gaudin, M. The Genetics of Artemisinin Content in Artemisia annua L. and the Breeding of High Yielding Cultivars. Curr. Med. Chem. 2001, 8, 1795–1801. [Google Scholar] [CrossRef]
- Anyinkeng, N.; Bechem, E.E.T.; Bizama, F.M. Evaluation of the Artemisinin Content of Artemisia annua L. Grown in Different Agro Ecological Zones of Cameroon. World J. Adv. Res. Rev. 2023, 20, 681–689. [Google Scholar] [CrossRef]
- Numonov, S.; Sharopov, F.; Salimov, A.; Sukhrobov, P.; Atolikshoeva, S.; Safarzoda, R.; Habasi, M.; Aisa, H.A. Assessment of Artemisinin Contents in Selected Artemisia Species from Tajikistan (Central Asia). Medicines 2019, 6, 23. [Google Scholar] [CrossRef]
- Pulice, G.; Pelaz, S.; Matías-Hernández, L. Molecular Farming in Artemisia annua, a Promising Approach to Improve Anti-Malarial Drug Production. Front. Plant Sci. 2016, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.; Jessing, K.K.; Jørgensen, N.O.G.; Cedergreen, N.; Kandeler, E.; Strobel, B.W. Distribution and Ecological Impact of Artemisinin Derived from Artemisia annua L. in an Agricultural Ecosystem. Soil Biol. Biochem. 2013, 57, 164–172. [Google Scholar] [CrossRef]
- Berman, A.R.; Birkenheuer, A.J.; Sorah, E.L.; Papich, M.G. Analysis of US Marketed Artemisinin Supplements for Use in Dogs. J. Vet. Pharmacol. Ther. 2025, 48, 56–60. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morua, E.; Cuyas, L.; Bethencourt-Estrella, C.J.; López-Arencibia, A.; Garrido Martínez, M.; Sañudo Otero, A.; Lorenzo-Morales, J.; Piñero, J.E.; Yetano Cunchillos, A.; Virto Resano, R.; et al. Standardized Artemisia annua Exhibits Dual Antileishmanial Activity and Immunomodulatory Potential In Vitro. Vet. Sci. 2025, 12, 950. https://doi.org/10.3390/vetsci12100950
Morua E, Cuyas L, Bethencourt-Estrella CJ, López-Arencibia A, Garrido Martínez M, Sañudo Otero A, Lorenzo-Morales J, Piñero JE, Yetano Cunchillos A, Virto Resano R, et al. Standardized Artemisia annua Exhibits Dual Antileishmanial Activity and Immunomodulatory Potential In Vitro. Veterinary Sciences. 2025; 12(10):950. https://doi.org/10.3390/vetsci12100950
Chicago/Turabian StyleMorua, Estefania, Laura Cuyas, Carlos J. Bethencourt-Estrella, Atteneri López-Arencibia, Maria Garrido Martínez, Ana Sañudo Otero, Jacob Lorenzo-Morales, José E. Piñero, Anabel Yetano Cunchillos, Raquel Virto Resano, and et al. 2025. "Standardized Artemisia annua Exhibits Dual Antileishmanial Activity and Immunomodulatory Potential In Vitro" Veterinary Sciences 12, no. 10: 950. https://doi.org/10.3390/vetsci12100950
APA StyleMorua, E., Cuyas, L., Bethencourt-Estrella, C. J., López-Arencibia, A., Garrido Martínez, M., Sañudo Otero, A., Lorenzo-Morales, J., Piñero, J. E., Yetano Cunchillos, A., Virto Resano, R., & Matías-Hernández, L. (2025). Standardized Artemisia annua Exhibits Dual Antileishmanial Activity and Immunomodulatory Potential In Vitro. Veterinary Sciences, 12(10), 950. https://doi.org/10.3390/vetsci12100950







