Characterization of the Hemagglutinin Gene of Morbillivirus canis in Domestic Dogs from the Mid-Western Area of Brazil
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
- Poconé, MT (−16.2715, −56.6062): samples MT1, MT2, MT3, and MT4;
- Cuiabá, MT (−15.5997, −56.0791): MT5 and MT6;
- Sinop, MT (−11.8574, −55.5012): MT7, MT8, MT9, MT10, MT11, and MT12;
- Ji-Paraná, RO (−10.8791, −61.9442): RO1 and RO2.
2.2. RNA Extraction
2.3. Primers
2.4. One-Step RT-PCR Reaction for Canine Distemper Virus Amplification (RT-PCR)
2.5. Nucleotide Sequencing, Phylogenetic Analysis, and Characterization of the H Gene
2.6. Phylogenetic and Genetic Population Network Analysis
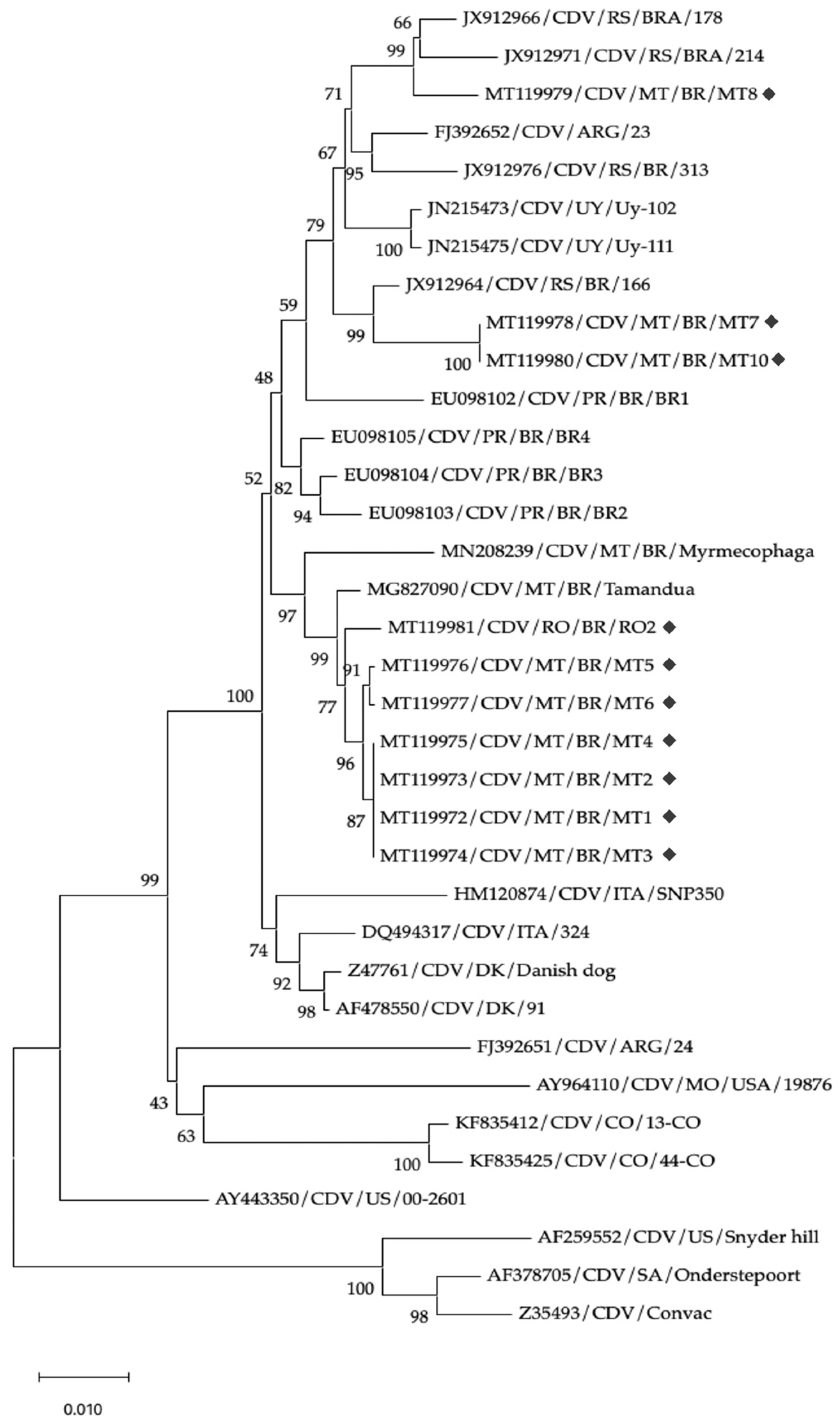
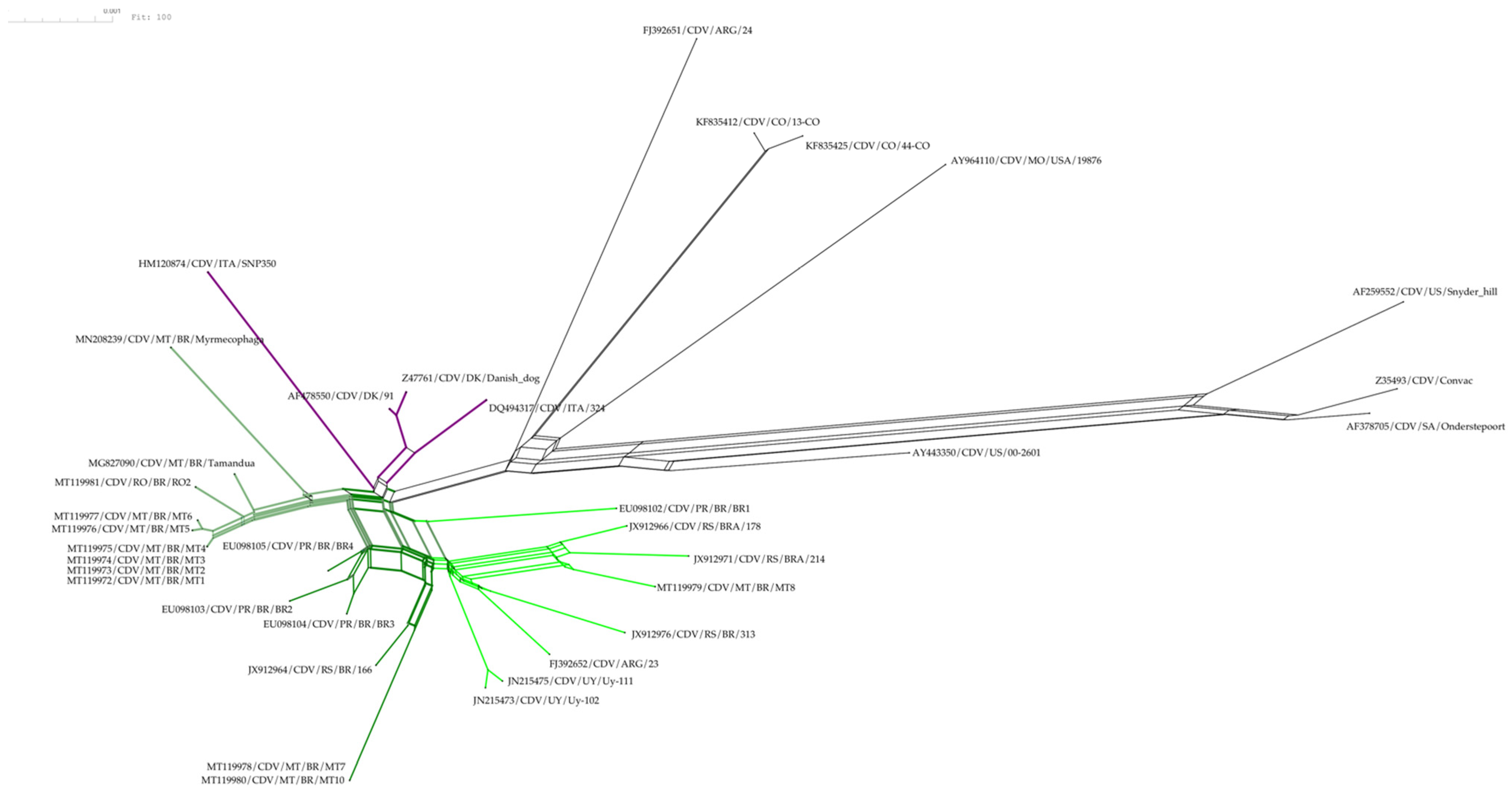
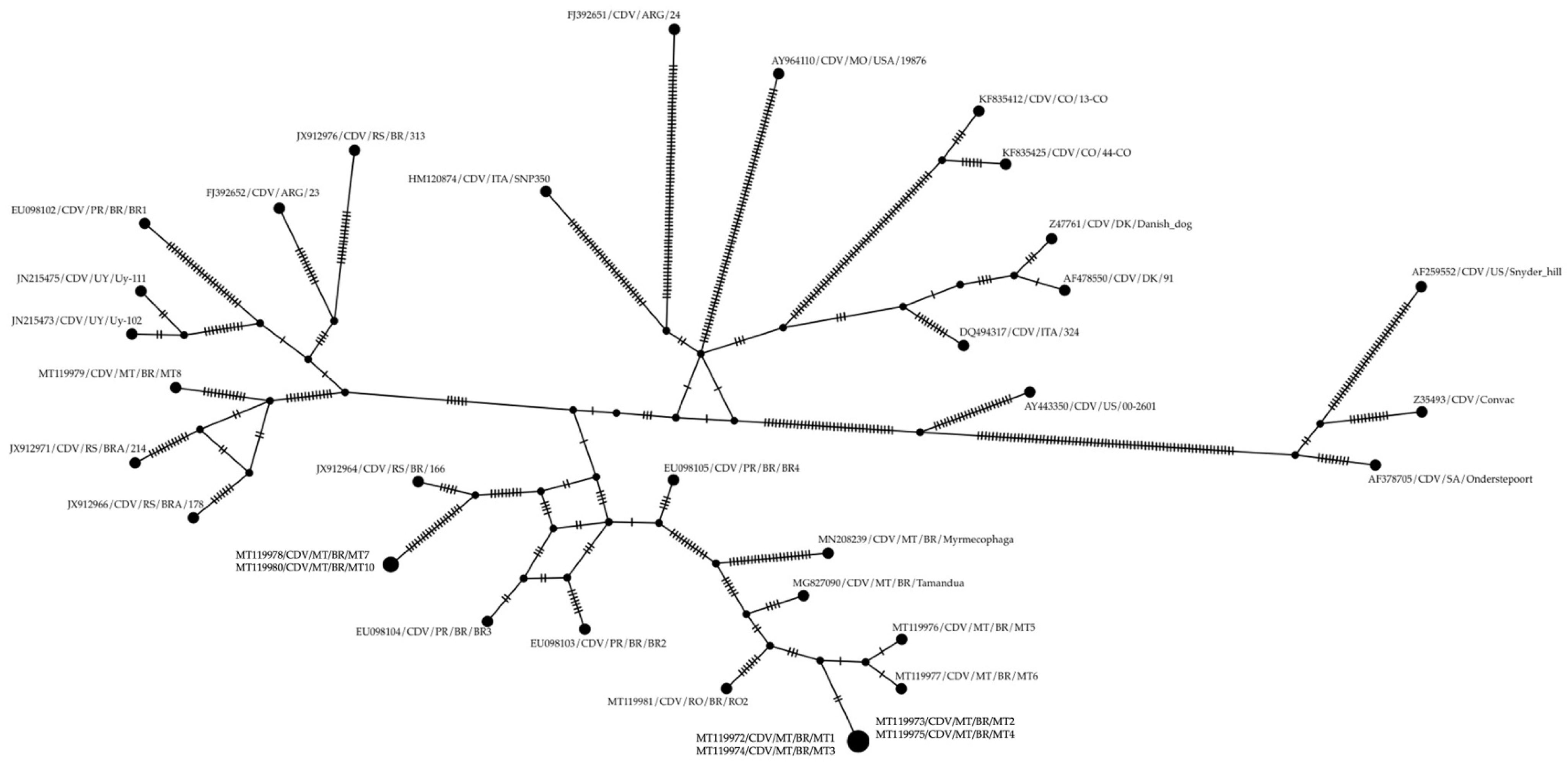
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Committee on Taxonomy of Viruses (ICTV). Morbillivirus. ICTV Virus Taxonomy. 2021. Available online: https://ictv.global/report/chapter/paramyxoviridae/paramyxoviridae/morbillivirus (accessed on 20 July 2025).
- Tipold, A. Diagnosis of inflammatory and infectious diseases of the central nervous system in dogs: A retrospective study. J. Vet. Intern. Med. 1995, 9, 304–314. [Google Scholar] [CrossRef]
- Freire, H.L.; Iara, Í.H.N.; Ribeiro, L.S.R.; Gonçalves, P.A.O.; Matta, D.H.; Torres, B.B.J. Neurological Manifestation of Canine Distemper Virus: Increased Risk in Young Shih Tzu and Lhasa Apso with Seasonal Prevalence in Autumn. Viruses 2025, 17, 820. [Google Scholar] [CrossRef]
- Lunardi, M.; Darold, G.M.; Amude, A.M.; Hesadley, S.A.; Sonne, L.; Yamauchi, K.C.I.; Boabaid, F.M.; Alfieri, A.F.; Alfieri, A.A. Canine distemper virus active infection in order Pilosa, family Myrmecophagidae, species Tamandua tetradactyla. Vet. Microbiol. 2018, 220, 7–11. [Google Scholar] [CrossRef]
- Granjeiro, M.D.B.; Kavasaki, M.L.; Morgado, T.O.; Pavelegini, L.A.D.; Barros, M.A.; Fontana, C.; Bianchini, M.A.; Souza, A.O.; Lima, A.R.G.O.S.; Lunardi, M.; et al. First report of a canine morbillivirus infection in a giant anteater (Myrmecophaga tridactyla) in Brazil. Vet. Med. Sci. 2020, 6, 606–611. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Ochikubo, F.; Matsubara, Y.; Tsuruoka, H.; Ishii, M.; Shirota, K.; Nomura, Y.; Sugiyama, M.; Yamanouchi, K. Natural infection with canine distemper virus in a Japanese monkey (Macaca fuscata). Vet. Microbiol. 1989, 20, 193–205. [Google Scholar] [CrossRef]
- Oni, O.; Wajjwalku, W.; Boodde, O.; Chumsing, W. Canine distemper virus antibodies in the Asian elephant (Elaphas maximus). Vet. Rec. 2006, 159, 420–421. [Google Scholar] [CrossRef]
- Kennedy, S.; Kuiken, T.; Jepson, P.D.; Deaville, R.; Forsyth, M.; Barrett, T.; van de Bildt, M.W.; Osterhaus, A.D.; Eybatov, T.; Duck, C.; et al. Mass die-off of Caspian seals caused by canine distemper virus. Emerg. Infect. Dis. 2000, 6, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Saenz, R.J. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar]
- Amude, A.M.; Alfieri, A.A.; Balarin, M.R.S.R. Cerebrospinal fluid from a 7-month-old dog with seizure-like episodes. Vet. Clin. Pathol. 2006, 35, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Germeraad, E.A.; Sanders, P.; Hagenaars, T.J.; de Jong, M.C.; Beerens, N.; Gonzales, J.L. Virus Shedding of Avian Influenza in Poultry: A Systematic Review and Meta-Analysis. Viruses 2019, 11, 812. [Google Scholar] [CrossRef]
- Andreychev, A.; Boyarova, E.; Brandler, O.; Tukhbatullin, A.; Kapustina, S. Terrestrial and Subterranean Mammals as Reservoirs of Zoonotic Diseases in the Central Part of European Russia. Diversity 2023, 15, 39. [Google Scholar] [CrossRef]
- Martella, V.; Elia, G.; Buonavoglia, C. Canine distemper virus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Budaszewski, R.D.F.; Pinto, L.D.; Weber, M.N.; Teles, E.C.; Alves, C.D.B.; Martella, V.; Ikuta, N.; Lunge, V.R.; Canal, C.W. Genotyping of canine distemper virus strains circulating in Brazil from 2008 to 2012. Virus Res. 2014, 180, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Budaszewski, R.D.F.; Hudacek, A.; Sawatsky, B.; Krämer, B.; Yin, X.; Schnell, M.J.; Messling, V.V. Inactivated Recombinant Rabies Viruses Displaying Canine Distemper Virus Glycoproteins Induce Protective Immunity against Both Pathogens. J. Virol. 2017, 91, 02077-16. [Google Scholar] [CrossRef]
- Duque-Valencia, J.; Forero-Muñoz, N.R.; Díaz, F.J.; Martins, E.; Barato, P.; Ruiz-Saenz, J. Phylogenetic evidence of the intercontinental circulation of a Canine distemper virus lineage in the Americas. Sci. Rep. 2019, 9, 15747. [Google Scholar] [CrossRef]
- An, D.J.; Yoon, S.H.; Park, J.Y.; No, I.S.; Park, B.K. Phylogenetic characterization of canine distemper virus isolates from naturally infected dogs and a marten in Korea. Vet. Microbiol. 2008, 132, 389–395. [Google Scholar] [CrossRef]
- Frisk, A.L.; König, M.; Moritz, A.; Baumgärtner, W. Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. J. Clin. Microbiol. 1999, 37, 3634–3643. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.C.; Kenter, M.; Vos, H.; Siebelink, K.; Huisman, W.; Van Amerongen, G.; Osterhaus, A.D.M.E. Canine distemper virus from diseased large felids: Biological properties and phylogenetic relationships. J. Gen. Virol. 1996, 77, 397–405. [Google Scholar] [CrossRef]
- Hashimoto, M.; Une, Y.; Mochizuki, M. Hemagglutinin genotype profiles of canine distemper virus from domestic dogs in Japan. Arch. Virol. 2001, 146, 149–155. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Bryant, D.; Moulton, V. Neighbor-Net: An Agglomerative Method for the Construction of Phylogenetic Networks. Mol. Biol. Evol. 2004, 21, 255–265. [Google Scholar] [CrossRef]
- Bryant, D.; Huson, D.H. NeighborNet: Improved Algorithms and Implementation. Front. Bioinform. 2023, 3, 1210958. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Bryant, D. Application of Phylogenetic Networks in Evolutionary Studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Hamming, R.W. Error Detecting and Error Correcting Codes. Bell Syst. Tech. J. 1950, 29, 147–160. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Vandevelde, M.; Zurbriggen, A. Demyelination in canine distemper virus infection: A review. Acta Neuropathol. 2005, 109, 56–68. [Google Scholar] [CrossRef]
- Pratakpiriya, W.; Ping Teh, A.P.; Radtanakatikanon, A.; Pirarat, N.; Thi Lan, N.; Takeda, M.; Techangamsuwan, S.; Yamaguchi, R. Expression of canine distemper virus receptor nectin-4 in the central nervous system of dogs. Sci. Rep. 2017, 23, 7, 349. [Google Scholar] [CrossRef]
- Tomé, C.L.; Rohden, J.B. The progress and education in history of Sinop—Mato Grosso: “it’s good to expand borders of our fatherland! [O Discurso do Progresso e a Educação na História de Sinop—Mato Grosso: “Como é Bom Alargar Fronteiras de Nossa Pátria!]. Hist. Educ. 2017, 21, 312–334. [Google Scholar] [CrossRef]
- Jorge, R.S.P.; Rocha, F.L.; May Júnior, J.A.; Maroto, G.R. Occurrence of pathogens in brazilian wild carnivores and its implications for conservation and public health [Ocorrência de patógenos em carnívoros selvagens brasileiros e suas implicações para a conservação e Saúde Pública]. Oecologia Aust. 2010, 14, 686–710. [Google Scholar] [CrossRef][Green Version]
- Taques, I.I.G.G.; Morgado, T.O.; Braga, I.A.; Paz, R.C.R.; Correa, S.H.R.; Fritzen, J.T.T.; Alfieri, A.A.; Aguiar, D.M. Antibodies against canine distemper virus, parvovirus and Ehrlichia spp. in wild captive carnivores in midwestern Brazil. Pesq. Vet. Bras. 2018, 38, 1681–1684. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Shaw, M.A.; Goodman, S.J. Pathogen evolution and disease emergence in carnivores. Proc. Biol. Sci. 2007, 274, 3165–3174. [Google Scholar] [CrossRef] [PubMed]
- Nikolin, V.M.; Olarte-Castillo, X.A.; Osterrieder, N.; Hofer, H.; Dubovi, E.; Mazzoni, C.J.; Brunner, E.; Goller, K.V.; Fyumagwa, R.D.; Moehlman, P.D.; et al. Canine distemper virus in the Serengeti ecosystem: Molecular adaptation to different carnivore species. Mol. Ecol. 2017, 26, 2111–2130. [Google Scholar] [CrossRef] [PubMed]
| Primer | Primer Nucleotide Sequence (5′–3′) | Gene | Base Pair | Reference |
|---|---|---|---|---|
| CDV-1F | ACAGGATTGCTGAGGACCTAT | N | 287 | Frisk et al. [18] |
| CDV-2R | CAAGATAACCATGTACGGTGC | N | Frisk et al. [18] | |
| RH3-F * | AGGGCTCAGGTACTCCAGC | H | 1937 | Harder et al. [19] |
| RH4-R * | AATGCTAGAGATGGTTTAATT | H | Harder et al. [19] | |
| H1F ** | ATGCTCTCCTACCAAGACAA | H | 789 | An et al. [17] |
| H1R ** | CATGTCATTCAGCCACCGTT | H | An et al. [17] | |
| H2F ** | AATATGCTAACCGCTATCTC | H | 523 | An et al. [17] |
| H2RB ** | TTTGGTTGCACATAGGGTAG | H | Budaszewski et al. [14] | |
| H3FB ** | CATATGATATATCCCGGGGC | H | 253 | Budaszewski et al. [14] |
| H3R ** | TCARGGWTTTKAACGRYYAC | H | An et al. [17] | |
| CDVF10B ** | TAYCATGAYAGYARTGGTTC | H | 870 | Hashimoto et al. [20] |
| CDVR10 ** | ARTYYTCRACACTGRTKGTG | H | Hashimoto et al. [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavasaki, M.L.; Souza, A.d.O.; Campos, A.N.d.S.; Taques, I.I.G.G.; Barros, R.V.P.d.; Gomes, S.d.S.P.; Pereira, N.A.; Magalhães, T.B.S.; Massoli Junior, E.V.; Pavelegini, L.A.D.; et al. Characterization of the Hemagglutinin Gene of Morbillivirus canis in Domestic Dogs from the Mid-Western Area of Brazil. Vet. Sci. 2025, 12, 948. https://doi.org/10.3390/vetsci12100948
Kavasaki ML, Souza AdO, Campos ANdS, Taques IIGG, Barros RVPd, Gomes SdSP, Pereira NA, Magalhães TBS, Massoli Junior EV, Pavelegini LAD, et al. Characterization of the Hemagglutinin Gene of Morbillivirus canis in Domestic Dogs from the Mid-Western Area of Brazil. Veterinary Sciences. 2025; 12(10):948. https://doi.org/10.3390/vetsci12100948
Chicago/Turabian StyleKavasaki, Mayara Lima, Aneliza de Oliveira Souza, Amanda Noeli da Silva Campos, Isis Indaiara Gonçalves Granjeiro Taques, Rachel Vieira Paes de Barros, Sofia de Souza Pereira Gomes, Nathalia Assis Pereira, Tayane Bruna Soares Magalhães, Edson Viana Massoli Junior, Lucas Avelino D. Pavelegini, and et al. 2025. "Characterization of the Hemagglutinin Gene of Morbillivirus canis in Domestic Dogs from the Mid-Western Area of Brazil" Veterinary Sciences 12, no. 10: 948. https://doi.org/10.3390/vetsci12100948
APA StyleKavasaki, M. L., Souza, A. d. O., Campos, A. N. d. S., Taques, I. I. G. G., Barros, R. V. P. d., Gomes, S. d. S. P., Pereira, N. A., Magalhães, T. B. S., Massoli Junior, E. V., Pavelegini, L. A. D., Campeiro Junior, L. D., Castro, B. G. d., Lunardi, M., & Aguiar, D. M. d. (2025). Characterization of the Hemagglutinin Gene of Morbillivirus canis in Domestic Dogs from the Mid-Western Area of Brazil. Veterinary Sciences, 12(10), 948. https://doi.org/10.3390/vetsci12100948







