Low Seroprevalence of Bovine Brucellosis in Communal Areas of Limpopo Province, South Africa
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
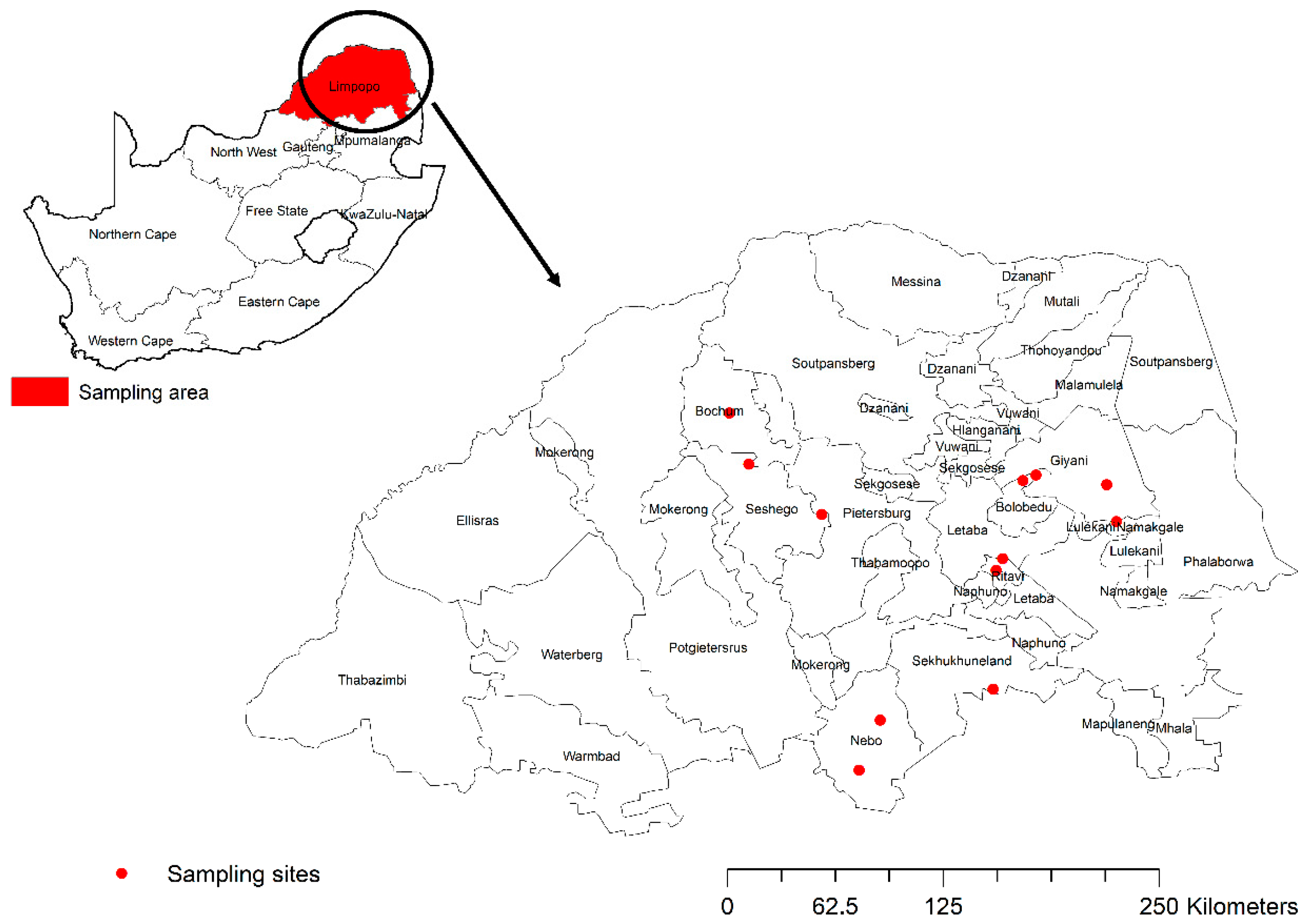
2.1. Study Design and Sampling Sites
2.2. Sample Size Determination and Sampling
2.3. Blood Sample Collection
2.4. Rose Bengal Blood Test (RBT)
2.5. Compliment Fixation Test (CFT)
2.6. Statistical Analysis
2.7. Ethical Statement
3. Results
3.1. Seroprevalence by RBT
3.2. Seroprevalence by CFT
4. Discussion and Conclusions
5. Limitations to the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolo, F.B.; Adesiyun, A.A.; Fasina, F.O.; Katsande, C.T.; Dogonyaro, B.B.; Potts, A.; Matle, I.; Gelaw, A.K.; Van Heerden, H. Seroprevalence and characterization of Brucella species in cattle slaughtered at Gauteng abattoirs, South Africa. Vet. Med. Sci. 2019, 5, 545–555. [Google Scholar] [CrossRef]
- Matle, I.; Ledwaba, B.; Madiba, K.; Makhado, L.; Jambwa, K.; Ntushelo, N. Characterisation of Brucella species and biovars in South Africa between 2008 and 2018 using laboratory diagnostic data. Vet. Med. Sci. 2021, 7, 1245–1253. [Google Scholar] [CrossRef]
- Ducrotoy, M.; Bertu, W.J.; Matope, G.; Cadmus, S.; Conde-Álvarez, R.; Gusi, A.M.; Welburn, S.; Ocholi, R.; Blasco, J.M.; Moriyón, I. Brucellosis in Sub-Saharan Africa: Current challenges for management, diagnosis and control. Acta Trop. 2017, 165, 179–193. [Google Scholar] [CrossRef]
- Van Drimmelen, G.C. The presence of Brucella melitensis infection in sheep in the Transvaal. Bull. I’Office Int. Ds Epizoot. 1965, 64, 745–756. [Google Scholar]
- Department of Agriculture Forestry & Fisheries (DAFF). Discussion Paper on the Review of Bovine Brucellosis Control in South Africa; DAFF: Pretoria, South Africa, 2017. [Google Scholar]
- Barbosa, A.A.; Figueiredo, A.C.S.; Palhao, M.P.; Viana, J.H.M.; Fernandes, C.A.C. Safety of vaccination against brucellosis with the rough strain in pregnant cattle. Trop. Anim. Health Prod. 2017, 49, 1779–1781. [Google Scholar] [CrossRef]
- Simpson, G.; Marcotty, T.; Rouille, E.; Matekwe, N.; Letesson, J.J.; Godfroid, J. Documenting the absence of brucellosis in cattle, goats and dogs in a “One Health” interface in the Mnisi community, Limpopo, South Africa. Trop. Anim. Health Prod. 2018, 50, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Godfroid, J. Brucellosis in wildlife. OIE Rev. Sci. Tech.-Off. Int. Epizoot. 2002, 21, 277–286. [Google Scholar] [CrossRef]
- Muendo, E.N.; Mbatha, P.M.; Macharia, J.; Abdoel, T.H.; Janszen, P.V.; Pastoor, R.; Smits, H.L. Infection of cattle in Kenya with Brucella abortus biovar 3 and Brucella melitensis biovar 1 genotypes. Trop. Anim. Health Prod. 2012, 44, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, U.W.; Bagnall, R.; Perrett, K.; Bosch, B.; Horner, R.; Gummow, B. A serological prevalence survey of Brucella abortus in cattle of rural communities in the province of KwaZulu-Natal, South Africa. J. S. Afr. Vet. Assoc. 2008, 79, 15–18. [Google Scholar] [CrossRef] [PubMed]
- WOAH. Chapter 3.1.4 Brucellosis (Brucella abortus, B. melitensis and B. suis (Infection with B. abortus, B. melitensis and B suis). In Terrestrial Animal Health Code: Recommendations Applicable to OIE Listed Diseases; WOAH: Paris, France, 2018; pp. 355–398. [Google Scholar]
- Hill, W. Standardization of the complement fixation test for brucellosis. Bull. OIE 1963, 60, 401–410. [Google Scholar]
- Herr, S.; Huchzermeyer, H.F.; Te Brugge, L.A.; Williamson, C.C.; Roos, J.A.; Schiele, G.J. The use of a single complement fixation test technique in bovine brucellosis, Johne’s disease, dourine, equine piroplasmosis and Q fever serology. Onderstepoort J. Vet. Res. 1985, 52, 279–282. [Google Scholar]
- Available online: https://www.openepi.com/Proportion/Proportion.htm (accessed on 28 March 2024).
- Mmbengwa, V.; Nyhodo, B.; Myeki, L.; Ngethu, X.; van Schalkwyk, H. Communal livestock farming in South Africa: Does this farming system create jobs for poverty stricken rural areas. Sylwan 2015, 159, 176–192. Available online: https://www.researchgate.net/publication/282648464 (accessed on 10 March 2021).
- Government Gazette. Republic of South Africa. 2002. Animal Health Act No. 23675, Volume 445, Cape Town. 30 July 2002. Available online: https://www.gov.za/sites/default/files/gcis_document/201409/a7-02.pdf (accessed on 28 April 2021).
- Marumo, B.; Hlokwe, T.M.; Kayoka-Kabongo, P.N. Seroprevalence of brucellosis in communal and smallholder cattle farming in Northwest Province, South Africa. Onderstepoort J. Vet. Res. 2023, 90, e2114. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.; Manoto, S.N.; McCrindle, C.M. Sero-prevalence of bovine brucellosis in the Bojanala Region, Northwest Province, South Africa 2009–2013. J. S. Afr. Vet. Assoc. 2020, 91, 1–6. [Google Scholar]
- Ndengu, M.; Matope, G.; de Garine-Wichatitsky, M.; Tivapasi, M.; Scacchia, M.; Bonfini, B.; Pfukenyi, D.M. Seroprevalence of brucellosis in cattle and selected wildlife species at selected livestock/wildlife interface areas of the Gonarezhou National Park, Zimbabwe. Prev. Vet. Med. 2017, 146, 158–165. [Google Scholar] [CrossRef]
- Mfune, R.L.; Mubanga, M.; Silwamba, I.; Sagamiko, F.; Mudenda, S.; Daka, V.; Godfroid, J.; Hangombe, B.M.; Muma, J.B. Seroprevalence of bovine brucellosis in selected districts of Zambia. Int. J. Environ. Res. Public Health 2021, 18, 1436. [Google Scholar] [CrossRef]
- Enström, S.; Nthiwa, D.; Bett, B.; Karlsson, A.; Alonso, S.; Lindahl, J.F. Brucella seroprevalence in cattle near a wildlife reserve in Kenya. BMC Res. Notes 2017, 10, 615. [Google Scholar] [CrossRef]
- Deresa, B.; Tulu, D.; Deressa, F.B. Epidemiological investigation of cattle abortion and its association with Brucellosis in Jimma Zone. Vet. Med. Res. Rep. 2020, 11, 87–98. [Google Scholar] [CrossRef]
- Mangena, M.; Gcebe, N.; Pierneef, R.; Thompson, P.N.; Adesiyun, A.A. Q fever: Seroprevalence, risk factors in slaughter livestock and genotypes of Coxiella burnetii in South Africa. Pathogens 2021, 10, 258. [Google Scholar] [CrossRef]
- Tolosa, T.; Regassa, F.; Belihu, K. Seroprevalence study of bovine brucellosis in extensive management system in selected sites of Jimma Zone, Western Ethiopia. Bull. Anim. Health Prod. Afr. 2008, 56. [Google Scholar] [CrossRef]
- Bayemi, P.H.; Webb, E.C.; Nsongka, M.V.; Unger, H.; Njakoi, H. Prevalence of Brucella abortus antibodies in serum of Holstein cattle in Cameroon. Trop. Anim. Health Prod. 2009, 41, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Boukary, A.R.; Saegerman, C.; Abatih, E.; Fretin, D.; Bada, R.A.; De Deken, R.; Harouna, H.A.; Yenikoye, A.; Thys, E. Seroprevalence and potential risk factors for Brucella spp. infection in traditional cattle, sheep and goats reared in urban, periurban and rural areas of Niger. PLoS ONE 2013, 8, e83175. [Google Scholar] [CrossRef] [PubMed]
| Variable | Samples Collected and Tested for RBT (Positive Reactor Group) | RBT Positive (No.) | Sero Prevalence % 95% CI |
|---|---|---|---|
| District municipality | Mopani (n = 823) | 48 | 5.83 (4.38–7.59) |
| Capricorn (n = 167) | 0 | 0.00 (0.00–0.26) | |
| Sekhukhune (n = 143) | 0 | 0.00 (0.00–0.26) | |
| Local municipality | Tzaneen (n = 599) | 27 | 4.51 (3.05.6.40) |
| Letaba (n = 224) | 21 | 9.38 (6.05–13.74) | |
| Blouberg (n = 100) | 0 | 0.00 (0.00–2.95) | |
| Ephraim Mogale (n = 43) | 0 | 0.00 (0.00–6.73) | |
| Makhuduthamaga (n = 100) | 0 | 0.00 (0.00–2.95) | |
| Polokwane (n = 67) | 0 | 0.00 (0.00–4.37) | |
| Sex | Female (n = 1103) | 48 | 04.35 (3.26–5.68) |
| Male (n = 30) | 0 | 0.00 (0.00–0.26) | |
| Age | >5 years (n = 479) | 25 | 5.22 (3.48–7.49) |
| <5 years (n = 654) | 23 | 3.52 (2.29–5.15) | |
| Breed | Nguni (n = 581) | 27 | 4.65 (3.21–6.72) |
| Mixed (n = 126) | 11 | 8.73 (4.87–15.25) | |
| Unidentified (n = 380) | 4 | 1.05 (0.35–2.61) | |
| Brahman (n = 46) | 6 | 13.04 (5.59–25.68) | |
| Birth history | Heifer (n = 139) | 12 | 8.63 (4.76–14.21) |
| <2 times (n = 338) | 13 | 3.85 (2.155–6.326) | |
| >2 times (n = 218) | 16 | 7.34 (4.41–11.14) | |
| Unidentified (n = 402) | 7 | 1.74 (0.76–3.41) | |
| Abortion (n = 4) | 0 | 0.00 (0.10–0.83) | |
| Expecting (n = 2) | 0 | 0.00 (0.10–0.83) | |
| Male (n = 30) | 0 | 0.00 (0.10–0.83) |
| Variable | Category | No. of Positive Animals | (%) |
|---|---|---|---|
| Frequency of birth | Not indicated Heifer Less than 2 More than 2 | 4 2 2 1 | 44.40 22.20 22.20 11.10 |
| Age | More than 5 Less than 5 | 6 3 | 66.70 33.30 |
| Breed | Nguni Mix Not indicated Brahman | 5 2 20 | 55.60 22.20 22.20 00.00 |
| Gender | Male Female | 09 | 0.00 100 |
| Municipality | Tzaneen Letaba | 5 4 | 55.60 44.40 |
| District | Mopani | 9 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madiba, K.; Gcebe, N.; Boshoff, C.; Sirdar, M.; Ramaselela, N.; Hlokwe, T. Low Seroprevalence of Bovine Brucellosis in Communal Areas of Limpopo Province, South Africa. Vet. Sci. 2025, 12, 942. https://doi.org/10.3390/vetsci12100942
Madiba K, Gcebe N, Boshoff C, Sirdar M, Ramaselela N, Hlokwe T. Low Seroprevalence of Bovine Brucellosis in Communal Areas of Limpopo Province, South Africa. Veterinary Sciences. 2025; 12(10):942. https://doi.org/10.3390/vetsci12100942
Chicago/Turabian StyleMadiba, Karabelo, Nomakorinte Gcebe, Carin Boshoff, Mohamed Sirdar, Ngoako Ramaselela, and Tiny Hlokwe. 2025. "Low Seroprevalence of Bovine Brucellosis in Communal Areas of Limpopo Province, South Africa" Veterinary Sciences 12, no. 10: 942. https://doi.org/10.3390/vetsci12100942
APA StyleMadiba, K., Gcebe, N., Boshoff, C., Sirdar, M., Ramaselela, N., & Hlokwe, T. (2025). Low Seroprevalence of Bovine Brucellosis in Communal Areas of Limpopo Province, South Africa. Veterinary Sciences, 12(10), 942. https://doi.org/10.3390/vetsci12100942





