Simple Summary
Gabapentin is popular in veterinary medicine due to its depressive effects on the central nervous system, analgesic properties, and behavioral impact. Stress or aggressive behavior can make handling cats during clinical examinations extremely difficult. This systematic review, based on twenty selected articles, aims to evaluate gabapentin’s impact on sedation, anxiety, behavioral modification, pain, and cardiovascular function in feline patients during veterinary appointments. The review shows that gabapentin mildly to moderately reduces anxiety in cats. However, responsiveness may vary depending on the dosage used. Gabapentin can be orally administered mixed with food and does not negatively impact the cardiovascular system. Overall, gabapentin may reduce stress in cats during veterinary examinations.
Abstract
Gabapentin is a drug frequently used in veterinary medicine because of its recognized analgesic, sedative, and behavioral properties. In recent years, its use has become particularly important in feline medicine. The clinical examination of a cat can be challenging due to various factors, such as patient compliance or inadequate handling techniques employed by veterinary staff, which can lead to fear-based aggressive behavior in cats. A systematic review based on the PRISMA statement was conducted from March to September 2024. Out of 543 articles, only 20 were included in the review. The objective of the systematic review was to describe the impact of gabapentin on sedation, anxiety, behavioral modification, pain, and cardiovascular function in feline patients during veterinary appointments. Gabapentin’s effects may be dose-dependent, though a specific dosage is not available. Administering gabapentin with wet or dry food is suggested. Furthermore, gabapentin has positive behavioral, analgesic, and sedative effects, ensuring an anxiolytic effect without altering any cardiovascular, echocardiographic, or hemodynamic aspects.
1. Introduction
Gabapentin (1-aminomethylcyclohexane acetic acid) is a drug that functions as an analogue of gamma amino butyric acid (GABA) without altering the binding, re-uptaking, or degradation of this neurotransmitter. It seems to exert its action by binding the alpha 2 delta-1 subunit of voltage-gated calcium GABA-associated receptors within the central nervous system [1]. By reducing calcium influx, it inhibits the release of excitatory neurotransmitters (e.g., substance P, glutamate, norepinephrine) [2]. Morphologically, it appears as a crystalline, hydrophilic substance with a bitter taste [3].
The optimal method of administration for gabapentin in cats is via the oral route. This is due to the lower bioavailability of transdermal administration [1].
The absorption rate is about 3 h after the ingestion in most species. In cats, it has a bioavailability average of 90% after oral administration, but a considerable degree of variation has been observed among patients. It was determined that peak levels in cats occurred approximately 100 min after the administration of the drug [4]. The volume of distribution is relatively low (Vdss of 0.65 L/kg). It is excreted by the kidney and entirely eliminated through the urine [5]. The clearance in cats is about 3 mL/min/kg, with a mean elimination half-life of 2.8 h [6]. This drug was first introduced in human medicine approximately 40 years ago to treat neuropathic pain and refractory partial seizures, with rare reported side effects [7]. In cats, gabapentin was used off-label at first as an anti-epileptic drug [8]. Nowadays, it is prevalently used to manage chronic and neuropathic pain [9]. Additionally, in cats, gabapentin showed efficacy in anxiety management [10].
Moreover, regarding its use as an analgesic in neuropathic conditions, this pharmaceutical agent has been demonstrated to be efficacious in the veterinary management of pain associated with spinal cord injury and similar disorders [2].
In recent years, gabapentin has also been used extensively in veterinary behavioral medicine with the aim of reducing stress levels in cats by making them more compliant during physical evaluation [11].
It is important to emphasize that this is still an off-label drug and its evidence in veterinary medicine is limited for neuropathic pain conditions other than behavioral and sedative effects.
Stress in human medicine is defined as a condition in which an environmental demand exceeds the body’s ability to regulate itself, especially when this event is unpredictable and uncontrollable [11]. In veterinary medicine, the term “stress” is a multifaceted concept with numerous definitions. From the perspective of health and disease, stress is frequently defined in terms of stressor. Therefore, a stressor could be any agent that instigates the activation of the central threat response system [12]. Buffington [13] proposed a taxonomy of feline stress duration, grounded in Shonkoff’s classification of stressor potential and response duration [12]. It was divided into moderate, mild, and toxic. Mild stress is context-dependent and may facilitate autoregulation during new situations. These reactions to stress occur within the safe, predictable environment of stable and supportive relationships (e.g., exposing the kitten to new stimuli at home). Moderate stress arises when threat is greater than the mild one, such as boredom or an acute illness. Unlike mild stress, the cat is exposed to moderate stress for a longer period but is able to regulate itself because its surroundings allow it to calm down. Finally, toxic stress, more known as chronic stress, is the most threatening one as it may not be possible for the cat to return to its basal state. In fact, some situations, such as mistreatment of the animal, chronic illness, a poorly performed physical evaluation, or a prolonged hospital stay, can lead to chronic stress.
In fact, the relationship between cats and veterinarians during the clinical examination is well known to be complicated and sometimes challenging, as being in a new environment combined with unfamiliar stimuli and smells causes the animal to experience high levels of stress. Therefore, cats show significantly lower stress values when examined at home compared to a hospital environment [14]. Furthermore, cats are not used to the restraint and manipulation required for the physical examination. Indeed, all of this could lead the cat to react by following the fight-or-flight behavioral pattern, attempting to flee or attack the operator. Furthermore, an inappropriate approach or inexperience could be responsible for anxiety or phobia on subsequent visits. All these events could lead to the development of avoidance or aggressive behavior, even towards the owner, prior to future veterinary appointments. In addition, the cat may experience discomfort from being placed in the carrier on the way to the waiting room and, finally, to the clinical examination table. This can significantly alter not only the cat’s behaviours but also clinical parameters and consequently diagnosis and treatment, not to mention the physical danger to the operator when dealing with these subjects [15]. To improve this issue, behavioral medicine can be employed. Unfortunately, appropriate cat handling is not often applied by veterinary staff and owners in most facilities, and this represents a threat to the welfare of the animal [16]. Consequently, euthanasia may be used when the animal’s behavior is deemed to be untreatable [17]. In order to improve the relationship between veterinarians and cats, there are both practical and pharmacological alternatives that help the animal to greatly reduce stress levels and, consequently, ensure a much more positive experience in unfamiliar situations. On one hand, it is possible to adopt manual practices such as avoiding scruffling the cat, having a kinder and more patient approach to it, and environmental enrichment of the living areas [18]. On the other hand, there are number of drugs on the market that aim significantly reduce stress levels and improve interaction with humans and veterinarians such as Alpha-casozepine [18], Trazodone, and gabapentin [19]. Due to the limited available bibliography and to standardize the data related to gabapentin, this review focuses exclusively on the latter drug.
This systematic review may provide clearer indication to the practitioner on the use of gabapentin in cats considering the different clinical needs. The aim of this review is to describe the impact of gabapentin on sedation, anxiety, behavioral modification, pain, and cardiovascular function in feline patients during veterinary appointments.
2. Materials and Methods
A systematic literature search was conducted based on the Preferred Reporting Items of Systematic reviews and Meta-Analysis, PRISMA 2020 statement [20]. Eligibility criteria were the following:
- Population: Domestic cats (Felis silvestris catus), regardless of breed, sex, or health status.
- Intervention: Oral administration of gabapentin at any dosage and frequency.
- Comparator: Placebo, no treatment, or other sedative, anxiolytic, or analgesic agents.
- Outcomes: Defined a priori as: behavioral changes; sedative effects; analgesic effects; cardiovascular responses; adverse effects.
- Study design: Randomized controlled trials (RCTs), non-randomized controlled trials, observational studies, case reports, systematic reviews, and meta-analyses. Editorials and abstracts were excluded.
The search strategy was submitted on three main databases: Pubmed, Web of Science, and Scopus, between 1 March 2024 and 30 September 2024. The keywords used for the research were “gabapentin”; “cats OR feline” using the Boolean operator “AND”, limited to studies published in Italian, English, Spanish, or French. Titles and abstracts were screened by two of the authors independently. Articles not related to the analgesic, behavioral, or sedative effect of gabapentin in cats were ruled out. Articles which administered gabapentin to cats via a route other than oral were ruled out. Articles that used gabapentin in combination with other drugs were also excluded (such as multimodal anesthesia). Articles that evaluated the single effect of gabapentin with other drugs with similar effects such as Alprazolam or Melatonin were included. Primary outcomes included sedative and behavioral effects. Secondary outcomes were cardiovascular and analgesic effects as well as adverse events. These outcomes were pre-defined prior to data collection. All studies published up to September 2024 were considered. No lower time limit was applied.
Risk of bias was assessed for each included study. For randomized controlled trials, the SYRCLE’s risk of bias tool was used [21]. For non-randomized studies, the ROBINS-I tool was applied [22]. Each study was evaluated independently by two reviewers with disagreement resolved by consensus.
3. Results
The initial literature research from the three databases pointed out a total of 543 records: 63 from Pubmed; 115 from Web of Science, and 365 from Scopus. After removal of duplicates (n), 515 records were screened. Out of these 515 records, 497 were removed because they were not related to the topic, such as those examining multimodal association of gabapentin with other drugs or gabapentin used for other purposes, such as anti-epileptic use. In the end, a total of 20 articles were considered eligible for this review and were included (Figure 1)
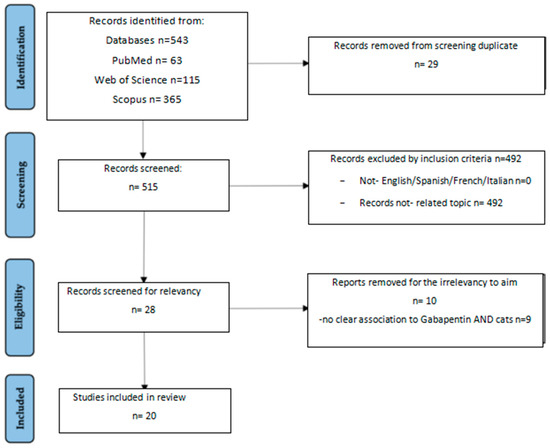
Figure 1.
Study selection based on PRISMA flow chart.
The 20 articles assessed in this review are reported by specific criteria (see Table 1).

Table 1.
Summary of the 20 selected studies, including author, year of publication, number of cats enrolled, gabapentin dosage, purpose of the study, study outcomes, number of references cited, article type, reported effects related to gabapentin administration, and scoring systems used.
The included studies comprised six RCTs, assessed using the SYRCLE risk of bias tool, and thirteen observational studies or non-randomized controlled studies, assessed using ROBINS-I. One narrative review was excluded from bias assessment. Study purpose varied, focusing on sedative effects, behavioral responses, analgesia, cardiovascular safety, and side effects. Sample sizes ranged from 3 to 75 cats. Gabapentin dosages ranged from 9 to 47 mg/Kg, typically administered orally 60–120 min before assessment. Among the RCTs, four were judged to have low risk of bias, while two had moderate concerns. Overall, the methodological quality of RCTs was high with adequate randomization and outcome assessment. The non-randomized studies generally exhibited moderate to serious risk of bias, particularly due to confounding, lack of control groups, and subjectivity in behavioral outcome assessment. Three studies were considered at serious risk of bias due to lack of comparator and retrospective design. A complete risk of bias is provided in Table 2.

Table 2.
Risk of bias assessment for the 20 included studies.
4. Discussions
This systematic review identified 20 articles focusing on the sedative, analgesic, behavioral, and cardiovascular effects, as well as the adverse effects, in cats. To the authors’ knowledge, no other systematic reviews on this topic were found in the databases used for the search (PubMed, Web of Science, and Scopus).
All RCTs reported that oral gabapentin significantly reduced stress levels and improved handling compliance, especially when administered 90–120 min before evaluation. The effect was dose-dependent in some studies [30], with enhanced side effects at ≥30 mg/kg [23]. Conversely, Kruszka [24] states that behavioral changes depend on the individual subjects and are not related to the dosage. Furthermore, the article emphasized a much calmer attitude when gabapentin was administered prior to transporting the cat from its familiar environment to the clinic. Behavioral scoring tools included the Cat Stress Score, Glasgow Composite Pain Scale, and Feline Grimace Scale. Several observational studies supported these findings, although individual variability was noted [24,36,37,40]. One article found that many cats classified as “fear-aggressive behavior” that were given a standard dosage of the drug [24] showed a noticeable behavioral change two hours after administration. The examined animals were much friendlier and more approachable toward the operator, with or without food present.
Four studies documented analgesic effects. Steagall [40] observed reduced postoperative pain following ovariohysterectomy when gabapentin was administered with buprenorphine. In an acute experimental model, Pypendop [23] found no effect of gabapentin on thermal antinociception. Lorenz [28] also reported that chronic pain decreased according to the animals’ activity after about one month of gabapentin administration in three cats with bone trauma. Guedes [38] reported improved pain scores in osteoarthritic cats after chronic administration. However, gabapentin alone did not demonstrate strong analgesic efficacy in the short term. Guedes [38] hypothesizes that, to observe a significant reduction in pain, gabapentin must be administered over a long period, emphasizing the role of the accumulation effect.
Four studies investigated cardiovascular parameters. Gabapentin did not significantly alter blood pressure, heart rate, or echocardiographic findings in healthy or hyperthyroid cats. One study reported a minor decrease in systolic function and T-wave polarity change, but these findings were not clinically significant [27,30,32,33]. However, it is possible to say that three of four studies had a moderate risk of bias; thus, the results may not be reliable.
Compared to placebo, gabapentin has consistent anxiolytic and sedative effects supported by behavioral and, in some cases, physiological data. Indeed, in almost all studies in which gabapentin was compared to placebo, it showed a great efficacy in reducing stress, improving compliance and promoting calmer behavior during the veterinary examination [25,29,39]. Papageorgiou [40] found that gabapentin and alprazolam produced comparable levels of sedation and anxiolysis. No significant differences were observed in post-operative outcomes, and post-operative profiles were similar. Thus, gabapentin offers a non-benzodiazepine alternative with similar efficacy but potentially safer long-term profile. Tuleski [30] demonstrated that gabapentin and melatonin significantly improved compliance during examination without affecting cardiovascular parameters. Comparative studies with Mirtazapine, Fantinatu [9], and Spano [41] found that both drugs increased appetite and improved demeanor, but gabapentin has a more direct impact on compliance and sedation. The combination of topical mirtazapine and oral gabapentin was synergistic, facilitating ingestion and enhancing calming effects.
According to some studies [24,25,29,31,32,39], side effects have been reported during gabapentin administration. The most common were ataxia, salivation, and stupor. These were often accompanied by protrusion of the third eyelid, fasciculations, vomiting, and diarrhea. Van Haaften [29] correlated these side effects with the dosage administered. Indeed, a higher dosage was associated with a greater likelihood of adverse effects emerging. Therefore, we can assume that the observed side effects are related to toxicity (dose-related or individual reaction), which resolved within eight to ten hours after administration [29]. Furthermore, no side effects were reported in cats with hyperthyroidism associated with gabapentin administration.
Outside the scope of this systematic review, we can make some considerations regarding dosages, intervals, and administration methods. There is no uniformity in dosing intervals in the reviewed literature. In fact, gabapentin administration schedules of once, twice, or three times a day were described. Furthermore, the administered dose of gabapentin varies. In most articles, the administered dose was 100 mg per cat, or 150 mg if the cat weighed more than 7 kg. Other articles administered a higher dosage of gabapentin than 150 mg per cat, such as 200 mg per cat or up to 30 mg/kg [23]. Only one article established the dose prior to the start of the study at 10 mg/kg [38]. The modality of gabapentin administration described in all the analyzed articles is uniform. It is, in fact, dispensed by the oral route, either via a mixture of wet or dry food [27] or providing it as it is [26]. Gabapentin was offered to patients in different ways: by offering the animal a bowl and assessing its intake capacity, by syringe with a Tom Cat catheter, or by combining it with wet food. Only one article [28] describes administering gabapentin in syrup form (gabapentin syrup 40 mg/mL, Nova Laboratories), which is an oil-based compounded liquid suspension. A case series reported the parenteral administration of gabapentin via a feeding tube [28].
5. Conclusions
This systematic review demonstrates that gabapentin has consistent and beneficial effects on key clinical outcomes in domestic cats undergoing veterinary procedures. Across the selected studies, gabapentin was shown to reliably induce sedation, reduce stress-related behaviors, and exert analgesic effects in both acute and chronic settings.
Behavioral improvements were particularly notable in fearful or aggressive cats, with increased compliance and reduced signs of anxiety during clinical examinations. Sedative effects were observed in the majority of studies, with clear facilitation of handling and physical evaluation. However, it is important to underline how side effects such as ataxia or sialorrhea may result as the dosage increases or due to individual reactions. In addition, gabapentin contributed to pain reduction, particularly in postoperative scenarios and chronic pain conditions, as evidenced by behavioral scoring systems and, in some cases, physiological indicators such as serum cortisol levels.
Importantly, gabapentin was well tolerated across the included studies. Reported adverse effects were generally mild and transient, with no significant alterations in cardiovascular or echocardiographic parameters.
In conclusion, gabapentin emerges as a multifunctional pharmacological option in feline veterinary medicine, providing consistent sedation, behavioral calming, and analgesia. These properties make it a valuable tool for enhancing feline welfare during clinical procedures. Further research is encouraged to support the development of standardized dosage and outcome measures and expand evidence on its long-term safety and efficacy.
Author Contributions
Conceptualization, M.V.L., F.G. and F.S.; methodology, M.V.L., M.P., C.P., F.G., and F.S.; software, M.V.L., M.P.; validation, C.P., F.G., F.S.; formal analysis, M.V.L., M.P., F.S.; investigation, M.V.L., F.S., F.G.; resources, F.S., F.G.; data curation, M.V.L.; writing—original draft preparation, M.V.L., F.S.; writing—review and editing, M.V.L., F.S., C.P., F.G., F.S.; visualization, M.V.L.; supervision, F.S., F.G.; project administration, F.S., F.G.; funding acquisition, F.S., F.G.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adrian, D.; Papich, M.G.; Baynes, R.; Stafford, E.; Lascelles, B.D.X. The pharmacokinetics of gabapentin in cats. J. Vet. Intern. Med. 2018, 32, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Plumb, D.C. Plumb’s Veterinary Drug Handbook, 7th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bassilios, N.; Launay-Vacher, V.; Khoury, N.; Rondeau, E.; Deray, G.; Sraer, J. Gabapentin neurotoxicity in a chronic haemodialysis patient. Nephrol. Dial. Transplant. 2001, 16, 2112–2113. [Google Scholar] [CrossRef]
- Rose, M.A.; Kam, P.C. Gabapentin: Pharmacology and its use in pain management. Anaesthesia 2002, 57, 451–462. [Google Scholar] [CrossRef]
- Siao, K.T.; Pypendop, B.H.; Ilkiw, J.E. Pharmacokinetics of gabapentin in cats. Am. J. Vet. Res. 2010, 71, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Noah, S. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int. J. Dermatol. 2003, 42, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, F.; Negro, V.; Ravasio, G.; Villa, R.; Draghi, S.; Cagnardi, P. Gabapentin: Clinical use and pharmacokinetics in dogs, cats, and horses. Animals 2023, 13, 2045. [Google Scholar] [CrossRef]
- Podell, M. Antiepileptic drug therapy and monitoring. Clin. Tech. Small Anim. Pract. 1998, 13, 185–192. [Google Scholar] [CrossRef]
- Fantinatu, M.; Trnka, J.; Signor, A.; Dumond, S.; Jourdan, G.; Verwaerde, P.; Priymenko, N. Appetite-stimulating effect of gabapentin vs mirtazapine in healthy cats post-ovariectomy. J. Feline Med. Surg. 2020, 22, 1176–1183. [Google Scholar] [CrossRef]
- Lazarus, R.S.; DeLongis, A.; Folkman, S.; Gruen, R. Stress and adaptational outcomes: The problem of confounded measures. Am. Psychol. 1985, 40, 770–779. [Google Scholar] [CrossRef]
- Shonkoff, J.P. Building a New Biodevelopmental Framework to Guide the Future of Early Childhood Policy. Child Dev. 2010, 81, 357–367. [Google Scholar] [CrossRef]
- Buffington, C.A.T.; Bain, M. Stress and feline health. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Nibblett, B.M.; Ketzis, J.K.; Grigg, E.K. Comparison of stress exhibited by cats examined in a clinic versus a home setting. Appl. Anim. Behav. Sci. 2015, 173, 68–75. [Google Scholar] [CrossRef]
- Rodan, I.; Sundahl, E.; Carney, H.; Gagnon, A.C.; Heath, S.; Landsberg, G.; Seksel, K.; Yin, S. AAFP and ISFM Feline-Friendly Handling Guidelines. J. Feline Med. Surg. 2011, 13, 364–375. [Google Scholar] [CrossRef]
- Overall, K.L. Evidence-based paradigm shifts in veterinary behavioral medicine. J. Am. Vet. Med. Assoc. 2019, 254, 798–807. [Google Scholar] [CrossRef]
- Kogan, L.R.; Hellyer, P.W.; Rishniw, M.; Schoenfeld-Tacher, R. Veterinary Behavior: Assessment of Veterinarians’ Training, Experience, and Comfort Level with Cases. J. Vet. Med. Educ. 2020, 47, 158–169. [Google Scholar] [CrossRef]
- Taylor, S.; St Denis, K.; Collins, S.; Dowgray, N.; Ellis, S.L.; Heath, S.; Rodan, I.; Ryan, L. 2022 ISFM/AAFP Cat Friendly Veterinary Environment Guidelines. J. Feline Med. Surg. 2022, 24, 1133–1163. [Google Scholar] [CrossRef]
- Landsberg, G.; Milgram, B.; Mougeot, I.; Kelly, S.; de Rivera, C. Therapeutic effects of an alpha-casozepine and L-tryptophan supplemented diet on fear and anxiety in the cat. J. Feline Med. Surg. 2016, 19, 594–602. [Google Scholar] [CrossRef]
- Erickson, A.; Harbin, K.; MacPherson, J.; Rundle, K.; Overall, K.L. A review of pre-appointment medications to reduce fear and anxiety in dogs and cats at veterinary visits. Can. Vet. J. 2021, 62, 952–960. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.; Wang, D.; Ren, P.; Hong, Q.; Kang, D. The ROBINS-I and the NOS had similar reliability but differed in applicability: A random sampling observational studies of systematic reviews/meta-analysis. J. Evid.-Based Med. 2021, 14, 112–122. [Google Scholar] [CrossRef]
- Pypendop, B.H.; Siao, K.T.; Ilkiw, J.E. Thermal antinociceptive effect of orally administered gabapentin in healthy cats. Am. J. Vet. Res. 2010, 71, 1027–1032. [Google Scholar] [CrossRef]
- Kruszka, M.; Graff, E.; Medam, T.; Masson, S. Clinical evaluation of the effects of a single oral dose of gabapentin on fear-based aggressive behaviors in cats during veterinary examinations. J. Am. Vet. Med. Assoc. 2021, 259, 1285–1291. [Google Scholar] [CrossRef]
- Pankratz, K.E.; Ferris, K.K.; Griffith, E.H.; Sherman, B.L. Use of single-dose oral gabapentin to attenuate fear responses in cage-trap confined community cats: A double-blind, placebo-controlled field trial. J. Feline Med. Surg. 2017, 20, 535–543. [Google Scholar] [CrossRef]
- Versteg, N.; Dias, T.P.; de Freitas, V.R.; das Neves, V.B.; Gomes, M.R.; Meinerz, A.R.M.; Jorge, S.; Rondelli, M.C.H.; Cleff, M.B. A comparative study between integrative practices and preappointment gabapentin on serum cortisol in cats. Vet. Res. Commun. 2024, 48, 3469–3474. [Google Scholar] [CrossRef]
- Allen, M.E.; LeBlanc, N.L.; Scollan, K.F. Hemodynamic, echocardiographic, and sedative effects of oral gabapentin in healthy cats. J. Am. Anim. Hosp. Assoc. 2021, 57, 278–284. [Google Scholar] [CrossRef]
- Lorenz, N.D.; Comerford, E.J.; Iff, I. Long-term use of gabapentin for musculoskeletal disease and trauma in three cats. J. Feline Med. Surg. 2012, 15, 507–512. [Google Scholar] [CrossRef] [PubMed]
- van Haaften, K.A.; Forsythe, L.R.; Stelow, E.A.; Bain, M.J. Effects of a single preappointment dose of gabapentin on signs of stress in cats during transportation and veterinary examination. J. Am. Vet. Med. Assoc. 2017, 251, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Ruviaro Tuleski, G.L.; Silveira, M.F.; Bastos, R.F.; Pscheidt, M.J.; Prieto, W.D.; Sousa, M.G. Behavioral and cardiovascular effects of a single dose of gabapentin or melatonin in cats: A randomized, double-blind, placebo-controlled trial. J. Feline Med. Surg. 2022, 24, e524–e534. [Google Scholar] [CrossRef] [PubMed]
- Veronezi, T.M.; Lopes, D.J.; Zardo, I.L.; Ferronatto, J.V.; Trojan, M.M.; Franck, K.R.; de Azevedo, A.F.; Spiering, A.G.; Nunes, L.N.; Fadel, L.; et al. Evaluation of the effects of gabapentin on the physiologic and echocardiographic variables of healthy cats: A prospective, randomized and blinded study. J. Feline Med. Surg. 2022, 24, e498–e504. [Google Scholar] [CrossRef]
- De Lombaert, M.C.; Lourenço, B.N.; Coleman, A.E.; Arne, A.M.; Berghaus, R.D.; Schmiedt, C.W. Effect of gabapentin on ambulatory, direct, systemic arterial blood pressure in apparently healthy cats in the at-home and in-clinic environments. J. Feline Med. Surg. 2023, 25, 1098612X231188770. [Google Scholar] [CrossRef]
- Quimby, J.M.; Jones, S.E.; Saffire, A.; Brusach, K.K.; Kurdziel, K.; George, Z.; Paschall, R.E.; Aarnes, T.K. Assessment of the effect of gabapentin on blood pressure in cats with and without chronic kidney disease. J. Feline Med. Surg. 2024, 26, 1098612X241240326. [Google Scholar] [CrossRef]
- Gurney, M.; Gower, L. Randomised clinical trial evaluating the effect of a single preappointment dose of gabapentin on signs of stress in hyperthyroid cats. J. Feline Med. Surg. 2022, 24, e85–e89. [Google Scholar] [CrossRef]
- Crowe, Y.C.; Groth, A.D.; Billson, F.M.; White, J.; Coall, S.M.; Yates, K.L.; Premont, J.E. Gabapentin reduces stress and does not affect ocular parameters in clinically normal cats. Vet. Ophthalmol. 2022, 25, 493–498. [Google Scholar] [CrossRef]
- Hudec, C.P.; Griffin, C.E. Changes in the stress markers cortisol and glucose before and during intradermal testing in cats after single administration of pre-appointment gabapentin. J. Feline Med. Surg. 2019, 22, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.G.P.; Meadows, J.M.; Pypendop, B.H.; Johnson, E.G.; Zaffarano, B. Assessment of the effects of gabapentin on activity levels and owner-perceived mobility impairment and quality of life in osteoarthritic geriatric cats. J. Am. Vet. Med. Assoc. 2018, 253, 579–585. [Google Scholar] [CrossRef]
- Eagan, B.H.; van Haaften, K.; Protopopova, A. Daily gabapentin improved behavior modification progress and decreased stress in shelter cats from hoarding environments in a double-blind randomized placebo-controlled clinical trial. J. Am. Vet. Med. Assoc. 2023, 61, 1305–1315. [Google Scholar] [CrossRef]
- Papageorgiou, V.; Ververidis, C.; Mylonakis, M.E.; Savvas, I.; Kazakos, G. Orally administered gabapentin and alprazolam induce comparable levels of anxiolysis and sedation in cats. J. Am. Vet. Med. Assoc. 2024, 262, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Spano, V.; Springer, C.M.; Christensen, E.L.; Albright, J.D. Effects of transdermal mirtazapine and oral gabapentin as pre-veterinary visit pharmaceuticals for shelter cats. J. Vet. Behav. 2023, 64–65, 47–53. [Google Scholar] [CrossRef]
- Steagall, P.V.; Monteiro, B.P. Acute pain in cats: Recent advances in clinical assessment. J. Feline Med. Surg. 2019, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).