Influence of Prednisolone Treatment on Serum Bile Acid Concentrations in Cats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
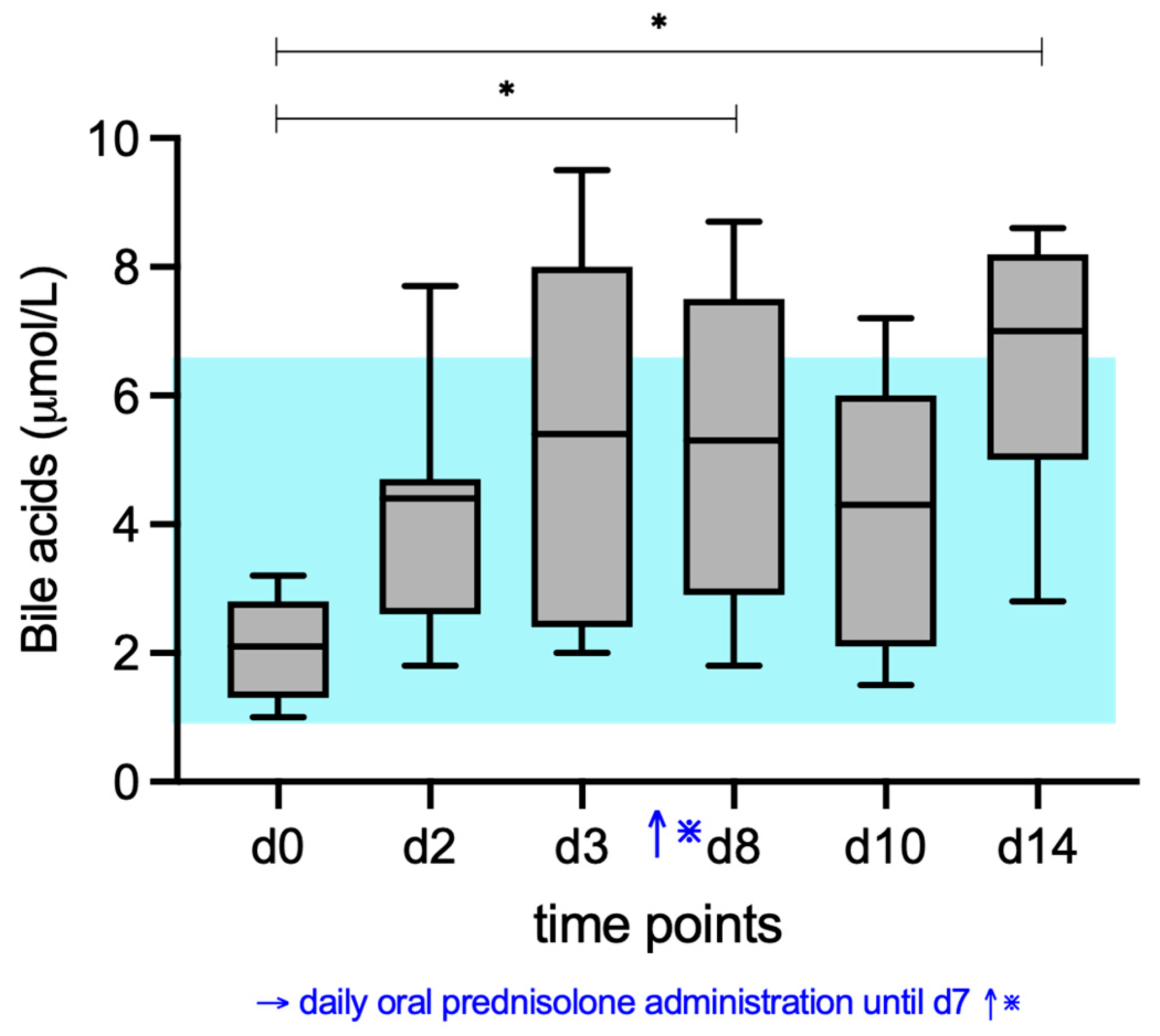
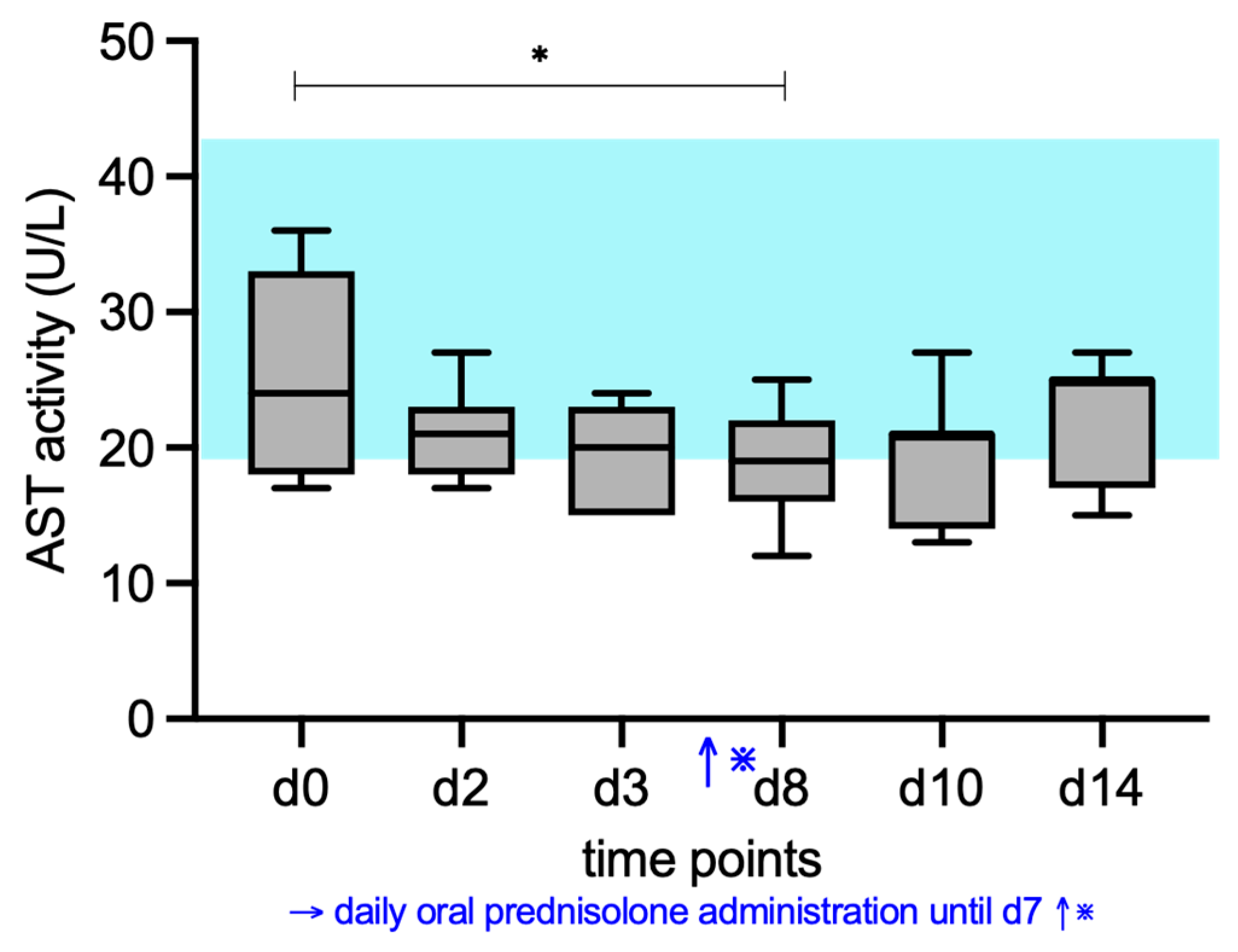
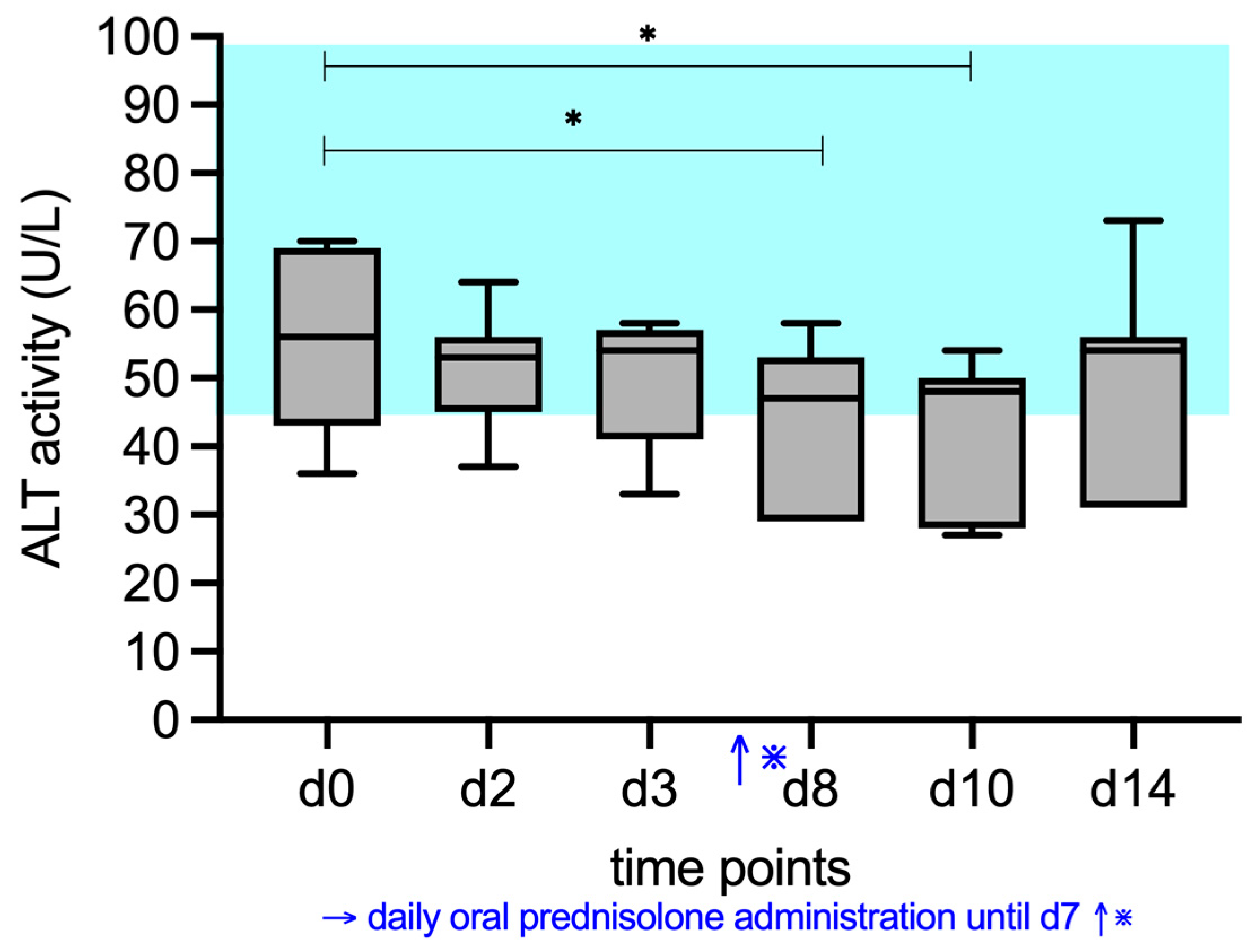
3. Results
3.1. Bile Acid Concentrations
3.2. ALP, AST, ALT, and GGT Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| GGP | γ-glutamyltransferase |
| RI | Reference interval |
| p | p-value |
| CBC | Complete blood count |
References
- Bayton, W.A.; Westgarth, C.; Scase, T.; Price, D.J.; Bexfield, N.H. Histopathological Frequency of Feline Hepatobiliary Disease in the UK. J. Small Anim. Pract. 2018, 59, 404–410. [Google Scholar] [CrossRef]
- Twedt, D.C. Twedt DC. Update on Feline Hepatobiliary Disease. J Feline Med Surg. 2006. J. Feline Med. Surg. 2016, 8, xi–xiv. [Google Scholar] [CrossRef]
- Center, S.A. Serum Bile Acids in Companion Animal—Medicine. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 625–657. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.M.A.; Penning, L.C.; Rothuizen, J.; Favier, R.P. Retrospective Comparison of Prednisolone and Ursodeoxycholic Acid for the Treatment of Feline Lymphocytic Cholangitis. Vet. J. 2013, 195, 205–209. [Google Scholar] [CrossRef]
- Otte, C.M.; Penning, L.C.; Rothuizen, J. Feline Biliary Tree and Gallbladder Disease: Aetiology, Diagnosis and Treatment. J. Feline Med. Surg. 2017, 19, 514–528. [Google Scholar] [CrossRef]
- Sharkey, L.C.; Ployngam, T.; Tobias, A.H.; Torres, S.M.F. Effects of a Single Injection of Methylprednisolone Acetate on Serum Biochemical Parameters in 11 Cats. Vet. Clin. Pathol. 2007, 36, 184–187. [Google Scholar] [CrossRef]
- Lowe, A.D.; Campbell, K.L.; Barger, A.; Schaeffer, D.J.; Borst, L. Clinical, Clinicopathological and Histological Changes Observed in 14 Cats Treated with Glucocorticoids. Vet. Rec. 2008, 162, 777–783. [Google Scholar] [CrossRef]
- Khelik, I.A.; Berger, D.J.; Mochel, J.P.; Seo, Y.-J.; Palerme, J.-S.; Ware, W.A.; Ward, J.L. Clinicopathologic, Hemodynamic, and Echocardiographic Effects of Short-Term Oral Administration of Anti-Inflammatory Doses of Prednisolone to Systemically Normal Cats. Am. J. Vet. Res. 2019, 80, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yan, W.; Zhou, K.; Cao, Y.; Cai, W. Glucocorticoid Treatment Alters Systemic Bile Acid Homeostasis by Regulating the Biosynthesis and Transport of Bile Salts. Dig. Liver Dis. 2016, 48, 771–779. [Google Scholar] [CrossRef]
- Out, C.; Dikkers, A.; Laskewitz, A.; Boverhof, R.; Van Der Ley, C.; Kema, I.P.; Wolters, H.; Havinga, R.; Verkade, H.J.; Kuipers, F.; et al. Prednisolone Increases Enterohepatic Cycling of Bile Acids by Induction of Asbt and Promotes Reverse Cholesterol Transport. J. Hepatol. 2014, 61, 351–357. [Google Scholar] [CrossRef]
- Pettersson, H.; Ekstrand, C.; Hillström, A.; Lilliehöök, I. Effect of 1 Mg/Kg Oral Prednisolone on Biochemical Analytes in Ten Dogs: A Cross-over Study. Comp. Clin. Pathol. 2021, 30, 519–528. [Google Scholar] [CrossRef]
- Solter, P.F.; Hoffmann, W.E.; Chambers, M.D.; Schaeffer, D.J.; Kuhlenschmidt, M.S. Hepatic Total 3a -Hydroxy Bile Acids Concentration and Enzyme Activities in Prednisone-Treated Dogs. Vet. Res. 1994, 55, 1086–1092. [Google Scholar]
- Pacheva, M.; Brugger, D.; Riond, B.; Dennler, M.; Kook, P.H. Effects of Prednisolone on 1,2-O-dilauryl-rac-glycero glutaric acid-(60-methylresorufin) ester-lipase Activity and Pancreatic Lipase Immunoreactivity in Healthy Cats. J. Vet. Intern. Med. 2024, 38, 1370–1376. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E.C.; Haddad, J.L.; Brown, D.C.; Morgan, M.J.; Van Winkle, T.J.; Rondeau, M.P. Feline Cholangitis: A Necropsy Study of 44 Cats (1986–2008). J. Feline Med. Surg. 2011, 13, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Fragkou, F.C.; Adamama-Moraitou, K.K.; Poutahidis, T.; Prassinos, N.N.; Kritsepi-Konstantinou, M.; Xenoulis, P.G.; Steiner, J.M.; Lidbury, J.A.; Suchodolski, J.S.; Rallis, T.S. Prevalence and Clinicopathological Features of Triaditis in a Prospective Case Series of Symptomatic and Asymptomatic Cats. J. Vet. Intern. Med. 2016, 30, 1031–1045. [Google Scholar] [CrossRef]
- Bennaim, M.; Shiel, R.E.; Forde, C.; Mooney, C.T. Evaluation of Individual Low-dose Dexamethasone Suppression Test Patterns in Naturally Occurring Hyperadrenocorticism in Dogs. J. Vet. Intern. Med. 2018, 32, 967–977. [Google Scholar] [CrossRef]
- Feldman, E.C.; Nelson, R.W.; Reusch, C.E.; Scott-Moncrieff, J.C.R. Canine and Feline Endocrinology, 4th ed.; Elsevier Saunders: St. Louis, MO, USA, 2015. [Google Scholar]
- Tinted, N.; Pongcharoenwanit, S.; Ongvisespaibool, T.; Wachirodom, V.; Jumnansilp, T.; Buckland, N.; Chuchalermporn, P.; Soontararak, S.; Pairor, S.; Steiner, J.M.; et al. Serum Bile Acids Concentrations and Liver Enzyme Activities after Low-Dose Trilostane in Dogs with Hyperadrenocorticism. Animals 2023, 13, 3244. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Z.; Xiong, X.; Wang, X.; Li, J.; Shi, G.; Yang, J.; Zhang, X.; Zhang, H.; Hong, J.; et al. Glucocorticoids Promote Hepatic Cholestasis in Mice by Inhibiting the Transcriptional Activity of the Farnesoid X Receptor. Gastroenterology 2012, 143, 1630–1640.e8. [Google Scholar] [CrossRef]
- Ettinger, S.J.; Feldman, E.C.; Cote, E. Ettinger’s Textbook of Veterinary Internal Medicine, 9th ed.; Elsevier: St. Louis, MO, USA, 2024. [Google Scholar]
- Hoffman, W.E.; Renegar, W.E.; Dorner, J.L. Alkaline Phosphatase and Alkaline Phosphatase Isoenzymes in the Cat. Vet. Clin. Pathol. 1977, 6, 21–24. [Google Scholar] [CrossRef]
- Scott, D.W.; Kirk, R.W.; Bentinck-Smith, J. Some Effects of Short-Term Methylprednisolone Therapy in Normal Cats. Cornell Vet. 1979, 69, 104–115. [Google Scholar]
- Nielsen, H.K.; Thomsen, K.; Eriksen, E.F.; Charles, P.; Storm, T.; Mosekilde, L. The Effects of High-Dose Glucocorticoid Administration on Serum Bone Gamma Carboxyglutamic Acid-Containing Protein, Serum Alkaline Phosphatase and Vitamin D Metabolites in Normal Subjects. Bone Miner. 1988, 4, 105–113. [Google Scholar]
- Pearce, G.; Tabensky, D.A.; Delmas, P.D.; Baker, H.W.G.; Seeman, E. Corticosteroid-Induced Bone Loss in Men. J. Clin. Endocrinol. Metab. 1998, 83, 801–806. [Google Scholar] [CrossRef]
- Stein, T.A.; Burns, G.P.; Wise, L. Diagnostic Value of Liver Function Tests in Bile Duct Obstruction. J. Surg. Res. 1989, 46, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Van Vleet, J.F. Tissue Gamma-Glutamyl Transpeptidase Activity and Hepatic Ultrastructural Alterations in Dogs with Experimentally Induced Glucocorticoid Hepatopathy. Merican J. Vet. Res. 1982, 43, 649–655. [Google Scholar]
- Center, S.A.; Erb, H.N.; Joseph, S.A. Measurement of Serum Bile Acids Concentrations for Diagnosis of Hepatobiliary Disease in Cats. J. Am. Vet. Med. Assoc. 1995, 207, 1048–1054. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheva, M.; Brugger, D.; Riond, B.; Kook, P.H. Influence of Prednisolone Treatment on Serum Bile Acid Concentrations in Cats. Vet. Sci. 2025, 12, 933. https://doi.org/10.3390/vetsci12100933
Pacheva M, Brugger D, Riond B, Kook PH. Influence of Prednisolone Treatment on Serum Bile Acid Concentrations in Cats. Veterinary Sciences. 2025; 12(10):933. https://doi.org/10.3390/vetsci12100933
Chicago/Turabian StylePacheva, Militsa, Daniel Brugger, Barbara Riond, and Peter Hendrik Kook. 2025. "Influence of Prednisolone Treatment on Serum Bile Acid Concentrations in Cats" Veterinary Sciences 12, no. 10: 933. https://doi.org/10.3390/vetsci12100933
APA StylePacheva, M., Brugger, D., Riond, B., & Kook, P. H. (2025). Influence of Prednisolone Treatment on Serum Bile Acid Concentrations in Cats. Veterinary Sciences, 12(10), 933. https://doi.org/10.3390/vetsci12100933






