Antimicrobial Susceptibility of Campylobacter spp. Isolated from Cattle in Mongolia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Isolation and Identification of Campylobacter spp.
2.3. Antimicrobial Susceptibility Test
2.4. Genetic Analysis
3. Results
3.1. Isolation and Identification of Campylobacter spp.
3.2. AMR of Campylobacter spp. Isolates
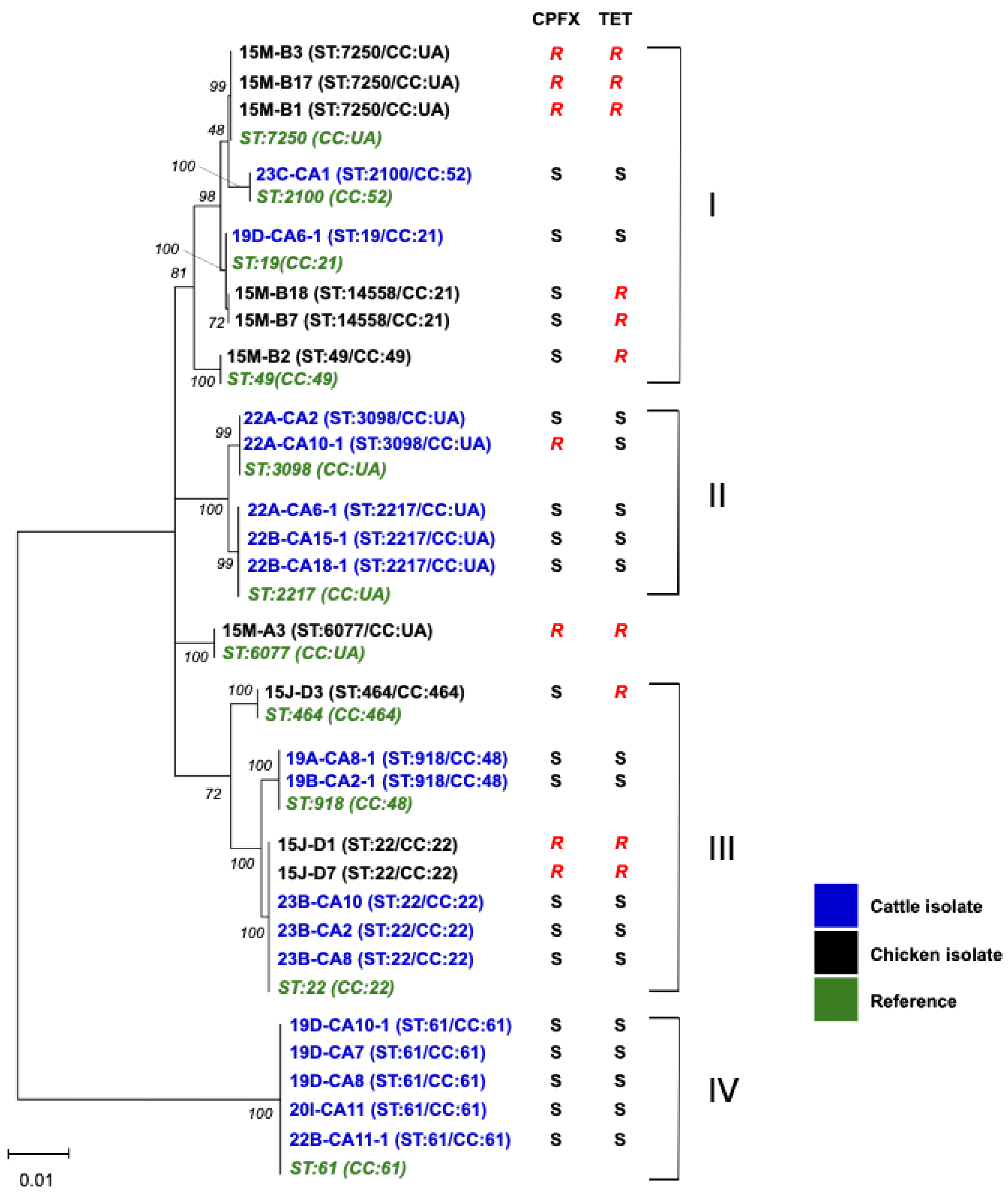
3.3. MLST and Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansen, A.; Stark, K.; Kunkel, J.; Schreier, E.; Ignatius, R.; Liesenfeld, O.; Werber, D.; Göbel, U.B.; Zeitz, M.; Schneider, T. Aetiology of community-acquired, acute gastroenteritis in hospitalised adults: A prospective cohort study. BMC Infect. Dis. 2008, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J.; Engberg, J. Clinical Aspects of Campylobacter jejuni and Campylobacter coli Infection, 3rd ed.; Nachamkin, I., Szymanski, C.M., Blaser, M.J., Eds.; ASM Press: Washington, DC, USA, 2008. [Google Scholar]
- Poropatich, K.O.; Walker, C.L.; Black, R.E. Quantifying the association between Campylobacter infection and Guillain-Barré syndrome: A systematic review. J. Health Popul. Nutr. 2010, 28, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.H.; Soudain, S.E.; Gregson, N.A.; Hughes, R.A. Campylobacter jejuni infection and Guillain-Barré syndrome. N. Engl. J. Med. 1995, 333, 1374–1379. [Google Scholar] [CrossRef]
- Keithlin, J.; Sargeant, J.; Thomas, M.K.; Fazil, A. Systematic review and meta-analysis of the proportion of Campylobacter cases that develop chronic sequelae. BMC Public Health 2014, 14, 1203. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Pijnacker, R.; Coipan, C.; Mulder, A.C.; Fernandes Veludo, A.; de Rijk, S.; van Hoek, A.; Buij, R.; Muskens, G.; Koene, M.; et al. Sources and transmission routes of campylobacteriosis: A combined analysis of genome and exposure data. J. Infect. 2021, 82, 216–226. [Google Scholar] [CrossRef]
- Sheppard, S.K.; Dallas, J.F.; Strachan, N.J.; MacRae, M.; McCarthy, N.D.; Wilson, D.J.; Gormley, F.J.; Falush, D.; Ogden, I.D.; Maiden, M.C.; et al. Campylobacter genotyping to determine the source of human infection. Clin. Infect. Dis. 2009, 48, 1072–1078. [Google Scholar] [CrossRef]
- Byambajav, Z.; Bulgan, E.; Hirai, Y.; Nakayama, M.; Tanaka, M.; Nitta, Y.; Suzuki, A.; Umemura, T.; Altankhuu, B.; Tsagaan, A.; et al. Research Note: Antimicrobial resistance of Campylobacter species isolated from chickens near Ulaanbaatar city, Mongolia. Poult. Sci. 2021, 100, 100916. [Google Scholar] [CrossRef]
- Ministry of Food, Agriculture and Light Industry. Available online: https://mofa.gov.mn/branch/huns/616fa7c073bc4a5fc70f2228 (accessed on 14 August 2025).
- Asakura, M.; Samosornsuk, W.; Hinenoya, A.; Misawa, N.; Nishimura, K.; Matsuhisa, A.; Yamasaki, S. Development of a cytolethal distending toxin (cdt) gene-based species-specific multiplex PCR assay for the detection and identification of Campylobacter jejuni, Campylobacter coli and Campylobacter fetus. FEMS Immunol. Med. Microbiol. 2008, 52, 260–266. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Gibreel, A.; Sjögren, E.; Kaijser, B.; Wretlind, B.; Sköld, O. Rapid emergence of high-level resistance to quinolones in Campylobacter jejuni associated with mutational changes in gyrA and parC. Antimicrob. Agents Chemother. 1998, 42, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Laatu, M.; Rautelin, H.; Hänninen, M.L. Susceptibility of Campylobacter hyointestinalis subsp. hyointestinalis to antimicrobial agents and characterization of quinolone-resistant strains. J. Antimicrob. Chemother. 2005, 55, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Leatherbarrow, A.J.; Griffiths, R.; Hart, C.A.; Kemp, R.; Williams, N.J.; Diggle, P.J.; Wright, E.J.; Sutherst, J.; Houghton, P.; French, N.P. Campylobacter lari: Genotype and antibiotic resistance of isolates from cattle, wildlife and water in an area of mixed dairy farmland in the United Kingdom. Environ. Microbiol. 2007, 9, 1772–1779. [Google Scholar] [CrossRef]
- van der Graaf-van Bloois, L.; Duim, B.; Looft, T.; Veldman, K.T.; Zomer, A.L.; Wagenaar, J.A. Antimicrobial resistance in Campylobacter fetus: Emergence and genomic evolution. Microb. Genom. 2023, 9, 934. [Google Scholar] [CrossRef]
- European Food Safety Authority/European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2022–2023. EFSA J. 2025, 23, e9237. [Google Scholar] [CrossRef]
- Mohan, V.; Strepis, N.; Mitsakakis, K.; Becker, K.; Chindelevitch, L.; Shivaperumal, N.; Swe-Han, K.S.; Hays, J.P. Antimicrobial resistance in Campylobacter spp. focussing on C. jejuni and C. coli—A narrative review. J. Glob. Antimicrob. Resist. 2025, 43, 372–389. [Google Scholar] [CrossRef]
- Sproston, E.L.; Wimalarathna, H.M.L.; Sheppard, S.K. Trends in fluoroquinolone resistance in Campylobacter. Microb. Genom. 2018, 4, e000198. [Google Scholar] [CrossRef]
- World Health Organization. WHO’s List of Medically Important Antimicrobials: A Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Luo, N.; Pereira, S.; Sahin, O.; Lin, J.; Huang, S.; Michel, L.; Zhang, Q. Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc. Natl. Acad. Sci. USA 2005, 102, 541–546. [Google Scholar] [CrossRef]
- Zhang, Q.; Sahin, O.; McDermott, P.F.; Payot, S. Fitness of antimicrobial-resistant Campylobacter and Salmonella. Microbes Infect. 2006, 8, 1972–1978. [Google Scholar] [CrossRef]
- Goulart, D.B.; Zhang, Q.; Sahin, O. Growth kinetics and fitness of fluoroquinolone resistant and susceptible Campylobacter jejuni strains of cattle origin. Front. Vet. Sci. 2023, 10, 1117975. [Google Scholar] [CrossRef]
- Smith, A.B.; Renter, D.G.; Cernicchiaro, N.; Shi, X.; Nickell, J.S.; Keil, D.J.; Nagaraja, T.G. A Randomized Trial to Assess the Effect of Fluoroquinolone Metaphylaxis on the Fecal Prevalence and Quinolone Susceptibilities of Salmonella and Campylobacter in Feedlot Cattle. Foodborne Pathog. Dis. 2017, 14, 600–607. [Google Scholar] [CrossRef]
- Cody, A.J.; McCarthy, N.M.; Wimalarathna, H.L.; Colles, F.M.; Clark, L.; Bowler, I.C.; Maiden, M.C.; Dingle, K.E. A longitudinal 6-year study of the molecular epidemiology of clinical campylobacter isolates in Oxfordshire, United kingdom. J. Clin. Microbiol. 2012, 50, 3193–3201. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hasards. Scientific Opinion on the public health risks related to the consumption of raw drinking milk. EFSA J. 2015, 13, 3940. [Google Scholar] [CrossRef]
- Ohno, Y.; Sekizuka, T.; Kuroda, M.; Ikeda, T. Outbreaks of Campylobacteriosis Caused by Drinking Raw Milk in Japan: Evidence of Relationship Between Milk and Patients by Using Whole Genome Sequencing. Foodborne Pathog. Dis. 2023, 20, 375–380. [Google Scholar] [CrossRef]
| Location (District of Ulaanbaatar) | Sampling Year | Farm ID | Number of Animals 1 | Number of Isolates | Number of Campylobacter spp. Isolated | |||
|---|---|---|---|---|---|---|---|---|
| C. jejuni | C. fetus | C. lari | C. hyointestinalis | |||||
| Songinokhairkhan | 2019 | 19A | 10 | 3 | 1 | 1 | - 2 | 1 |
| Songinokhairkhan | 2019 | 19B | 10 | 4 | 2 | 1 | - | 1 |
| Songinokhairkhan | 2019 | 19C | 10 | 3 | - | - | - | 3 |
| Songinokhairkhan | 2019 | 19D | 10 | 4 | 4 | - | - | - |
| Baganuur | 2020 | 20I | 10 | 1 | 1 | - | - | - |
| Songinokhairkhan | 2022 | 22A | 10 | 4 | 3 | - | 1 | - |
| Songinokhairkhan | 2022 | 22B | 10 | 5 | 3 | - | - | 2 |
| Songinokhairkhan | 2023 | 23A | 10 | 2 | 1 | 1 | - | - |
| Songinokhairkhan | 2023 | 23B | 10 | 5 | 5 | - | - | - |
| Songinokhairkhan | 2023 | 23C | 10 | 4 | 3 | 1 | - | - |
| Total | 100 | 35 | 23 | 4 | 1 | 7 | ||
| Sample ID (1) | Campylobacter spp. | ST (2) | CC (2) | MIC (μg/mL) (3) | gyrA Codon 86 | |||
|---|---|---|---|---|---|---|---|---|
| TET | EM | CPFX | NA | |||||
| 19A-CA8-1 | C. jejuni | 918 | 48 | 0.14 | 0.5 | 0.79 | 1.25 | Thr |
| 19B-CA2-1 | C. jejuni | 918 | 48 | 0.048 | 0.63 | 0.14 | 1 | Thr |
| 19B-CA8 | C. jejuni | NT (4) | NT | 0.26 | 0.24 | 0.079 | 1.5 | Thr |
| 19D-CA6-1 | C. jejuni | 19 | 21 | 0.0945 | 0.75 | 0.157 | 2 | Thr |
| 19D-CA7 | C. jejuni | 61 | 61 | 0.237 | 0.75 | 0.313 | 7 | Thr |
| 19D-CA8 | C. jejuni | 61 | 61 | 0.594 | 1.5 | 0.25 | 8 | Thr |
| 19D-CA10-1 | C. jejuni | 61 | 61 | 0.444 | 1 | 0.765 | 4.75 | Thr |
| 20I-CA11 | C. jejuni | 61 | 61 | 0.094 | 2 | 0.125 | 5.5 | Thr |
| 22A-CA2 | C. jejuni | 3098 | UA (4) | 0.074 | 2.13 | 0.07 | 0.75 | Thr |
| 22A-CA6-1 | C. jejuni | 2217 | UA | 0.547 | 2.13 | 0.05 | 0.5 | Thr |
| 22A-CA10-1 | C. jejuni | 3098 | UA | 0.059 | 0.75 | >32 | 20 | Ile |
| 22B-CA11-1 | C. jejuni | 61 | 61 | 0.079 | 1.13 | 0.172 | 3 | Thr |
| 22B-CA15-1 | C. jejuni | 2217 | UA | 0.133 | 0.813 | 0.01 | 0.025 | Thr |
| 22B-CA18-1 | C. jejuni | 2217 | UA | 0.137 | 2.14 | 0.016 | 0.5 | Thr |
| 23A-CA1 | C. jejuni | NT | NT | 0.035 | 0.315 | 0.035 | 1.75 | Thr |
| 23B-CA2 | C. jejuni | 22 | 22 | 0.158 | 1.125 | 0.125 | 2 | Thr |
| 23B-CA5 | C. jejuni | NT | NT | 0.142 | 1 | 0.094 | 2 | Thr |
| 23B-CA8 | C. jejuni | 22 | 22 | 0.11 | 0.75 | 0.094 | 2 | Thr |
| 23B-CA9 | C. jejuni | NT | NT | 0.095 | 1 | 0.064 | 1.5 | Thr |
| 23B-CA10 | C. jejuni | 22 | 22 | 0.127 | 0.875 | 0.094 | 2 | Thr |
| 23C-CA1 | C. jejuni | 2100 | 52 | 0.21 | 1.3 | 0.136 | 4 | Thr |
| 23C-CA6 | C. jejuni | NT | NT | 0.158 | 1.5 | 0.079 | 2 | Thr |
| 23C-CA8 | C. jejuni | NT | NT | 0.056 | 0.75 | 0.158 | 4 | Thr |
| 19A-CA4-1 | C. hyointestinalis | NT | NT | 0.38 | 1.5 | 0.19 | 144 | NT |
| 19B-CA2-2 | C. hyointestinalis | NT | NT | 0.38 | 2 | 0.19 | 144 | NT |
| 19C-CA3 | C. hyointestinalis | NT | NT | 0.158 | 1.25 | 0.25 | 48 | NT |
| 19C-CA7-1 | C. hyointestinalis | NT | NT | 0.064 | 0.75 | 3 | 256 | NT |
| 19C-CA9 | C. hyointestinalis | NT | NT | 0.19 | 0.845 | 0.25 | 40 | NT |
| 22B-CA11-2 | C. hyointestinalis | NT | NT | 0.094 | 0.625 | 0.125 | >256 | NT |
| 22B-CA13-1 | C. hyointestinalis | NT | NT | 0.095 | 0.438 | 0.079 | 32 | NT |
| 19A-CA1-1 | C. fetus | NT | NT | 0.57 | 1 | 0.5 | 160 | NT |
| 19B-CA4-1 | C. fetus | NT | NT | 0.314 | 0.75 | 0.262 | >256 | NT |
| 23A-CA7 | C. fetus | NT | NT | 0.315 | 0.625 | 0.138 | >256 | NT |
| 23C-CA7 | C. fetus | NT | NT | 0.274 | 0.625 | 0.208 | 192 | NT |
| 22A-CA5-1 | C. lari | NT | NT | 0.158 | 2.25 | 5.5 | >256 | NT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulgan, E.; Byambajav, Z.; Naranchimeg, B.; Chantsal, B.; Bolormaa, T.; Sandagdorj, B.; Nyam-Osor, P.; Kikuchi, E.; Suzuki, A.; Toyting-Hiraishi, J.; et al. Antimicrobial Susceptibility of Campylobacter spp. Isolated from Cattle in Mongolia. Vet. Sci. 2025, 12, 931. https://doi.org/10.3390/vetsci12100931
Bulgan E, Byambajav Z, Naranchimeg B, Chantsal B, Bolormaa T, Sandagdorj B, Nyam-Osor P, Kikuchi E, Suzuki A, Toyting-Hiraishi J, et al. Antimicrobial Susceptibility of Campylobacter spp. Isolated from Cattle in Mongolia. Veterinary Sciences. 2025; 12(10):931. https://doi.org/10.3390/vetsci12100931
Chicago/Turabian StyleBulgan, Erdenebat, Zolzaya Byambajav, Batsukh Naranchimeg, Batsaikhan Chantsal, Tsognemekh Bolormaa, Badrakh Sandagdorj, Purevdorj Nyam-Osor, Eisaku Kikuchi, Akio Suzuki, Jirachaya Toyting-Hiraishi, and et al. 2025. "Antimicrobial Susceptibility of Campylobacter spp. Isolated from Cattle in Mongolia" Veterinary Sciences 12, no. 10: 931. https://doi.org/10.3390/vetsci12100931
APA StyleBulgan, E., Byambajav, Z., Naranchimeg, B., Chantsal, B., Bolormaa, T., Sandagdorj, B., Nyam-Osor, P., Kikuchi, E., Suzuki, A., Toyting-Hiraishi, J., Sato, T., & Horiuchi, M. (2025). Antimicrobial Susceptibility of Campylobacter spp. Isolated from Cattle in Mongolia. Veterinary Sciences, 12(10), 931. https://doi.org/10.3390/vetsci12100931





