Inhibition of Retinoic Acid Receptor Gamma Improves Bovine Embryo Development
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Oocyte Collection and In Vitro Maturation (IVM)
2.2. In Vitro Fertilization (IVF)
2.3. Single-Embryo SMART-Seq
2.4. bESC Culture
2.5. Cell Metabolism Analysis
2.6. Statistical Analysis
3. Results
3.1. LY2955303 Enhances Bovine IVF Embryo Development
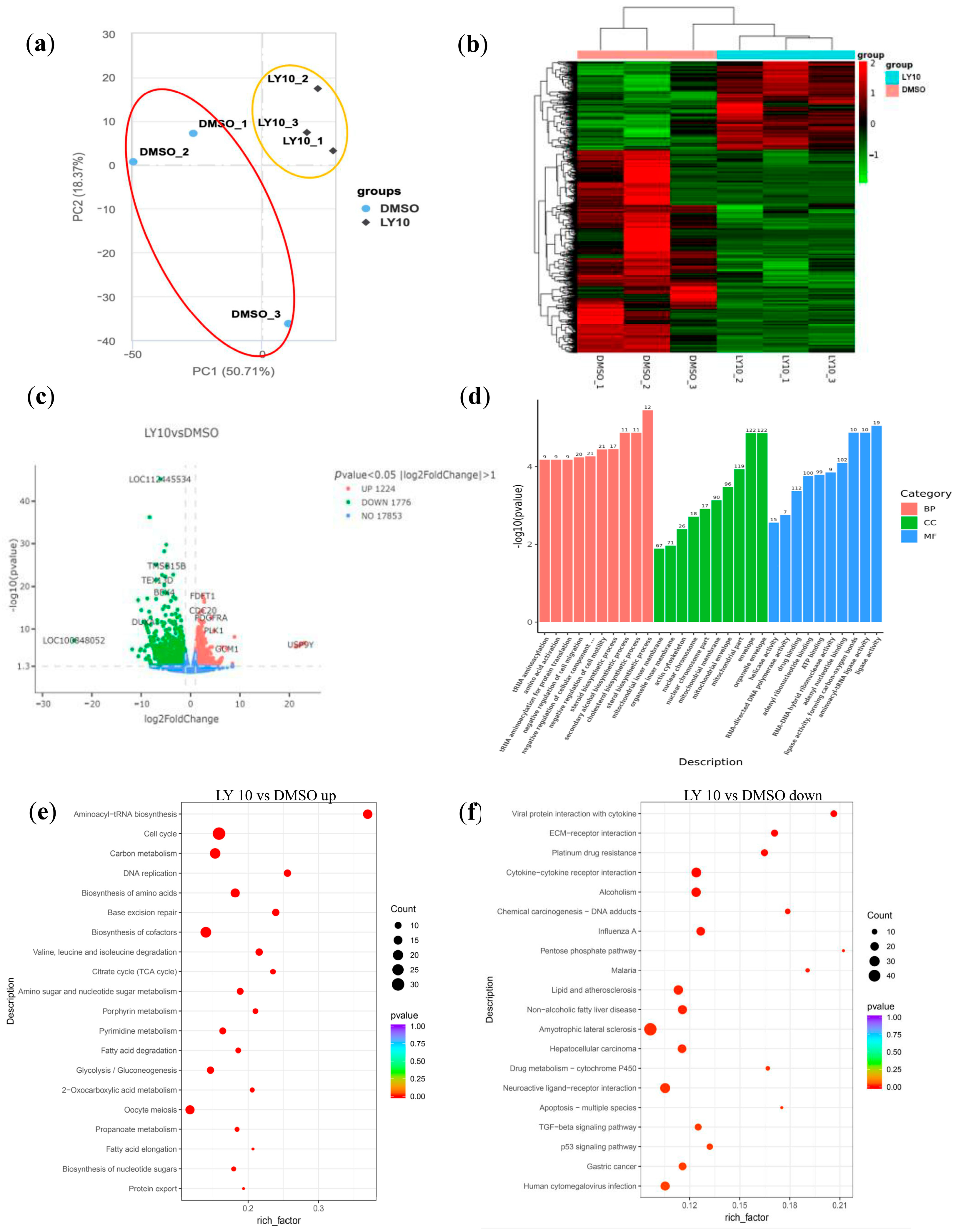
3.2. LY2955303 Treatment Alters mRNA Expression Profiles in Bovine Blastocysts
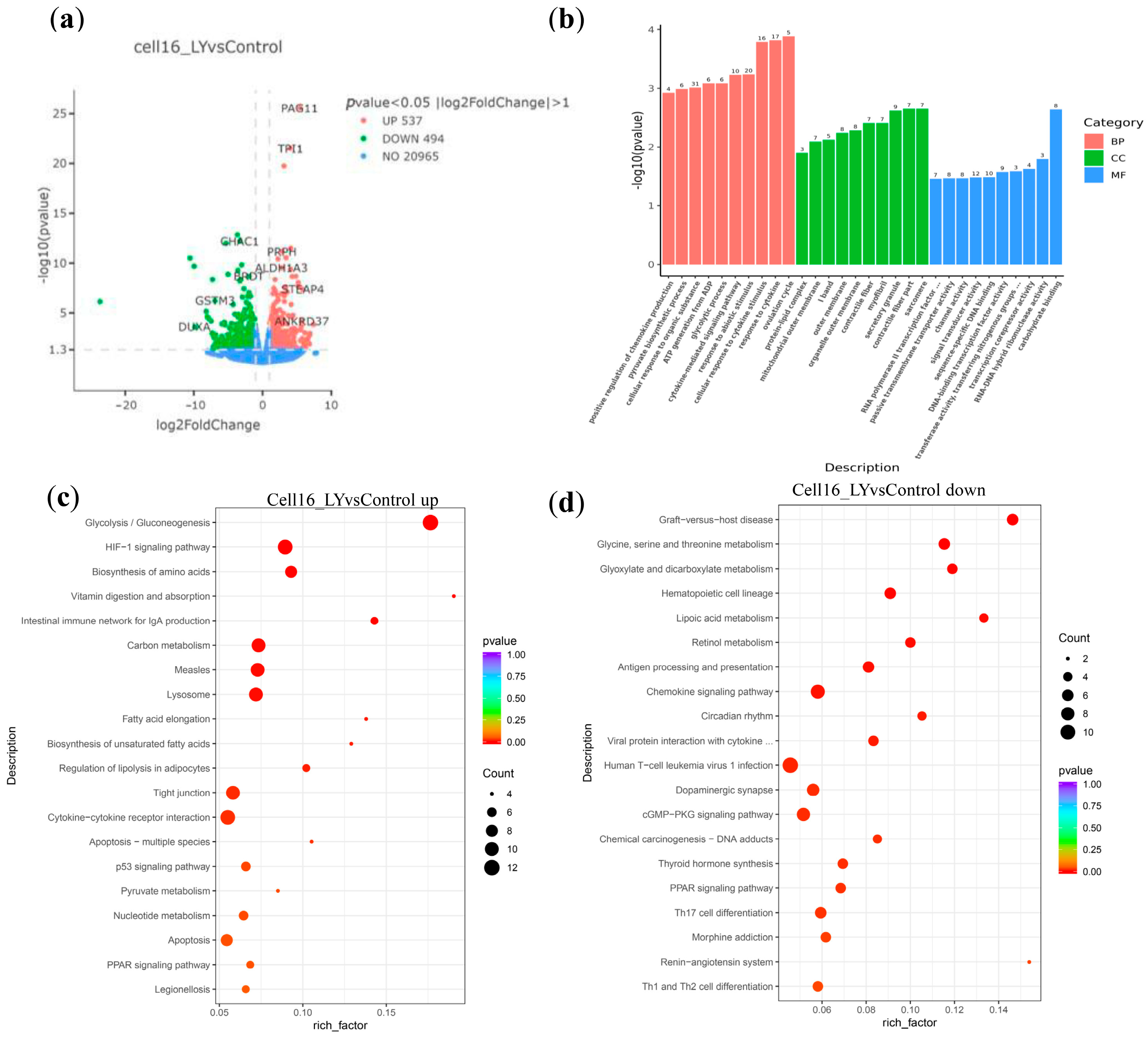
3.3. LY Promotes Post-ZGA Bovine Embryo Energy Metabolism
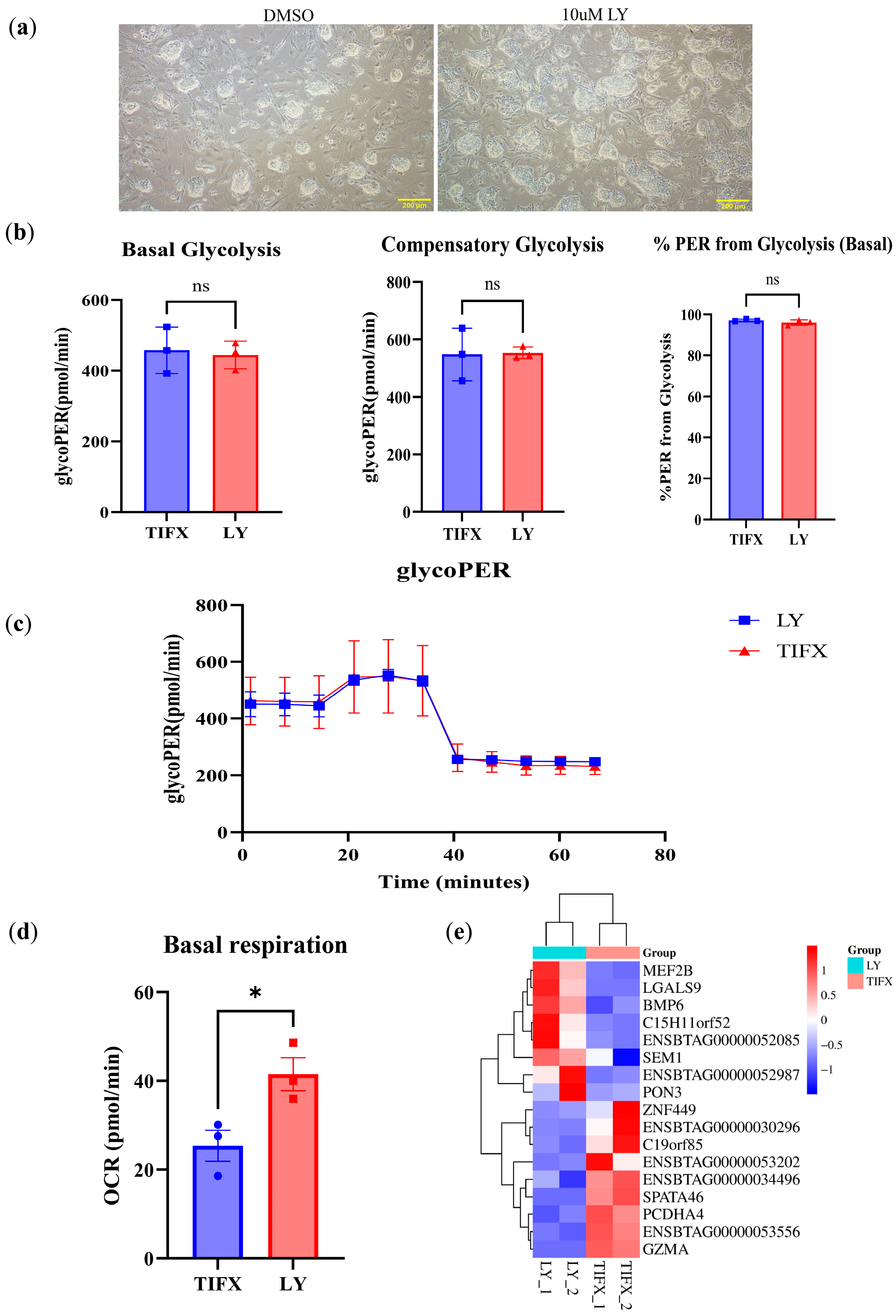
3.4. LY Enhances Oxidative Phosphorylation in Bovine Embryonic Stem Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RA | retinoic acid |
| RARs | retinoic acid receptors |
| RXRs | retinoid X receptors |
| ZGA | zygotic genome activation |
| ESCs | embryonic stem cells |
| PSCs | pluripotent stem cells |
| IVF | in vitro fertilization |
| SHO | slaughterhouse ovaries |
| OPU | ovum pick-up |
| IVM | in vitro maturation |
| COCs | cumulus–oocyte complexes |
References
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015, 16, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; Dolle, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Allenby, G.; Bocquel, M.T.; Saunders, M.; Kazmer, S.; Speck, J.; Rosenberger, M.; Lovey, A.; Kastner, P.; Grippo, J.F.; Chambon, P.; et al. Retinoic acid receptors and retinoid X receptors: Interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. USA 1993, 90, 30–34. [Google Scholar] [CrossRef]
- Egea, P.F.; Rochel, N.; Birck, C.; Vachette, P.; Timmins, P.A.; Moras, D. Effects of ligand binding on the association properties and conformation in solution of retinoic acid receptors RXR and RAR. J. Mol. Biol. 2001, 307, 557–576. [Google Scholar] [CrossRef]
- Iturbide, A.; Ruiz Tejada Segura, M.L.; Noll, C.; Schorpp, K.; Rothenaigner, I.; Ruiz-Morales, E.R.; Lubatti, G.; Agami, A.; Hadian, K.; Scialdone, A.; et al. Retinoic acid signaling is critical during the totipotency window in early mammalian development. Nat. Struct. Mol. Biol. 2021, 28, 521–532. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Ren, Y.; Wang, X.; Lyu, Y.; Xie, B.; Sun, Y.; Yuan, X.; Liu, H.; Yang, W.; et al. Derivation of totipotent-like stem cells with blastocyst-like structure forming potential. Cell Res. 2022, 32, 513–529. [Google Scholar] [CrossRef]
- Gao, R.; Yang, G.; Wang, M.; Xiao, J.; Yi, S.; Huang, Y.; Guo, Z.; Kang, Y.; Fu, Q.; Wang, M.; et al. Defining a TFAP2C-centered transcription factor network during murine peri-implantation. Dev. Cell 2024, 59, 1146–1158.e1146. [Google Scholar] [CrossRef]
- Das, M.; Pethe, P. Differential expression of retinoic acid alpha and beta receptors in neuronal progenitors generated from human embryonic stem cells in response to TTNPB (a retinoic acid mimetic). Differentiation 2021, 121, 13–24. [Google Scholar] [CrossRef]
- Koterazawa, Y.; Koyanagi-Aoi, M.; Uehara, K.; Kakeji, Y.; Aoi, T. Retinoic acid receptor γ activation promotes differentiation of human induced pluripotent stem cells into esophageal epithelium. J. Gastroenterol. 2020, 55, 763–774. [Google Scholar] [CrossRef]
- Wu, J.; Xu, J.; Liu, B.; Yao, G.; Wang, P.; Lin, Z.; Huang, B.; Wang, X.; Li, T.; Shi, S.; et al. Chromatin analysis in human early development reveals epigenetic transition during ZGA. Nature 2018, 557, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Landeo, L.; Zuniga, M.; Gastelu, T.; Artica, M.; Ruiz, J.; Silva, M.; Ratto, M.H. Oocyte Quality, In Vitro Fertilization and Embryo Development of Alpaca Oocytes Collected by Ultrasound-Guided Follicular Aspiration or from Slaughterhouse Ovaries. Animals 2022, 12, 1102. [Google Scholar] [CrossRef]
- Graf, A.; Krebs, S.; Zakhartchenko, V.; Schwalb, B.; Blum, H.; Wolf, E. Fine mapping of genome activation in bovine embryos by RNA sequencing. Proc. Natl. Acad. Sci. USA 2014, 111, 4139–4144. [Google Scholar] [CrossRef]
- Li, N.; Yang, Z.; Su, Y.; Ma, W.; Zhao, J.; Wang, X.; Wan, W.; Xie, S.; Li, H.; Wang, M.; et al. Establishing Bovine Embryonic Stem Cells and Dissecting Their Self-Renewal Mechanisms. Int. J. Mol. Sci. 2025, 26, 3536. [Google Scholar] [CrossRef] [PubMed]
- Nolte, C.; De Kumar, B.; Krumlauf, R. Hox genes: Downstream “effectors” of retinoic acid signaling in vertebrate embryogenesis. Genesis 2019, 57, e23306. [Google Scholar] [CrossRef] [PubMed]
- Martinvalet, D.; Dykxhoorn, D.M.; Ferrini, R.; Lieberman, J. Granzyme A cleaves a mitochondrial complex I protein to initiate caspase-independent cell death. Cell 2008, 133, 681–692. [Google Scholar] [CrossRef]
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y.; et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020, 368, eaaz7548. [Google Scholar] [CrossRef]
- Hoang, N.M.; Liu, Y.; Bates, P.D.; Heaton, A.R.; Lopez, A.F.; Liu, P.; Zhu, F.; Chen, R.; Kondapelli, A.; Zhang, X.; et al. Targeting DNMT3A-mediated oxidative phosphorylation to overcome ibrutinib resistance in mantle cell lymphoma. Cell Rep. Med. 2024, 5, 101484. [Google Scholar] [CrossRef]
- Sakhnevych, S.S.; Yasinska, I.M.; Fasler-Kan, E.; Sumbayev, V.V. Mitochondrial Defunctionalization Supresses Tim-3-Galectin-9 Secretory Pathway in Human Colorectal Cancer Cells and Thus Can Possibly Affect Tumor Immune Escape. Front. Pharmacol. 2019, 10, 342. [Google Scholar] [CrossRef]
- Gomez, E.; Caamano, J.N.; Rodriguez, A.; De Frutos, C.; Facal, N.; Diez, C. Bovine early embryonic development and vitamin A. Reprod. Domest. Anim. 2006, 41 (Suppl. S2), 63–71. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Diez, C.; Ikeda, S.; Royo, L.J.; Caamano, J.N.; Alonso-Montes, C.; Goyache, F.; Alvarez, I.; Facal, N.; Gomez, E. Retinoids during the in vitro transition from bovine morula to blastocyst. Hum. Reprod. 2006, 21, 2149–2157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef]
- Cha, Y.; Han, M.J.; Cha, H.J.; Zoldan, J.; Burkart, A.; Jung, J.H.; Jang, Y.; Kim, C.H.; Jeong, H.C.; Kim, B.G.; et al. Metabolic control of primed human pluripotent stem cell fate and function by the miR-200c-SIRT2 axis. Nat. Cell Biol. 2017, 19, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Krisher, R.L.; Herrick, J.R. Bovine embryo production in vitro: Evolution of culture media and commercial perspectives. Anim. Reprod. 2024, 21, e20240051. [Google Scholar] [CrossRef] [PubMed]
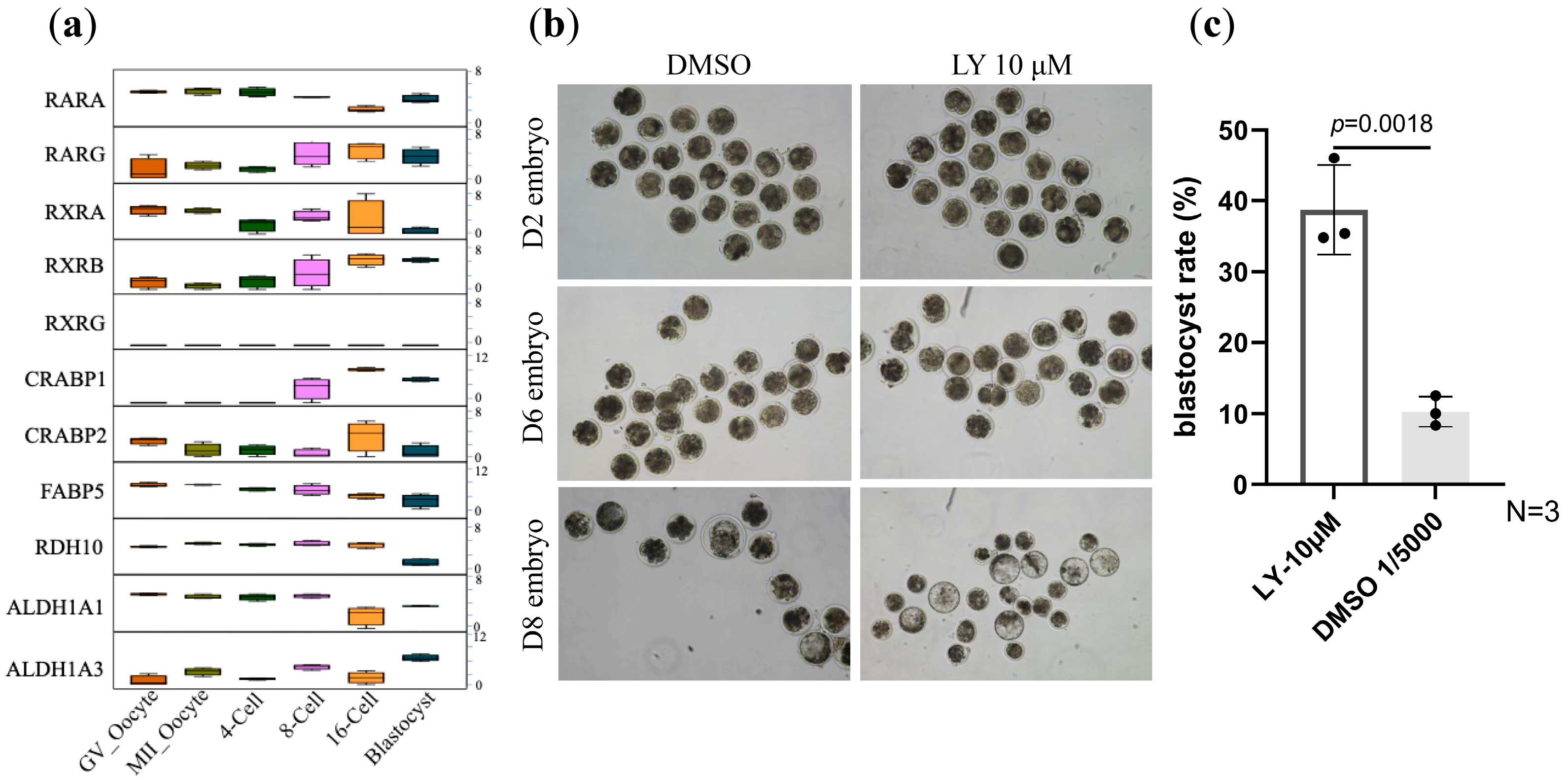

| Groups | Presumptive Zygotes in Culture | Blastocyst | Blastocyst Rate (%) |
|---|---|---|---|
| LY 10 μM | 54 | 20 | 38.7 ± 7.3 |
| DMSO | 30 | 3 | 10.3 ± 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wan, W.; Su, Y.; Xie, S.; Jiang, F.; Yang, Z.; Xiehe, S.; Ma, W.; Yue, L.; Li, N.; et al. Inhibition of Retinoic Acid Receptor Gamma Improves Bovine Embryo Development. Vet. Sci. 2025, 12, 924. https://doi.org/10.3390/vetsci12100924
Wang X, Wan W, Su Y, Xie S, Jiang F, Yang Z, Xiehe S, Ma W, Yue L, Li N, et al. Inhibition of Retinoic Acid Receptor Gamma Improves Bovine Embryo Development. Veterinary Sciences. 2025; 12(10):924. https://doi.org/10.3390/vetsci12100924
Chicago/Turabian StyleWang, Xiangyan, Wenjing Wan, Yue Su, Shengcan Xie, Fenfen Jiang, Zhen Yang, Shuangyi Xiehe, Wei Ma, Linxiu Yue, Ningxiao Li, and et al. 2025. "Inhibition of Retinoic Acid Receptor Gamma Improves Bovine Embryo Development" Veterinary Sciences 12, no. 10: 924. https://doi.org/10.3390/vetsci12100924
APA StyleWang, X., Wan, W., Su, Y., Xie, S., Jiang, F., Yang, Z., Xiehe, S., Ma, W., Yue, L., Li, N., Wang, A., Guo, J., Li, X., Liu, X., & Tang, Y. (2025). Inhibition of Retinoic Acid Receptor Gamma Improves Bovine Embryo Development. Veterinary Sciences, 12(10), 924. https://doi.org/10.3390/vetsci12100924







