Extended Survival in a Dog with Primary Bone Hemangiosarcoma Following Treatment with Neoadjuvant Oncolytic Virotherapy and Standard of Care
Simple Summary
Abstract
1. Introduction
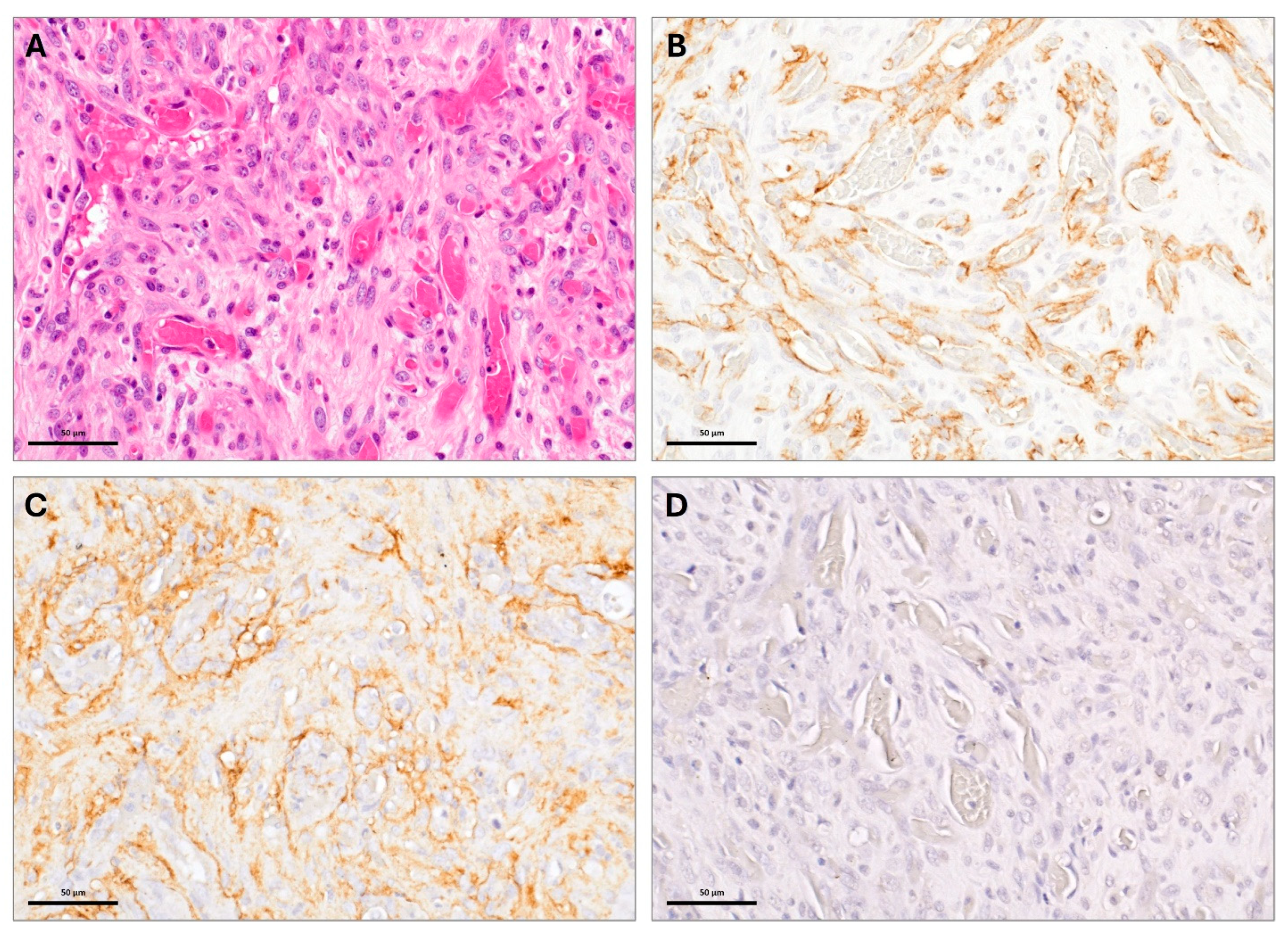
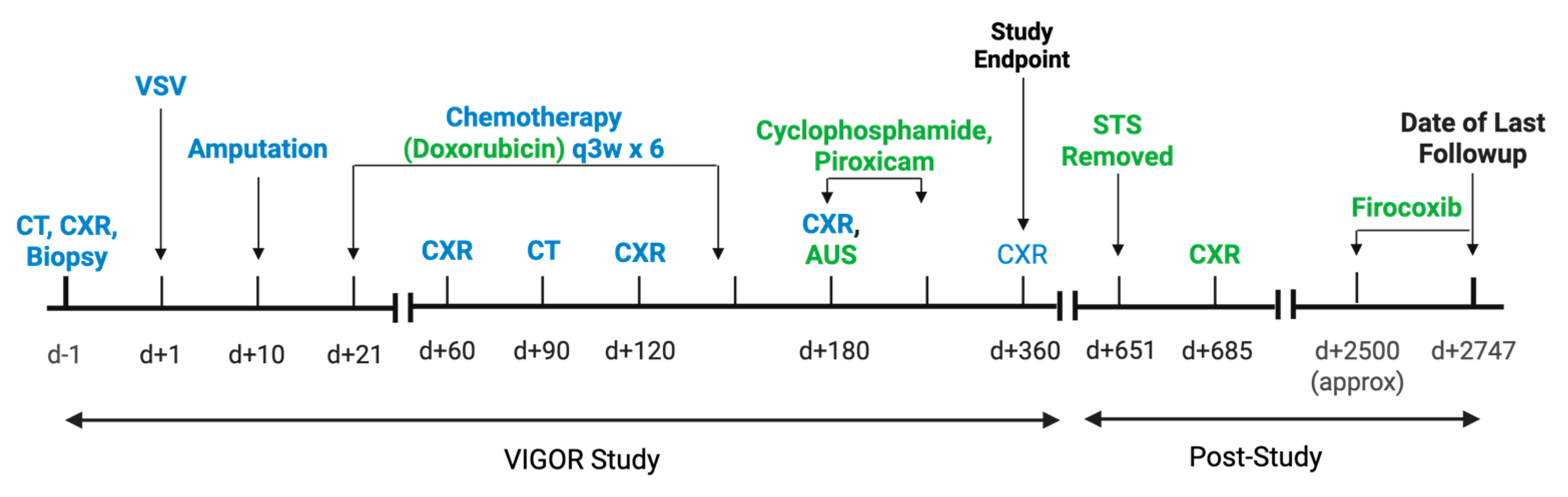
2. Case Description
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamerato-Kozicki, A.R.; Helm, K.M.; Jubala, C.M.; Cutter, G.C.; Modiano, J.F. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp. Hematol. 2006, 34, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Brodey, R.S.; Riser, W.H. Canine Osteosarcoma. A Clinicopathologic Study of 194 cases. Clin. Orthop. Relat. Res. 1969, 62, 54–64. [Google Scholar] [PubMed]
- Vail, D.M.; Thamm, D.H.; Liptak, J.M. Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Elsevier: Edinburgh, NY, USA, 2020; ISBN 978-0-323-59496-7. [Google Scholar]
- Jongeward, S.J. Primary Bone Tumors. Vet. Clin. N. Am. Small Anim. Pract. 1985, 15, 609–641. [Google Scholar] [CrossRef] [PubMed]
- Cagle, L.A.; Maisel, M.; Conrado, F.O.; Wait, C.; Peper, K.; Lochhead, T.; Vo, T.; Dolan, J.K.; De Oliveira, H.H.; Bosch, S.; et al. Telangiectatic osteosarcoma in four dogs: Cytologic, histopathologic, cytochemical, and immunohistochemical findings. Vet. Clin. Pathol 2024, 53, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.A.; Kamstock, D.A.; Selmic, L.E.; Pass, W.; Szivek, A.; Mison, M.B.; Boston, S.E.; Fox, L.E.; Robat, C.; Grimes, J.A.; et al. Primary appendicular hemangiosarcoma and telangiectatic osteosarcoma in 70 dogs: A Veterinary Society of Surgical Oncology retrospective study. Vet. Surg. 2018, 47, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Makielski, K.M.; Sarver, A.L.; Henson, M.S.; Stuebner, K.M.; Borgatti, A.; Suksanpaisan, L.; Preusser, C.; Tabaran, A.-F.; Cornax, I.; O’Sullivan, M.G.; et al. Neoadjuvant systemic oncolytic vesicular stomatitis virus is safe and may enhance long-term survivorship in dogs with naturally occurring osteosarcoma. Mol. Ther. Oncolytics 2023, 31, 100736. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, P.C. A Retrospective Study of Visceral and Nonvisceral Hemangiosarcoma and Hemangiomas in Domestic Animals. J. Vet. Diagn. Investig. 2004, 16, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.A.; Bacon, N.J.; Kamstock, D.A. Use of routine histopathology and factor VIII-related antigen/von Willebrand factor immunohistochemistry to differentiate primary hemangiosarcoma of bone from telangiectatic osteosarcoma in 54 dogs. Vet. Comp. Oncol. 2017, 15, 1232–1239. [Google Scholar] [CrossRef]
- Hauben, E.I.; Weeden, S.; Pringle, J.; Van Marck, E.A.; Hogendoorn, P.C.W. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur. J. Cancer 2002, 38, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.B.; Leite, J.D.S.; Fonseca, A.B.M.; Ferreira, A.M.R. Vimentin, osteocalcin and osteonectin expression in canine primary bone tumors: Diagnostic and prognostic implications. Mol. Biol. Rep. 2018, 45, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Wehrle-Martinez, A.S.; Dittmer, K.E.; Aberdein, D.; Thompson, K.G. Osteocalcin and Osteonectin Expression in Canine Osteosarcoma. Vet. Pathol. 2016, 53, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, L.; Fondevila, D.; Rabanal, R.M.; Vilafranca, M. Immunohistochemical Detection of CD31 Antigen in Normal and Neoplastic Canine Endothelial Cells. J. Comp. Pathol. 1995, 112, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Göritz, M.; Müller, K.; Krastel, D.; Staudacher, G.; Schmidt, P.; Kühn, M.; Nickel, R.; Schoon, H.-A. Canine Splenic Haemangiosarcoma: Influence of Metastases, Chemotherapy and Growth Pattern on Post-splenectomy Survival and Expression of Angiogenic Factors. J. Comp. Pathol. 2013, 149, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.I.; Rochell, R.E.; Penza, V.; Naik, S. Translation of oncolytic viruses in sarcoma. Mol. Ther. Oncol. 2024, 32, 200822. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Antonescu, C.R.; Bowler, T.; Munhoz, R.; Chi, P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; Movva, S.; Dholakia, R.; et al. Objective Response Rate Among Patients with Locally Advanced or Metastatic Sarcoma Treated with Talimogene Laherparepvec in Combination with Pembrolizumab: A Phase 2 Clinical Trial. JAMA Oncol 2020, 6, 402. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labé, C.; Chehadeh, A.; Winter, A.; Pracht, S.; Stuebner, K.M.; Lewellen, M.; Eskander, B.; O’Sullivan, M.G.; Tabaran, A.-F.; Ober, C.; et al. Extended Survival in a Dog with Primary Bone Hemangiosarcoma Following Treatment with Neoadjuvant Oncolytic Virotherapy and Standard of Care. Vet. Sci. 2025, 12, 921. https://doi.org/10.3390/vetsci12100921
Labé C, Chehadeh A, Winter A, Pracht S, Stuebner KM, Lewellen M, Eskander B, O’Sullivan MG, Tabaran A-F, Ober C, et al. Extended Survival in a Dog with Primary Bone Hemangiosarcoma Following Treatment with Neoadjuvant Oncolytic Virotherapy and Standard of Care. Veterinary Sciences. 2025; 12(10):921. https://doi.org/10.3390/vetsci12100921
Chicago/Turabian StyleLabé, Courtney, Andrea Chehadeh, Amber Winter, Sara Pracht, Kathy M. Stuebner, Mitzi Lewellen, Bishoy Eskander, M. Gerard O’Sullivan, Alexandru-Flaviu Tabaran, Christopher Ober, and et al. 2025. "Extended Survival in a Dog with Primary Bone Hemangiosarcoma Following Treatment with Neoadjuvant Oncolytic Virotherapy and Standard of Care" Veterinary Sciences 12, no. 10: 921. https://doi.org/10.3390/vetsci12100921
APA StyleLabé, C., Chehadeh, A., Winter, A., Pracht, S., Stuebner, K. M., Lewellen, M., Eskander, B., O’Sullivan, M. G., Tabaran, A.-F., Ober, C., Henson, M. S., Seelig, D., Russell, S. J., Modiano, J. F., Naik, S., & Makielski, K. M. (2025). Extended Survival in a Dog with Primary Bone Hemangiosarcoma Following Treatment with Neoadjuvant Oncolytic Virotherapy and Standard of Care. Veterinary Sciences, 12(10), 921. https://doi.org/10.3390/vetsci12100921





