Preventive Immunology for Livestock and Zoonotic Infectious Diseases in the One Health Era: From Mechanistic Insights to Innovative Interventions
Simple Summary
Abstract
1. Introduction
2. Immunological Foundations of Preventive Immunology
2.1. Innate and Adaptive Immunity
2.2. Species Differences Matter
2.3. Pathogen Immune Evasion Guides Prevention
2.4. Correlates of Protection and T-Helper Polarization
2.5. Adjuvants and PRR Agonists
2.6. Mucosal Immunity and Delivery
2.7. Maternal and Early-Life Immunity
2.8. Single-Cell and Systems Immunology in Livestock
2.9. Zoonotic Transmission Pathways and Human Health Implications
3. Vaccine Innovations: From Classical to Next-Generation Approaches
3.1. Classical Platforms and DIVA Logic
3.2. Recombinant Viral Vectors (Cell-Mediated Immunity on Demand)
3.3. Nucleic-Acid Platforms (mRNA, DNA) in Livestock
3.4. Nanovaccines as Smart Adjuvants
3.5. Thermostability and Delivery for Low-Resource Settings
3.6. Data-Assisted, Systems-Vaccinology Design (Smarter, Faster Pipelines)
3.7. Practical Gaps and What to Watch
| Platform | Examples/Typical Use | Main Benefits | Field Readiness | Cold-Chain and Delivery | Key Constraints and Considerations | References |
|---|---|---|---|---|---|---|
| Classical and DIVA vaccines | gE-deleted BoHV-1 for IBR; gE/TK-deleted pseudorabies in swine | Well understood; reliable protection; compatible with surveillance (DIVA) | Widely used; licensed for eradication programs | Standard refrigeration; often multiple doses | Latent infection/reactivation in herpesviruses; slower to update antigens; DIVA testing requires planning | [132] |
| Recombinant viral vectors | Adenovirus, poxvirus, multi-gene-deleted herpesvirus; some multivalent designs | Can be retargeted more quickly than classical vaccines; strong T-cell responses | Used for specific backbones; broader licensing still limited | Refrigerated; injection; needs quality checks for genetic stability | Genetic stability must be monitored; reactivation control for herpes backbones; manufacturing can be complex and costly | [132,166] |
| mRNA and DNA vaccines | LNP-mRNA (e.g., CSFV E2); circular mRNA; DNA prime then protein or mRNA boost | Fast design and updates; flexible prime–boost combinations | Early field stage in livestock (proof of concept in swine; few licenses in food animals) | Usually frozen or refrigerated for mRNA; fill-finish capacity is a bottleneck | Regulatory pathways still developing; needs better thermostability and lower cost per dose; scale-up for GMP manufacturing | [148] |
| Nanovaccines (as adjuvants or carriers) | Co-delivery of antigen with innate agonists; mucosal targeting; dose-sparing | Improves antigen delivery and can shape the immune response; may allow room-temperature stability in some designs | Early to mid-development; limited field validation so far | May allow ambient stability depending on formulation | Manufacturing and quality control are demanding; cost and regulatory familiarity are current barriers | [146,167] |
| Thermostable and delivery innovations | NDV I-2 for poultry in LMICs; vacuum-foam drying; microneedle patches | Better stability and simpler use; supports large-scale campaigns | High for NDV I-2; early to mid-development for vacuum-foam drying and microneedles | Ambient-stable candidates; devices (patches) require reliable supply | Need regulatory and supply chain scaling for devices; unit cost must be acceptable for routine programs | [149,165,168] |
| Oral vaccines | Rabies baits for wildlife and free-roaming dogs | Non-invasive; practical for wide coverage in the field | Established for wildlife/dog programs | Often stable in the field; success depends on distribution and acceptance | Monitor ecological safety and program performance; community acceptance matters | [169] |
| Computational (data-assisted) design | Structure-aware B- and T-cell epitope selection; graph/transformer models; checked with single-cell readouts | Shortens early selection; helps aim for cross-protective candidates | Early stage (needs more prospective livestock validation) | Not applicable (design step only) | Requires external reference benchmarks and prospective animal-to-field studies before broad claims | [162] |
4. Beyond Vaccines: Emerging Preventive Immunological Strategies
4.1. Monoclonal Antibodies and Passive Immunization
4.2. Immunomodulators and Cytokine Therapies
4.3. Probiotics, Prebiotics, and Gut Microbiota Modulation
4.4. Phage-Based Immunomodulation
4.5. Gene Editing and CRISPR-Based Preventive Approaches
4.6. Diagnostics as Preventive Tools
4.7. Limitations, Failure Cases, and Conflicting Evidence
5. One Health Integration and Environmental Dimensions
5.1. Environmental Reservoirs and Spillover Interfaces
5.2. AMR as an Environmental Challenge
5.3. Integrated Surveillance: From Wastewater to Farm Biosecurity
5.4. Governance, Implementation, and Metrics
6. Translational and Policy Challenges
6.1. Regulatory and Approval Pathways
6.2. Economic and Logistical Barriers
6.3. Ethical, Societal, and Acceptance Issues
6.4. Workforce and Capacity Gaps
6.5. Policy Integration and One Health Governance
7. Future Directions and Research Gaps
7.1. AI and ML
7.2. Climate Change and AMR
7.3. Integrated One Health Surveillance
7.4. Whole-Genome Sequencing (WGS)
7.5. Socioeconomic and Regulatory Barriers
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; UNEP; WHO; WOAH. One Health Joint Plan of Action (2022–2026): Working Together for the Health of Humans, Animals, Plants and the Environment; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240059139 (accessed on 11 October 2025).
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S. The one health concept: 10 years old and a long road ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Robinson, T.P.; Bu, D.; Carrique-Mas, J.; Fèvre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 377–380. [Google Scholar] [CrossRef]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Mariner, J.C.; House, J.A.; Mebus, C.A.; Sollod, A.E.; Chibeu, D.; Jones, B.A.; Roeder, P.L.; Admassu, B.; van’t Klooster, G.G. Rinderpest eradication: Appropriate technology and social innovations. Science 2012, 337, 1309–1312. [Google Scholar] [CrossRef]
- Taylor, L.H.; Wallace, R.M.; Balaram, D.; Lindenmayer, J.M.; Eckery, D.C.; Mutonono-Watkiss, B.; Parravani, E.; Nel, L.H. The role of dog population management in rabies elimination—A review of current approaches and future opportunities. Front. Vet. Sci. 2017, 4, 109. [Google Scholar] [CrossRef] [PubMed]
- WHO. Zero by 30: The Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/handle/10665/272756 (accessed on 12 October 2025).
- Klein, E.Y.; Impalli, I.; Poleon, S.; Denoel, P.; Cipriano, M.; Van Boeckel, T.P.; Pecetta, S.; Bloom, D.E.; Nandi, A. Global trends in antibiotic consumption during 2016–2023 and future projections through 2030. Proc. Natl. Acad. Sci. USA 2024, 121, e2411919121. [Google Scholar] [CrossRef]
- Mulchandani, R.; Tiseo, K.; Nandi, A.; Klein, E.; Gandra, S.; Laxminarayan, R.; Van Boeckel, T. Global trends in inappropriate use of antibiotics, 2000–2021: Scoping review and prevalence estimates. BMJ Public Health 2025, 3, e002411. [Google Scholar] [CrossRef]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: Antibiotic Use Data for 2022. Available online: https://www.who.int/publications/i/item/9789240108127 (accessed on 10 October 2025).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Mahony, T.J.; Briody, T.E.; Ommeh, S.C. Can the revolution in mRNA-based vaccine technologies solve the intractable health issues of current ruminant production systems? Vaccines 2024, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Fazel, F.; Doost, J.S.; Raj, S.; Boodhoo, N.; Karimi, K.; Sharif, S. The mRNA vaccine platform for veterinary species. Vet. Immunol. Immunopathol. 2024, 274, 110803. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, J.; Li, G.; Yu, L. Research advances in replication-deficient viral vector vaccines. Front. Vet. Sci. 2025, 12, 1535328. [Google Scholar] [CrossRef]
- Kumar, V.; Verma, A.; Singh, R.; Garg, P.; Sharma, S.K.; Singh, H.N.; Mishra, S.K.; Kumar, S. Recombinant vaccines: Current updates and future prospects. Asian Pac. J. Trop. Med. 2024, 17, 338–350. [Google Scholar] [CrossRef]
- He, L.; Pan, R.; Liang, R.; Li, B.; Zhang, P.; He, S.; Li, B.; Li, Y. Nanomaterial Adjuvants for Veterinary Vaccines: Mechanisms and Applications. Research 2025, 8, 0761. [Google Scholar] [CrossRef] [PubMed]
- Hussain, W.; Chaman, S.; Koser, H.N.; Aun, S.M.; Bibi, Z.; Pirzadi, A.N.; Hussain, J.; Zubaria, Z.; Nabi, G.; Ullah, M.W. Nanoparticle-Mediated Mucosal Vaccination: Harnessing Nucleic Acids for Immune Enhancement: Nanoparticle-Mediated Mucosal Vaccination. Curr. Microbiol. 2024, 81, 279. [Google Scholar] [CrossRef]
- Nobrega, F.L.; Costa, A.R.; Kluskens, L.D.; Azeredo, J. Revisiting phage therapy: New applications for old resources. Trends Microbiol. 2015, 23, 185–191. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Miller, S.A. Clinical metagenomics. Nat. Rev. Genet. 2019, 20, 341–355. [Google Scholar] [CrossRef]
- Gwinn, M.; MacCannell, D.R.; Khabbaz, R.F. Integrating advanced molecular technologies into public health. J. Clin. Microbiol. 2017, 55, 703–714. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed]
- Kour, S.; Agrawal, R.; Sharma, N.; Tikoo, A.; Pande, N.; Sawhney, A. Artificial intelligence and its application in animal disease diagnosis. J. Anim. Res. 2022, 12, 1–10. [Google Scholar]
- Xiao, S.; Dhand, N.K.; Wang, Z.; Hu, K.; Thomson, P.C.; House, J.K.; Khatkar, M.S. Review of applications of deep learning in veterinary diagnostics and animal health. Front. Vet. Sci. 2025, 12, 1511522. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, P.; Yin, Y.; Du, X.; Cao, G.; Wu, S.; Zhao, Y. Molecular breeding of livestock for disease resistance. Virology 2023, 587, 109862. [Google Scholar] [CrossRef]
- Popova, J.; Bets, V.; Kozhevnikova, E. Perspectives in genome-editing techniques for livestock. Animals 2023, 13, 2580. [Google Scholar] [CrossRef]
- Mrutu, R.I.; Umar, K.M.; Abdulhamid, A.; Agaba, M.; Abdussamad, A.M. Microbial Engineering to Mitigate Methane Emissions in Ruminant Livestock--A Review. arXiv 2023, arXiv:2307.14372. [Google Scholar]
- Menary, J.; Fuller, S.S. New genomic techniques, old divides: Stakeholder attitudes towards new biotechnology regulation in the EU and UK. PLoS ONE 2024, 19, e0287276. [Google Scholar] [CrossRef]
- Milazzo, A.; Liu, J.; Multani, P.; Steele, S.; Hoon, E.; Chaber, A.-L. One health implementation: A systematic scoping review using the quadripartite one health joint plan of action. One Health 2025, 20, 101008. [Google Scholar] [CrossRef]
- Adisasmito, W.B.; Almuhairi, S.; Behravesh, C.B.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Becerra, N.C.; Charron, D.F.; Chaudhary, A.; Zanella, J.R.C. One Health: A new definition for a sustainable and healthy future. PLoS Pathog. 2022, 18, e1010537. [Google Scholar]
- FAO. No More Deaths from Rinderpest. 2011. Available online: https://www.fao.org/newsroom/detail/No-more-deaths-from-rinderpest/en (accessed on 11 October 2025).
- IAEA. Global Rinderpest Eradication Strengthens Early and Rapid Disease Diagnostic Networks. 2011. Available online: https://www.iaea.org/newscenter/news/global-rinderpest-eradication-strengthens-early-and-rapid-disease-diagnostic-networks (accessed on 11 October 2025).
- WOAH. The Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030 (Zero by 30). 2018. Available online: https://openknowledge.fao.org/items/26f02540-79ef-4d3b-a321-6926fc3c3fe1 (accessed on 11 October 2025).
- WHO-SEARO. Mass Dog Vaccination—Using the One Health Approach to Prevent Rabies. Available online: https://www.who.int/southeastasia/news/feature-stories/detail/mass-dog-vaccination-using-the-one-health-approach-to-prevent-rabies (accessed on 11 October 2025).
- Cleaveland, S.; Thumbi, S.; Sambo, M.; Lugelo, A.; Lushasi, K.; Hampson, K.; Lankester, F. Proof of concept of mass dog vaccination for the control and elimination of canine rabies. Rev. Sci. Et Tech. (Int. Off. Epizoot.) 2018, 37, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; McEntee, C.P. Vaccine adjuvants: Tailoring innate recognition to send the right message. Immunity 2024, 57, 772–789. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Irla, M. Toll-like receptors (TLRs) in the trained immunity era. eLife 2025, 14, e106443. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Q.; Zhang, Z.; Zhang, Y.; Wang, S.; Han, Y.; Zhu, H.; He, H. Expression profile of Toll-like receptors and cytokines in the cecal tonsil of chickens challenged with Eimeria tenella. Parasitol. Res. 2024, 123, 347. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lan, C.; Benlagha, K.; Camara, N.O.S.; Miller, H.; Kubo, M.; Heegaard, S.; Lee, P.; Yang, L.; Forsman, H. The interaction of innate immune and adaptive immune system. MedComm 2024, 5, e714. [Google Scholar] [CrossRef]
- Syeda, M.Z.; Hong, T.; Huang, C.; Huang, W.; Mu, Q. B cell memory: From generation to reactivation: A multipronged defense wall against pathogens. Cell Death Discov. 2024, 10, 117. [Google Scholar] [CrossRef]
- Giri, S.; Batra, L. Memory Cells in Infection and Autoimmunity: Mechanisms, Functions, and Therapeutic Implications. Vaccines 2025, 13, 205. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.L.; Damani-Yokota, P.; Yirsaw, A.; Loonie, K.; Teixeira, A.F.; Gillespie, A. Special features of γδ T cells in ruminants. Mol. Immunol. 2021, 134, 161–169. [Google Scholar] [CrossRef]
- Samuel, B.E.R.; Diaz, F.E.; Maina, T.W.; Corbett, R.J.; Tuggle, C.K.; McGill, J.L. Evidence of innate training in bovine γδ T cells following subcutaneous BCG administration. Front. Immunol. 2024, 15, 1423843. [Google Scholar] [CrossRef]
- Báez-Magaña, M.; Alva-Murillo, N.; Ochoa-Zarzosa, A.; López-Meza, J.E. Trained immunity in farm animals. Vet. Res. 2025, 56, 166. [Google Scholar] [CrossRef]
- Schat, K.A. The importance of the bursa of Fabricius, B cells and T cells for the pathogenesis of Marek’s disease: A review. Viruses 2022, 14, 2015. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Hou, Y.; Sun, M.; Xia, T.; Wu, X. Evasion of host defense by Brucella. Cell Insight 2024, 3, 100143. [Google Scholar] [CrossRef]
- Lu, P.; Luo, B.; Wang, Q.; Wang, L.; Chen, M.; Jia, J.; Yang, M.; Pan, J.; Liu, J.; Li, Z. Progress in brucellosis immune regulation inflammatory mechanisms and diagnostic advances. Eur. J. Med. Res. 2025, 30, 830. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.M.; He, K.; Pandey, A.; Cabello, A.; Zhang, D.; Yang, J.; Gomez, G.; Liu, Y.; Chang, H.; Li, X. Brucella activates the host RIDD pathway to subvert BLOS1-directed immune defense. eLife 2022, 11, e73625. [Google Scholar] [CrossRef] [PubMed]
- Bear, A.; Locke, T.; Rowland-Jones, S.; Pecetta, S.; Bagnoli, F.; Darton, T.C. The immune evasion roles of Staphylococcus aureus protein A and impact on vaccine development. Front. Cell. Infect. Microbiol. 2023, 13, 1242702. [Google Scholar] [CrossRef]
- Paiva, T.O.; Geoghegan, J.A.; Dufrêne, Y.F. High-force catch bonds between the Staphylococcus aureus surface protein SdrE and complement regulator factor H drive immune evasion. Commun. Biol. 2023, 6, 302. [Google Scholar] [CrossRef] [PubMed]
- Goding, J.W. Use of staphylococcal protein A as an immunological reagent. J. Immunol. Methods 1978, 20, 241–253. [Google Scholar] [CrossRef]
- Patti, J.M.; Allen, B.L.; McGavin, M.J.; Hook, M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 1994, 48, 585–618. [Google Scholar] [CrossRef]
- Thammavongsa, V.; Kim, H.K.; Missiakas, D.; Schneewind, O. Staphylococcal manipulation of host immune responses. Nat. Rev. Microbiol. 2015, 13, 529–543. [Google Scholar] [CrossRef]
- De Jong, N.W.; Van Kessel, K.P.; Van Strijp, J.A. Immune evasion by Staphylococcus aureus. Microbiol. Spectr. 2019, 7, 1–27. [Google Scholar] [CrossRef]
- Khairullah, A.R.; Moses, I.B.; Kusala, M.K.J.; Tyasningsih, W.; Ayuti, S.R.; Rantam, F.A.; Fauziah, I.; Silaen, O.S.M.; Puspitasari, Y.; Aryaloka, S. Unveiling insights into bovine tuberculosis: A comprehensive review. Open Vet. J. 2024, 14, 1330–1344. [Google Scholar] [CrossRef]
- Blanco, F.C.; Gravisaco, M.J.; Bigi, M.M.; García, E.A.; Marquez, C.; McNeil, M.; Jackson, M.; Bigi, F. Identifying bacterial and host factors involved in the interaction of Mycobacterium bovis with the bovine innate immune cells. Front. Immunol. 2021, 12, 674643. [Google Scholar] [CrossRef]
- Kennedy, H.E.; Welsh, M.D.; Bryson, D.G.; Cassidy, J.P.; Forster, F.I.; Howard, C.J.; Collins, R.A.; Pollock, J.M. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1+ γδ T cells. Infect. Immun. 2002, 70, 1488–1500. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.-Y.; Hu, Z. Immune correlates of protection as a game changer in tuberculosis vaccine development. npj Vaccines 2024, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.K.; Jia, F.; Wright, Q.G.; Islam, M.T.; Bean, A.; Layton, D.; Williams, D.T.; Lynch, S.E. Defining correlates of protection for mammalian livestock vaccines against high-priority viral diseases. Front. Immunol. 2024, 15, 1397780. [Google Scholar] [CrossRef]
- Sterle, H.M.; Putz, E.J.; Olsen, S.; Palmer, M.; Boggiatto, P.M. Effect of co-vaccination of cattle with RB51 and BCG on vaccine-specific CD4+ T cell responses. Front. Immunol. 2025, 16, 1664398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-j.; Tong, T.; Xin, X.-q.; Sheng, Y.-j.; Fu, S.-s.; Zheng, C.-k.; Xu, Z.-z.; Jiao, X.-a.; Chen, X. Establishment and evaluation of an interferon-gamma enzyme-linked immunospot method for the detection of Brucella-infected cattle and goats. J. Dairy Sci. 2025, 108, 7472–7480. [Google Scholar] [CrossRef] [PubMed]
- Klepp, L.I.; Blanco, F.C.; Bigi, M.M.; Vázquez, C.L.; García, E.A.; Sabio y García, J.; Bigi, F. B Cell and Antibody Responses in Bovine Tuberculosis. Antibodies 2024, 13, 84. [Google Scholar] [CrossRef]
- O’Grady, J.F.; McHugo, G.P.; Ward, J.A.; Hall, T.J.; Faherty O’Donnell, S.L.; Correia, C.N.; Browne, J.A.; McDonald, M.; Gormley, E.; Riggio, V. Integrative genomics sheds light on the immunogenetics of tuberculosis in cattle. Commun. Biol. 2025, 8, 479. [Google Scholar] [CrossRef]
- Sterle, H.M.; Putz, E.J.; Olsen, S.C.; Boggiatto, P.M. Induction of CD4 T cell memory responses following BCG vaccination in cattle. Front. Vet. Sci. 2024, 11, 1491424. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Z.; Fu, Y.-X.; Peng, H. Mucosal vaccine development for respiratory viral infections. hLife 2024, 2, 50–63. [Google Scholar] [CrossRef]
- Hoffmann, J.P.; Srivastava, A.; Yang, H.; Iwanaga, N.; Remcho, T.P.; Hewes, J.L.; Sharoff, R.; Song, K.; Norton, E.B.; Kolls, J.K. Vaccine-elicited IL-1R signaling results in Th17 TRM-mediated immunity. Commun. Biol. 2024, 7, 433. [Google Scholar] [CrossRef]
- Zhang, Z.; Hong, W.; Zhang, Y.; Li, X.; Que, H.; Wei, X. Mucosal immunity and vaccination strategies: Current insights and future perspectives. Mol. Biomed. 2025, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Oboge, H.; Riitho, V.; Nyamai, M.; Omondi, G.P.; Lacasta, A.; Githaka, N.; Nene, V.; Aboge, G.; Thumbi, S. Safety and efficacy of toll-like receptor agonists as therapeutic agents and vaccine adjuvants for infectious diseases in animals: A systematic review. Front. Vet. Sci. 2024, 11, 1428713. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, C.; Bertholet, S.; Philpott, D.J.; De Gregorio, E. Unleashing the potential of NOD-and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2014, 111, 12294–12299. [Google Scholar] [CrossRef]
- Mbow, M.L.; De Gregorio, E.; Valiante, N.M.; Rappuoli, R. New adjuvants for human vaccines. Curr. Opin. Immunol. 2010, 22, 411–416. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Dar, A.; Allan, B.; Gomis, S.; Potter, A.; Mutwiri, G. Immunotherapeutic potential of CpG oligonucleotides in chickens. J. Poult. Sci. 2009, 46, 69–80. [Google Scholar] [CrossRef][Green Version]
- Sun, M.; Pratama, A.C.; Qiu, H.; Liu, Z.; He, F. Toward innovative veterinary nanoparticle vaccines. Anim. Dis. 2024, 4, 14. [Google Scholar] [CrossRef]
- Dotiwala, F.; Upadhyay, A.K. Next generation mucosal vaccine strategy for respiratory pathogens. Vaccines 2023, 11, 1585. [Google Scholar] [CrossRef]
- Chamorro, B.M.; Luca, K.D.; Swaminathan, G.; Rochereau, N.; Majorel, J.; Poulet, H.; Chanut, B.; Piney, L.; Mundt, E.; Paul, S. Mucosal Vaccination with Live Attenuated Bordetella bronchiseptica Protects against Challenge in Wistar Rats. Vaccines 2023, 11, 982. [Google Scholar] [CrossRef]
- Koolaparambil Mukesh, R.; Hill, T.; Kaiser, F.; Prado-Smith, J.; Schulz, J.E.; Gallogly, S.; Herbold, L.; Bauer, K.; Smith, B.J.; Myers, L. Intranasal booster induces durable mucosal immunity against SARS-CoV-2 in mice. Sci. Rep. 2025, 15, 24224. [Google Scholar] [CrossRef]
- Christo, S.N.; Park, S.L.; Mueller, S.N.; Mackay, L.K. The multifaceted role of tissue-resident memory T cells. Annu. Rev. Immunol. 2024, 42, 317–345. [Google Scholar] [CrossRef]
- Zhong, K.; Chen, X.; Zhang, J.; Jiang, X.; Zhang, J.; Huang, M.; Bi, S.; Ju, C.; Luo, Y. Recent advances in oral vaccines for animals. Vet. Sci. 2024, 11, 353. [Google Scholar] [CrossRef]
- Silva, F.G.; Silva, S.R.; Pereira, A.M.; Cerqueira, J.L.; Conceição, C. A comprehensive review of bovine colostrum components and selected aspects regarding their impact on neonatal calf physiology. Animals 2024, 14, 1130. [Google Scholar] [CrossRef]
- Renaud, D.L.; Steele, M.A. What can’t colostrum do? Exploring the effects of supplementing colostrum after the first day of life: A narrative review. JDS Commun. 2025, 6, 469–473. [Google Scholar] [CrossRef]
- Fernandez-Novo, A.; Kolkman, I.; Driesse, M.; Yarnall, M.; Cerviño, M.; Dieguez, F.J.; Astiz, S. Factors associated with an excellent transfer of passive immunity: Multisite, cross-sectional study conducted in different European countries on dairy cattle. Front. Vet. Sci. 2025, 12, 1515196. [Google Scholar] [CrossRef]
- Denholm, K. Benefits of extended colostrum feeding in dairy calves and how to implement it on farm. Practice 2024, 46, 380–387. [Google Scholar] [CrossRef]
- Silva, A.P.; Cezar, A.M.; de Toledo, A.F.; Coelho, M.G.; Tomaluski, C.R.; Virgínio Júnior, G.F.; Bittar, C.M. Enrichment of medium-quality colostrum by adding colostrum replacer, combined or not with transition milk in the feeding of dairy calves. Sci. Rep. 2024, 14, 5533. [Google Scholar] [CrossRef]
- Rittipornlertrak, A.; Modethed, W.; Sangkakam, K.; Muenthaisong, A.; Vinitchaikul, P.; Boonsri, K.; Pringproa, K.; Punyapornwithaya, V.; Kreausukon, K.; Sthitmatee, N. Persistence of passive immunity in calves receiving colostrum from cows vaccinated with a live attenuated lumpy skin disease vaccine and the performance of serological tests. Front. Vet. Sci. 2024, 11, 1303424. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, M.; Baakhtari, M.; Ramah, A.; Imatake, S.; Ahmadi, P.; Deguchi, Y.; Uematsu, M.; Nakama, Y.; Imabeppu, K.; Nomura, Y. Comparative analysis of maternal colostrum and colostrum replacer effects on immunity, growth, and health of Japanese Black calves. Animals 2024, 14, 346. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Alexandre, P.A.; Brice, A.M.; Hine, B.C.; Ingham, A.; Legrand, T.P.; Royle, C.; Niemeyer, D.; Reverter, A.; Denman, S.E. Single-cell transcriptomics uncovers key immune drivers of vaccine efficacy in cattle. BMC Genom. 2025, 26, 750. [Google Scholar] [CrossRef]
- Wiarda, J.E.; Davila, K.M.S.; Trachsel, J.M.; Loving, C.L.; Boggiatto, P.; Lippolis, J.D.; Putz, E.J. Single-cell RNA sequencing characterization of Holstein cattle blood and milk immune cells during a chronic Staphylococcus aureus mastitis infection. Sci. Rep. 2025, 15, 12689. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, S.; Jia, M.; Chen, X.; Qi, W.; Gu, F.; Valencak, T.G.; Liu, J.-X.; Sun, H.-Z. Advances in single-cell transcriptomics in animal research. J. Anim. Sci. Biotechnol. 2024, 15, 102. [Google Scholar] [CrossRef]
- Werling, D.; Jungi, T.W. TOLL-like receptors linking innate and adaptive immune response. Vet. Immunol. Immunopathol. 2003, 91, 1–12. [Google Scholar] [CrossRef]
- Keestra, A.M.; de Zoete, M.R.; Bouwman, L.I.; van Putten, J.P. Chicken TLR21 is an innate CpG DNA receptor distinct from mammalian TLR9. J. Immunol. 2010, 185, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.; van der Meer, J.W.; Mhlanga, M.M.; Mulder, W.J. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.; Palmer, M.; Thacker, T.; Bannantine, J.; Vordermeier, H.; Hewinson, R.; Greenwald, R.; Esfandiari, J.; McNair, J.; Pollock, J. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clin. Vaccine Immunol. 2006, 13, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Mackay, L.K. Tissue-resident memory T cells: Local specialists in immune defence. Nat. Rev. Immunol. 2016, 16, 79–89. [Google Scholar] [CrossRef]
- Guzman, E.; Hope, J.; Taylor, G.; Smith, A.L.; Cubillos-Zapata, C.; Charleston, B. Bovine γδ T cells are a major regulatory T cell subset. J. Immunol. 2014, 193, 208–222. [Google Scholar] [CrossRef]
- Ratcliffe, M.J. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev. Comp. Immunol. 2006, 30, 101–118. [Google Scholar] [CrossRef]
- Dawson, H.D.; Loveland, J.E.; Pascal, G.; Gilbert, J.G.; Uenishi, H.; Mann, K.M.; Sang, Y.; Zhang, J.; Carvalho-Silva, D.; Hunt, T. Structural and functional annotation of the porcine immunome. BMC Genom. 2013, 14, 332. [Google Scholar] [CrossRef]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, A.; Moreno, E.; Gorvel, J.P. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol. Rev. 2011, 240, 211–234. [Google Scholar] [CrossRef]
- Thammavongsa, V.; Missiakas, D.M.; Schneewind, O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 2013, 342, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Cell Wall-Anchored Surface Proteins of Staphylococcus aureus. In Staphylococcus Aureus: Interplay Between Bacteria and Hosts; Springer: Berlin/Heidelberg, Germany, 2024; pp. 41–80. [Google Scholar]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef] [PubMed]
- Mutwiri, G.; Pontarollo, R.; Babiuk, S.; Griebel, P.; Mena, A.; Tsang, C.; Alcon, V.; Nichani, A.; Ioannou, X.; Gomis, S. Biological activity of immunostimulatory CpG DNA motifs in domestic animals. Vet. Immunol. Immunopathol. 2003, 91, 89–103. [Google Scholar] [CrossRef]
- Ren, X.; Cao, N.; Tian, L.; Liu, W.; Zhu, H.; Rong, Z.; Yao, M.; Li, X.; Qian, P. A self-assembled nanoparticle vaccine based on pseudorabies virus glycoprotein D induces potent protective immunity against pseudorabies virus infection. Vet. Microbiol. 2023, 284, 109799. [Google Scholar] [CrossRef]
- Godden, S.M.; Lombard, J.E.; Woolums, A.R. Colostrum management for dairy calves. Vet. Clin. North Am. Food Anim. Pract. 2019, 35, 535. [Google Scholar] [CrossRef]
- Khan, T.S.; Akram, N.; Faisal, Z.; Saeed, F.; Rasheed, A.; Ahmed, F.; Afzaal, M. Bovine colostrum: Therapeutic potential and clinical evidence. Int. Dairy J. 2024, 157, 105996. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Cai, G.; Wang, Y.; Yang, W.; Li, Y.; Zhao, X.; Li, R.; Gao, Y.; Tuo, W. Single-cell transcriptomic and chromatin accessibility analyses of dairy cattle peripheral blood mononuclear cells and their responses to lipopolysaccharide. BMC Genom. 2022, 23, 338. [Google Scholar] [CrossRef]
- Pereira, C.R.; Cotrim de Almeida, J.V.F.; Cardoso de Oliveira, I.R.; Faria de Oliveira, L.; Pereira, L.J.; Zangeronimo, M.G.; Lage, A.P.; Dorneles, E.M.S. Occupational exposure to Brucella spp.: A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2020, 14, e0008164. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.C.; McCarthy, T.; Capuano, A.W.; Setterquist, S.F.; Olsen, C.W.; Alavanja, M.C.; Lynch, C.F. Swine workers and swine influenza virus infections. Emerg. Infect. Dis. 2007, 13, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Terajima, J.; Izumiya, H.; Hara-Kudo, Y.; Ohnishi, M. Shiga Toxin (Verotoxin)-producingEscherichia coli and Foodborne Disease: A Review. Food Saf. 2017, 5, 35–53. [Google Scholar] [CrossRef]
- Joseph, A.; Cointe, A.; Mariani Kurkdjian, P.; Rafat, C.; Hertig, A. Shiga toxin-associated hemolytic uremic syndrome: A narrative review. Toxins 2020, 12, 67. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Im, J.; Lee, J.-S.; Jeon, H.J.; Mogeni, O.D.; Kim, J.H.; Rakotozandrindrainy, R.; Baker, S.; Marks, F. The global burden and epidemiology of invasive non-typhoidal Salmonella infections. Hum. Vaccines Immunother. 2019, 15, 1421–1426. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Myers, K.P.; Olsen, C.W.; Setterquist, S.F.; Capuano, A.W.; Donham, K.J.; Thacker, E.L.; Merchant, J.A.; Gray, G.C. Are swine workers in the United States at increased risk of infection with zoonotic influenza virus? Clin. Infect. Dis. 2006, 42, 14–20. [Google Scholar] [CrossRef]
- CDC. Highly Pathogenic Avian Influenza A(H5N1): Technical Report—29 December 2023. 2023. Available online: https://www.cdc.gov/bird-flu/php/technical-report/h5n1-122923.html (accessed on 11 October 2025).
- Alegbeleye, O.O.; Sant’Ana, A.S. Manure-borne pathogens as an important source of water contamination: An update on the dynamics of pathogen survival/transport as well as practical risk mitigation strategies. Int. J. Hyg. Environ. Health 2020, 227, 113524. [Google Scholar] [CrossRef]
- Bell, R.L.; Kase, J.A.; Harrison, L.M.; Balan, K.V.; Babu, U.; Chen, Y.; Macarisin, D.; Kwon, H.J.; Zheng, J.; Stevens, E.L. The persistence of bacterial pathogens in surface water and its impact on global food safety. Pathogens 2021, 10, 1391. [Google Scholar] [CrossRef]
- Million, M.; Roblot, F.; Carles, D.; d’Amato, F.; Protopopescu, C.; Carrieri, M.P.; Raoult, D. Reevaluation of the risk of fetal death and malformation after Q fever. Clin. Infect. Dis. 2014, 59, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Ghanem-Zoubi, N.; Paul, M. Q fever during pregnancy: A narrative review. Clin. Microbiol. Infect. 2020, 26, 864–870. [Google Scholar] [CrossRef]
- Wheelhouse, N.; Kemp, S.; Halliday, J.E.; Tingas, E.A.; Duncan, W.C.; Horne, A.W. Q fever and early pregnancy failure: A Scottish case–control study. Reprod. Fertil. 2022, 3, L1–L2. [Google Scholar] [CrossRef]
- Minas, A.; Minas, M.; Stournara, A.; Tselepidis, S. The “effects” of Rev-1 vaccination of sheep and goats on human brucellosis in Greece. Prev. Vet. Med. 2004, 64, 41–47. [Google Scholar] [CrossRef]
- COELHO, A.; Pinto, M.d.L.; DIEZ, J.G.; Coelho, A.C. Impact of B. melitensis Rev-1 vaccination on brucellosis prevalence. Turk. J. Vet. Anim. Sci. 2015, 39, 261–270. [Google Scholar] [CrossRef]
- Lake, R.; Campbell, D.; Hathaway, S.; Ashmore, E.; Cressey, P.; Horn, B.; Pirikahu, S.; Sherwood, J.; Baker, M.; Shoemack, P. Source attributed case-control study of campylobacteriosis in New Zealand. Int. J. Infect. Dis. 2021, 103, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Singh, M.; Sharif, S.; Sharma, S.; Kulkarni, R.R.; Alizadeh, M.; Yitbarek, A.; Helmy, Y.A. Intervention strategies to control Campylobacter at different stages of the food chain. Microorganisms 2023, 11, 113. [Google Scholar] [CrossRef]
- Grassly, N.C.; Shaw, A.G.; Owusu, M. Global wastewater surveillance for pathogens with pandemic potential: Opportunities and challenges. Lancet Microbe 2024, 6, 100939. [Google Scholar] [CrossRef] [PubMed]
- WHO. Wastewater and Environmental Surveillance for One or More Pathogens: Guidance on Prioritization, Implementation and Integration. 2024. Available online: https://www.who.int/publications/m/item/wastewater-and-environmental-surveillance-for-one-or-more-pathogens--guidance-on-prioritization--implementation-and-integration (accessed on 11 October 2025).
- Arnold, K.E.; Laing, G.; McMahon, B.J.; Fanning, S.; Stekel, D.J.; Pahl, O.; Coyne, L.; Latham, S.M.; McIntyre, K.M. The need for One Health systems-thinking approaches to understand multiscale dissemination of antimicrobial resistance. Lancet Planet. Health 2024, 8, e124–e133. [Google Scholar] [CrossRef]
- Petrini, S.; Righi, C.; Iscaro, C.; Viola, G.; Gobbi, P.; Scoccia, E.; Rossi, E.; Pellegrini, C.; De Mia, G.M. Evaluation of passive immunity induced by immunisation using two inactivated gE-deleted marker vaccines against infectious bovine rhinotracheitis (IBR) in calves. Vaccines 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Petrini, S.; Martucciello, A.; Righi, C.; Cappelli, G.; Torresi, C.; Grassi, C.; Scoccia, E.; Costantino, G.; Casciari, C.; Sabato, R. Assessment of different infectious bovine rhinotracheitis marker vaccines in calves. Vaccines 2022, 10, 1204. [Google Scholar] [CrossRef]
- Pavulraj, S.; Stout, R.W.; Paulsen, D.B.; Chowdhury, S.I. A Quadruple Gene-Deleted Live BoHV-1 Subunit RVFV Vaccine Vector Reactivates from Latency and Replicates in the TG Neurons of Calves but Is Not Transported to and Shed from Nasal Mucosa. Viruses 2024, 16, 1497. [Google Scholar] [CrossRef]
- Li, L.; Du, Y.; Zhang, Y.; Li, P.; Liu, X.; Zhang, X.; Li, J.; Zhang, T.; Li, X.; Xiao, D. Comprehensive evaluation of the safety and immunogenicity of a gene-deleted variant pseudorabies virus attenuated vaccine. Vet. Res. 2022, 53, 73. [Google Scholar] [CrossRef]
- Wang, T.; Xiao, Y.; Yang, Q.; Wang, Y.; Sun, Z.; Zhang, C.; Yan, S.; Wang, J.; Guo, L.; Yan, H. Construction of a gE-Deleted Pseudorabies Virus and Its Efficacy to the New-Emerging Variant PRV Challenge in the Form of Killed Vaccine. BioMed Res. Int. 2015, 2015, 684945. [Google Scholar] [CrossRef][Green Version]
- Kinker, D.R.; Swenson, S.L.; Wu, L.-L.; Zimmerman, J.J. Evaluation of serological tests for the detection of pseudorabies gE antibodies during early infection. Vet. Microbiol. 1997, 55, 99–106. [Google Scholar] [CrossRef]
- Abukhadra, B.A.; Abd El Rahman, S.; Soltan, M.A.; Elhafi, G.E.; Mosad, S.M. Preliminary molecular study for DIVA trial of antigenically characterized circulating bovine herpesvirus subtype 1.1 in Egypt. Virology 2024, 593, 110012. [Google Scholar] [CrossRef]
- Iscaro, C.; Cambiotti, V.; Petrini, S.; Feliziani, F. Control programs for infectious bovine rhinotracheitis (IBR) in European countries: An overview. Anim. Health Res. Rev. 2021, 22, 136–146. [Google Scholar] [CrossRef]
- Petrini, S.; Righi, C.; Costantino, G.; Scoccia, E.; Gobbi, P.; Pellegrini, C.; Pela, M.; Giammarioli, M.; Viola, G.; Sabato, R. Assessment of BoAHV-1 Seronegative latent carrier by the administration of two infectious Bovine rhinotracheitis live marker vaccines in calves. Vaccines 2024, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Pavulraj, S.; Stout, R.W.; Chowdhury, S.I. A Novel BoHV-1-Vectored Subunit RVFV Vaccine Induces a Robust Humoral and Cell-Mediated Immune Response Against Rift Valley Fever in Sheep. Viruses 2025, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.I. Bovine Herpesvirus Type 1 (BoHV-1) Vector Against Bovine Respiratory Disease Complex. U.S. Patent No. 11,083,787, 2021. [Google Scholar]
- Montbrau, C.; Gibert, M.; Solé, M.; Barril, I.; Roca, M.; Acal, L.; Vázquez, B.; Mallorqui, J.; March, R. Respiratory Efficacy of a Multivalent Marker Vaccine Against Bovine Viral Diarrhoea Virus Types 1 and 2, Infectious Bovine Rhinotracheitis Virus, Bovine Respiratory Syncytial Virus, and Bovine Parainfluenza-3 Virus in Young Calves. Vaccines 2025, 13, 999. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, X.; Song, C.; Feng, K.; Ke, H.; Yin, L.; Fu, J.; Yan, Z.; Lin, W.; Zhang, X. Safety and immunogenicity of a broad-spectrum HVT vector vaccine for avian infectious bursal disease. Poult. Sci. 2025, 105926. [Google Scholar] [CrossRef]
- Bude, S.A.; Lu, Z.; Zhao, Z.; Zhang, Q. Pseudorabies Virus Glycoproteins E and B Application in Vaccine and Diagnosis Kit Development. Vaccines 2024, 12, 1078. [Google Scholar] [CrossRef]
- Jogi, H.R.; Smaraki, N.; Rajak, K.K.; Yadav, A.K.; Bhatt, M.; Einstien, C.; Revathi, A.; Thakur, R.; Kamothi, D.J.; Dedeepya, P. Revolutionizing Veterinary Health with Viral Vector-Based Vaccines. Indian J. Microbiol. 2024, 64, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.-J.; Tang, Y.; Yang, F.; Tian, H.; Peng, Q.; Tang, M.-L.; Chen, Y.-Z.; Xia, T.; Li, S.; Zheng, H.-X. A single-dose mRNA vaccine encoding the classical swine fever virus E2-ECD induces durable protective immunity in rabbits. Vet. Res. 2025, 56, 152. [Google Scholar] [CrossRef]
- Liu, C.; Zhai, W.; Nie, W.; Zhong, C.; Diao, F.; Yin, B. Circular mRNA-LNP vaccine encoding self-assembled E2-TMD-mi3 nanoparticles licit enhanced CSFV-specific immunity over commercial subunit vaccine. Front. Immunol. 2025, 16, 1604677. [Google Scholar] [CrossRef]
- Ling, T.; Xin, Z.; Huan-Huan, L.; Ya-Ting, Z.; Ya-Mei, L.; Rong-Li, G.; Damiani, A.M.; Sai-Sai, C.; Chuan-Jian, Z.; Rui, D. A self-amplifying mRNA vaccine expressing PRV gD induces robust immunity against virulent mutants. npj Vaccines 2025, 10, 193. [Google Scholar] [CrossRef]
- Liu, J.; Xia, Y.; Tian, C.; Chen, Z.; Guo, W.; Liu, Y.; Wen, J.; Xie, Z.; Lin, J.; Li, J. E2-based mRNA vaccine encapsulated in lipid nanoparticles protects pigs against classical swine fever virus. J. Virol. 2025, 99, e0097825. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X. Beyond the Needle: Innovative Microneedle-Based Transdermal Vaccination. Medicines 2025, 12, 4. [Google Scholar] [CrossRef]
- Mahmood, M.S.; Siddique, F.; Hussain, I.; Ahmad, S.; Rafique, A. Thermostable vaccines for Newcastle disease: A review. World’s Poult. Sci. J. 2014, 70, 829–838. [Google Scholar] [CrossRef]
- Napit, R.; Poudel, A.; Pradhan, S.M.; Manandhar, P.; Ghaju, S.; Sharma, A.N.; Joshi, J.; Tha, S.; Dhital, K.; Rajbhandari, U. Newcastle disease burden in Nepal and efficacy of Tablet I2 vaccine in commercial and backyard poultry production. PLoS ONE 2023, 18, e0280688. [Google Scholar] [CrossRef]
- Habibi, H.; Firouzi, S.; Nili, H.; Asasi, K.; Mosleh, N. Efficacy of thermostable Newcastle disease virus strain I-2 in broiler chickens challenged with highly virulent Newcastle virus. Arch. Razi Inst. 2020, 75, 31. [Google Scholar]
- Lyu, F.; Zhao, Y.-H.; Zuo, X.-X.; Nyide, B.; Deng, B.-H.; Zhou, M.-X.; Hou, J.; Jiao, J.-J.; Zeng, M.-Q.; Jie, H.-Y.; et al. Thermostable vacuum foam dried Newcastle disease vaccine: Process optimization and pilot-scale study. Appl. Microbiol. Biotechnol. 2024, 108, 359. [Google Scholar] [CrossRef]
- Lyu, F.; Zhao, Y.-H.; Lu, Y.; Zuo, X.-X.; Deng, B.-H.; Zeng, M.-Q.; Wang, J.-N.; Olaniran, A.; Hou, J.; Khoza, T. Vacuum foam drying method improved the thermal stability and long-term shelf life of a live attenuated newcastle disease virus vaccine. AAPS PharmSciTech 2022, 23, 291. [Google Scholar] [CrossRef]
- Caudill, C.; Perry, J.L.; Iliadis, K.; Tessema, A.T.; Lee, B.J.; Mecham, B.S.; Tian, S.; DeSimone, J.M. Transdermal vaccination via 3D-printed microneedles induces potent humoral and cellular immunity. Proc. Natl. Acad. Sci. USA 2021, 118, e2102595118. [Google Scholar] [CrossRef]
- Choi, I.-J.; Cha, H.-R.; Kwon, D.; Kang, A.; Kim, J.S.; Kim, J.; Choi, J.-E.; Chung, H.W.; Park, S.; Shim, D.H. Development and Evaluation of Five-in-One Vaccine Microneedle Array Patch for Diphtheria, Tetanus, Pertussis, Hepatitis B, and Haemophilus Influenzae Type b: Immunological Efficacy and Long-Term Stability. Pharmaceutics 2024, 16, 1631. [Google Scholar] [CrossRef]
- Mistilis, M.J.; Joyce, J.C.; Esser, E.S.; Skountzou, I.; Compans, R.W.; Bommarius, A.S.; Prausnitz, M.R. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv. Transl. Res. 2017, 7, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wei, Z.; Yuan, Q.; Chen, S.; Yu, W.; Lu, Y.; Gao, J.; Yang, Y. Identifying B-cell epitopes using AlphaFold2 predicted structures and pretrained language model. Bioinformatics 2023, 39, btad187. [Google Scholar] [CrossRef] [PubMed]
- Israeli, S.; Louzoun, Y. Single-residue linear and conformational B cell epitopes prediction using random and ESM-2 based projections. Brief. Bioinform. 2024, 25, bbae084. [Google Scholar] [CrossRef] [PubMed]
- Bravi, B. Development and use of machine learning algorithms in vaccine target selection. npj Vaccines 2024, 9, 15. [Google Scholar] [CrossRef]
- Lyons, A.; Brown, J.; Davenport, K.M. Single-cell sequencing technology in ruminant livestock: Challenges and opportunities. Curr. Issues Mol. Biol. 2024, 46, 5291–5306. [Google Scholar] [CrossRef]
- Villanueva-Flores, F.; Sanchez-Villamil, J.I.; Garcia-Atutxa, I. AI-driven epitope prediction: A system review, comparative analysis, and practical guide for vaccine development. npj Vaccines 2025, 10, 207. [Google Scholar] [CrossRef]
- Petro-Turnquist, E.M.; Madapong, A.; Pekarek, M.; Steffen, D.; Weaver, E.A. Epitope-optimized vaccine elicits enduring immunity against swine influenza A virus. Nat. Commun. 2025, 16, 4046. [Google Scholar] [CrossRef]
- Petro-Turnquist, E.; Pekarek, M.J.; Weaver, E.A. Swine influenza A virus: Challenges and novel vaccine strategies. Front. Cell. Infect. Microbiol. 2024, 14, 1336013. [Google Scholar] [CrossRef]
- Raut, R.; Shrestha, R.; Adhikari, A.; Fatima, A.; Naeem, M. Revolutionizing Veterinary Vaccines: Overcoming Cold-Chain Barriers Through Thermostable and Novel Delivery Technologies. Appl. Microbiol. 2025, 5, 83. [Google Scholar] [CrossRef]
- Aida, V.; Pliasas, V.C.; Neasham, P.J.; North, J.F.; McWhorter, K.L.; Glover, S.R.; Kyriakis, C.S. Novel vaccine technologies in veterinary medicine: A herald to human medicine vaccines. Front. Vet. Sci. 2021, 8, 654289. [Google Scholar] [CrossRef]
- Song, H.; Abdullah, S.W.; Pei, C.; Shi, X.; Chen, X.; Ma, Y.; Yin, S.; Sun, S.; Huang, Y.; Guo, H. Self-assembling E2-based nanoparticles improve vaccine thermostability and protective immunity against CSFV. Int. J. Mol. Sci. 2024, 25, 596. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, A.; Mantegazza, L.; Hendrickx, S.; Capua, I. Thermostable vaccines in veterinary medicine: State of the art and opportunities to be seized. Vaccines 2022, 10, 245. [Google Scholar] [CrossRef]
- Wallace, R.M.; Undurraga, E.A.; Blanton, J.D.; Cleaton, J.; Franka, R. Elimination of dog-mediated human rabies deaths by 2030: Needs assessment and alternatives for progress based on dog vaccination. Front. Vet. Sci. 2017, 4, 9. [Google Scholar] [CrossRef]
- Morel, B.; Favrot, C.; Mirande, L.; Grünwald-Gruber, C.; Stordeur, V.; Vezina, L.P.; Faye, L.; Gomord, V. Exploring the Potentiality of a Plant Platform for Monoclonal Antibody Production in Veterinary Medicine. Vaccines 2024, 12, 620. [Google Scholar] [CrossRef]
- Nadal, D.; Bote, K.; Masthi, R.; Narayana, A.; Ross, Y.; Wallace, R.; Abela, B. Rabies post-exposure prophylaxis delivery to ensure treatment efficacy and increase compliance. IJID One Health 2023, 1, 100006. [Google Scholar] [CrossRef] [PubMed]
- da Silva Barcelos, L.; Ford, A.K.; Frühauf, M.I.; Botton, N.Y.; Fischer, G.; Maggioli, M.F. Interactions Between Bovine Respiratory Syncytial Virus and Cattle: Aspects of Pathogenesis and Immunity. Viruses 2024, 16, 1753. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Okumura, S.; Kobayashi, R.; Maeda, Y.; Takahashi, F.; Tanabe, T. Bovine respiratory syncytial virus enhances the attachment of Trueperella pyogenes to cells. J. Vet. Med. Sci. 2024, 86, 1068–1075. [Google Scholar] [CrossRef]
- Thomas, L.H.; Cook, R.S.; Wyld, S.G.; Furze, J.M.; Taylor, G. Passive protection of gnotobiotic calves using monoclonal antibodies directed at different epitopes on the fusion protein of bovine respiratory syncytial virus. J. Infect. Dis. 1998, 177, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Guzman, E.; Taylor, G. Immunology of bovine respiratory syncytial virus in calves. Mol. Immunol. 2015, 66, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Beukenhorst, A.L.; Frallicciardi, J.; Rice, K.L.; Koldijk, M.H.; Moreira de Mello, J.C.; Klap, J.M.; Hadjichrysanthou, C.; Koch, C.M.; da Costa, K.A.; Temperton, N. A pan-influenza monoclonal antibody neutralizes H5 strains and prophylactically protects through intranasal administration. Sci. Rep. 2024, 14, 3818. [Google Scholar] [CrossRef]
- Eriksson, M.; Nylen, S.; Grönvik, K.-O. Passive immunization of mice with IgY anti-H5N1 protects against experimental influenza virus infection and allows development of protective immunity. Vaccine 2024, 42, 126133. [Google Scholar] [CrossRef]
- Wallach, M.G.; Webby, R.J.; Islam, F.; Walkden-Brown, S.; Emmoth, E.; Feinstein, R.; Gronvik, K.-O. Cross-protection of chicken immunoglobulin Y antibodies against H5N1 and H1N1 viruses passively administered in mice. Clin. Vaccine Immunol. 2011, 18, 1083–1090. [Google Scholar] [CrossRef]
- NIH. Single Dose of Broadly Neutralizing Antibody Protects Macaques from H5N1 Influenza. 2025. Available online: https://www.nih.gov/news-events/news-releases/single-dose-broadly-neutralizing-antibody-protects-macaques-h5n1-influenza (accessed on 10 October 2025).
- Lei, J.; Miao, Y.; Bi, W.; Xiang, C.; Li, W.; Zhang, R.; Li, Q.; Yang, Z. Porcine epidemic diarrhea virus: Etiology, epidemiology, antigenicity, and control strategies in China. Animals 2024, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-A.; Li, H.-X.; Zheng, L.-L.; Ma, S.-J.; Wang, P.-L.; Zhao, L.; Chen, H.-Y. Development and identification of porcine monoclonal antibodies against PEDV from single B cells. Vet. Immunol. Immunopathol. 2025, 285, 110951. [Google Scholar] [CrossRef]
- Sun, B.; Mao, D.; Chen, J.; Bi, X.; Zou, L.; Bai, J.; Liu, R.; Hao, P.; Wang, Q.; Zhong, L. Preparation and Characterization of Monoclonal Antibodies Against the Porcine Rotavirus VP6 Protein. Vet. Sci. 2025, 12, 710. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Elazab, S.T.; Huang, J.; Hsu, W.H. Current Review of Monoclonal Antibody Therapeutics in Small Animal Medicine. Animals 2025, 15, 472. [Google Scholar] [CrossRef]
- Dassanayake, R.P.; Menghwar, H.; Bickel, K.A.; Holthausen, D.J.; Ma, H.; Diaz-San Segunda, F.; Rodriguez-Calzada, M.; Medina, G.N.; Attreed, S.; Falkenberg, S.M. Antiviral activity of bovine type III interferon against bovine viral diarrhea virus is greatly reduced in bovine turbinate cells due to limited expression of IFN lambda receptor 1 (IL-28Rα). Front. Immunol. 2024, 15, 1441908. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martin, E.; Weiss, M.; Diaz-San Segundo, F.; Pacheco, J.M.; Arzt, J.; Grubman, M.J.; de los Santos, T. Bovine type III interferon significantly delays and reduces the severity of foot-and-mouth disease in cattle. J. Virol. 2012, 86, 4477–4487. [Google Scholar] [CrossRef] [PubMed]
- Díaz-San Segundo, F.; Weiss, M.; Perez-Martín, E.; Koster, M.J.; Zhu, J.; Grubman, M.J.; De los Santos, T. Antiviral activity of bovine type III interferon against foot-and-mouth disease virus. Virology 2011, 413, 283–292. [Google Scholar] [CrossRef]
- Schmidt, A.; Paudyal, B.; Villanueva-Hernandez, S.; Mcnee, A.; Vatzia, E.; Carr, B.V.; Schmidt, S.; Mccarron, A.; Martini, V.; Schroedel, S. Effect of mucosal adjuvant IL-1β on heterotypic immunity in a pig influenza model. Front. Immunol. 2023, 14, 1181716. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, Z.; Zhan, X.; Yang, W.; Yao, J.; Xu, X. Immune pre-stimulation by injected yeast beta-glucan as a strategy to prevent calf diarrhea and bovine respiratory disease during the first 74 days of age. Front. Cell. Infect. Microbiol. 2025, 15, 1586121. [Google Scholar] [CrossRef]
- Wang, J.; Yan, F.; Xiong, M.; Dong, J.; Yang, W.; Xu, X. Effects of Yeast β-Glucan Supplementation on Calf Intestinal and Respiratory Health. Animals 2025, 15, 997. [Google Scholar] [CrossRef]
- Reis, M.E.; De Toledo, A.; Da Silva, A.; Poczynek, M.; Cantor, M.; Júnior, G.V.; Greco, L.; Bittar, C.M.M. Effect of supplementation with algae β-glucans on performance, health, and blood metabolites of Holstein dairy calves. J. Dairy Sci. 2022, 105, 7998–8007. [Google Scholar] [CrossRef]
- McGill, J.L.; Loving, C.L.; Kehrli, M.E., Jr. Future of Immune Modulation in Animal Agriculture. Annu. Rev. Anim. Biosci. 2024, 13, 255–275. [Google Scholar] [CrossRef]
- Mandviwala, A.S.; Liman, K.; Huckriede, A.L.; Arankalle, V.A.; Patil, H.P. Evaluation of dual pathogen recognition receptor agonists as adjuvants for respiratory syncytial virus-virus-like particles for pulmonary delivery. Front. Immunol. 2025, 16, 1561297. [Google Scholar] [CrossRef]
- Yue, T.; Lu, Y.; Ding, W.; Xu, B.; Zhang, C.; Li, L.; Jian, F.; Huang, S. The Role of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Livestock and Poultry Gut Health: A Review. Metabolites 2025, 15, 478. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Rossow, H.A.; Williams, D.R.; Cheong, S.; Okella, H.; Widmer, L.; Okello, E. Effect of Multi-Species Probiotic Supplementation on Fecal Microbiota in Pre-Weaned Holstein Dairy Calves in California. Microorganisms 2025, 13, 1810. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.N.; Chaudhary, P.; Kumar, S.; Grover, C.R.; Mondal, G. Effect of synbiotics on growth performance, gut health, and immunity status in pre-ruminant buffalo calves. Sci. Rep. 2023, 13, 10184. [Google Scholar] [CrossRef] [PubMed]
- Thanippilly, A.J.; Kumar, S.; Varada, V.V.; Balaga, S.; Mondal, G.; Tyagi, N.; Samanta, A.K. Optimizing synbiotic formulations with Ligilactobacillus salivarius BF17 for enhanced gut health in Murrah buffalo calves. Curr. Res. Biotechnol. 2024, 8, 100250. [Google Scholar] [CrossRef]
- Roque, F.; Lopes, M.H.S.; Raffi, P.; Oliveira, R.; Caparroz, M.; Longhini, G.; Granghelli, C.; Faria, D.; Araujo, L.; Araujo, C. Postbiotics: An Alternative for Improving Health and Performance of Poultry Production. Microorganisms 2025, 13, 1472. [Google Scholar] [CrossRef]
- Saeed, M.; Afzal, Z.; Afzal, F.; Khan, R.U.; Elnesr, S.S.; Alagawany, M.; Chen, H. Use of postbiotic as growth promoter in poultry industry: A review of current knowledge and future prospects. Food Sci. Anim. Resour. 2023, 43, 1111. [Google Scholar] [CrossRef]
- Murtaza, N.; Nawaz, M.; Yaqub, T.; Mehmood, A.K. Impact of Limosilactobacillus fermentum probiotic treatment on gut microbiota composition in sahiwal calves with rotavirus diarrhea: A 16S metagenomic analysis study. BMC Microbiol. 2024, 24, 114. [Google Scholar] [CrossRef]
- Kayasaki, F.; Okagawa, T.; Konnai, S.; Kohara, J.; Sajiki, Y.; Watari, K.; Ganbaatar, O.; Goto, S.; Nakamura, H.; Shimakura, H. Direct evidence of the preventive effect of milk replacer–based probiotic feeding in calves against severe diarrhea. Vet. Microbiol. 2021, 254, 108976. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fan, X.; Mao, X.; Yu, B.; He, J.; Yan, H.; Wang, J. The protective role of prebiotics and probiotics on diarrhea and gut damage in the rotavirus-infected piglets. J. Anim. Sci. Biotechnol. 2024, 15, 61. [Google Scholar] [CrossRef]
- Oyanguren, M.; Molina, E.; Mugica, M.; Ladero-Auñon, I.; Fuertes, M.; Fernández, M.; Benavides, J.; Elguezabal, N. Probiotic bacteria can modulate immune responses to paratuberculosis vaccination. Front. Cell. Infect. Microbiol. 2024, 14, 1394070. [Google Scholar] [CrossRef]
- Arrazuria, R.; Oyanguren, M.; Molina, E.; Mugica, M.; Gunapati, B.; Subbian, S.; Lavin, J.; Anguita, J.; Elguezabal, N. Dual effects of probiotic administration prior to Mycobacterium avium subsp. paratuberculosis infection are associated with immunological and microbiota shifts. Sci. Rep. 2025, 15, 23341. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The ISAPP consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, X.; Hu, M.; Hou, J.; Du, Y.; Si, W.; Yang, L.; Xu, L.; Xu, Q. Modulating gastrointestinal microbiota to alleviate diarrhea in calves. Front. Microbiol. 2023, 14, 1181545. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, S.; Gao, D.; Xu, Y.; Jiang, W.; Chen, T.; Xiao, J.; Wang, J.; Hou, G.; Li, S. The Bifidobacterium-dominated fecal microbiome in dairy calves shapes the characteristic growth phenotype of host. npj Biofilms Microbiomes 2024, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Torkashvand, N.; Kamyab, H.; Aarabi, P.; Shahverdi, A.R.; Torshizi, M.A.K.; Khoshayand, M.R.; Sepehrizadeh, Z. Evaluating the effectiveness and safety of a novel phage cocktail as a biocontrol of Salmonella in biofilm, food products, and broiler chicken. Front. Microbiol. 2024, 15, 1505805. [Google Scholar] [CrossRef]
- Thanki, A.M.; Hooton, S.; Whenham, N.; Salter, M.G.; Bedford, M.R.; O’Neill, H.V.M.; Clokie, M.R. A bacteriophage cocktail delivered in feed significantly reduced Salmonella colonization in challenged broiler chickens. Emerg. Microbes Infect. 2023, 12, 2217947. [Google Scholar] [CrossRef]
- Segundo-Arizmendi, N.; Flores-Coria, A.P.; Gómez-Garcia, J.; Hernández, E.; Meneses-Acosta, A. Bacteriophages against Salmonella Enteritidis: Challenges and Opportunities. Front. Bioeng. Biotechnol. 2025, 13, 1605263. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Pyzik, E. Selected Mechanisms of Action of Bacteriophages in Bacterial Infections in Animals. Viruses 2025, 17, 101. [Google Scholar] [CrossRef]
- Cianci, R.; Caldarelli, M.; Brani, P.; Bosi, A.; Ponti, A.; Giaroni, C.; Baj, A. Cytokines Meet Phages: A Revolutionary Pathway to Modulating Immunity and Microbial Balance. Biomedicines 2025, 13, 1202. [Google Scholar] [CrossRef] [PubMed]
- Zborowsky, S.; Seurat, J.; Balacheff, Q.; Ecomard, S.; Mulet, C.; Minh, C.N.N.; Titécat, M.; Evrard, E.; Rodriguez-Gonzalez, R.A.; Marchi, J. Macrophage-induced reduction of bacteriophage density limits the efficacy of in vivo pulmonary phage therapy. Nat. Commun. 2025, 16, 5725. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.; Wu, L.; Xiong, Y.; Xia, L.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z. Development and mouse model evaluation of a new phage cocktail intended as an alternative to antibiotics for treatment of Staphylococcus aureus-induced bovine mastitis. J. Dairy Sci. 2024, 107, 5974–5987. [Google Scholar] [CrossRef]
- Torres-Boncompte, J.; Garcia-Llorens, J.; Cortés, P.; Martinez-Sánchez, A.; Llagostera, M.; Campoy, S.; Soriano, J.M.; Catalá-Gregori, P.; Sevilla-Navarro, S. In Ovo phage administration to mitigate Salmonella Typhimurium colonization in broiler chickens–A new firewall strategy for the poultry industry. Food Control 2025, 180, 111637. [Google Scholar] [CrossRef]
- Gencay, Y.E.; Jasinskytė, D.; Robert, C.; Semsey, S.; Martínez, V.; Petersen, A.Ø.; Brunner, K.; de Santiago Torio, A.; Salazar, A.; Turcu, I.C. Engineered phage with antibacterial CRISPR–Cas selectively reduce E. coli burden in mice. Nat. Biotechnol. 2024, 42, 265–274. [Google Scholar] [CrossRef] [PubMed]
- CRISPR Medicine News. Clinical Update: SNIPR Biome Reports Positive Phase 1 Results for CRISPR-Armed Phage Therapy. 2023. Available online: https://crisprmedicinenews.com/news/clinical-update-snipr-biome-reports-positive-phase-1-results-for-crispr-armed-phage-therapy/ (accessed on 10 October 2025).
- Królikowska, D.; Szymańska, M.; Krzyżaniak, M.; Guziński, A.; Matusiak, R.; Kajdanek, A.; Kaczorek-Łukowska, E.; Maszewska, A.; Wójcik, E.A.; Dastych, J. A New Approach for Phage Cocktail Design in the Example of Anti-mastitis Solution. Pathogens 2024, 13, 839. [Google Scholar] [CrossRef]
- Jesudason, T. Advancing the use of bacteriophages to tackle AMR. Lancet Microbe 2025, 6, 101204. [Google Scholar] [CrossRef]
- Melaku, M.; Wang, J.; Xie, Y.; Ali, A.; Yi, B.; Ma, T.; Zhong, R.; Chen, L.; Zhang, H. Reviving hope: Phage therapy application for antimicrobial resistance in farm animal production over the past decade. Anim. Feed Sci. Technol. 2025, 324, 116333. [Google Scholar] [CrossRef]
- Nale, J.; McEwan, N. Bacteriophage therapy to control bovine mastitis: A review. Antibiotics 2023, 12, 1307. [Google Scholar] [CrossRef]
- Brouillette, E.; Millette, G.; Chamberland, S.; Roy, J.-P.; Ster, C.; Kiros, T.; Hickey, S.; Hittle, L.; Woolston, J.; Malouin, F. Effective treatment of staphylococcus aureus intramammary infection in a murine model using the bacteriophage cocktail staphLyse™. Viruses 2023, 15, 887. [Google Scholar] [CrossRef]
- Agapé, L.; Menanteau, P.; Kempf, F.; Schouler, C.; Boulesteix, O.; Riou, M.; Chaumeil, T.; Velge, P. Prophylactic phage administration reduces Salmonella Enteritidis infection in newly hatched chicks. MicrobiologyOpen 2024, 13, e70002. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Jończyk-Matysiak, E.; Kniotek, M.; Letkiewicz, S. Therapeutic phages as modulators of the immune response: Practical implications. Clin. Infect. Dis. 2023, 77, S433–S439. [Google Scholar] [CrossRef] [PubMed]
- Pye, H.V.; Krishnamurthi, R.; Cook, R.; Adriaenssens, E.M. Phage diversity in one health. Essays Biochem. 2024, 68, 607–619. [Google Scholar]
- Faltus, T. The medicinal phage-regulatory roadmap for phage therapy under EU pharmaceutical legislation. Viruses 2024, 16, 443. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, C.; Tsakri, D.; Panagiotopoulos, A.-P.; Saldari, C.; Sagona, A.P.; Tsakris, A. Armed Phages: A New Weapon in the Battle Against Antimicrobial Resistance. Viruses 2025, 17, 911. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, C.; Galina Pantoja, L.; Beaton, B.; Chen, C.-Y.; Culbertson, M.; Harms, P.; Holl, J.; Sosnicki, A.; Reddy, S.; Rotolo, M. Pigs lacking the SRCR5 domain of CD163 protein demonstrate heritable resistance to the PRRS virus and no changes in animal performance from birth to maturity. Front. Genome Ed. 2024, 6, 1322012. [Google Scholar] [CrossRef]
- Burkard, C.; Lillico, S.G.; Reid, E.; Jackson, B.; Mileham, A.J.; Ait-Ali, T.; Whitelaw, C.B.A.; Archibald, A.L. Precision engineering for PRRSV resistance in pigs: Macrophages from genome edited pigs lacking CD163 SRCR5 domain are fully resistant to both PRRSV genotypes while maintaining biological function. PLoS Pathog. 2017, 13, e1006206. [Google Scholar] [CrossRef]
- Dolezel, M.; Eckerstorfer, M.F.; Miklau, M.; Greiter, A.; Heissenberger, A.; Hörtenhuber, S.; Burn, S.-J.; Zollitsch, W.; Kastenhofer, K.; Hagen, K. Governance Perspectives on Genetically Modified Animals for Agriculture and Aquaculture: Challenges for the Assessment of Environmental Risks and Broader Societal Concerns. Animals 2025, 15, 2731. [Google Scholar] [CrossRef]
- Whitworth, K.M.; Rowland, R.R.; Ewen, C.L.; Trible, B.R.; Kerrigan, M.A.; Cino-Ozuna, A.G.; Samuel, M.S.; Lightner, J.E.; McLaren, D.G.; Mileham, A.J. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat. Biotechnol. 2016, 34, 20–22. [Google Scholar] [CrossRef]
- Salgado, B.; Rivas, R.B.; Pinto, D.; Sonstegard, T.S.; Carlson, D.F.; Martins, K.; Bostrom, J.R.; Sinebo, Y.; Rowland, R.R.; Brandariz-Nuñez, A. Genetically modified pigs lacking CD163 PSTII-domain-coding exon 13 are completely resistant to PRRSV infection. Antivir. Res. 2024, 221, 105793. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Xiang, H.-Y.; Niu, M.; Deng, J.-C.; Li, X.-Y.; Hao, W.-J.; Ou-Yang, H.-S.; Liu, T.-Y.; Tang, X.-C. Genetically modified pigs with CD163 point mutation are resistant to HP-PRRSV infection. Zool. Res. 2024, 45, 833. [Google Scholar] [CrossRef]
- Idoko-Akoh, A.; Goldhill, D.H.; Sheppard, C.M.; Bialy, D.; Quantrill, J.L.; Sukhova, K.; Brown, J.C.; Richardson, S.; Campbell, C.; Taylor, L. Creating resistance to avian influenza infection through genome editing of the ANP32 gene family. Nat. Commun. 2023, 14, 6136. [Google Scholar] [CrossRef]
- Workman, A.M.; Heaton, M.P.; Vander Ley, B.L.; Webster, D.A.; Sherry, L.; Bostrom, J.R.; Larson, S.; Kalbfleisch, T.S.; Harhay, G.P.; Jobman, E.E. First gene-edited calf with reduced susceptibility to a major viral pathogen. PNAS Nexus 2023, 2, pgad125. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.M.; Heaton, M.P.; Vander Ley, B.L. CD46 Gene Editing Confers Ex Vivo BVDV Resistance in Fibroblasts from Cloned Angus Calves. Viruses 2025, 17, 775. [Google Scholar] [CrossRef]
- USDA Agricultural Research Service. Scientists Use Gene-Editing Technology to Produce First Calf Resistant to Major Viral disease. 2023. Available online: https://www.ars.usda.gov/news-events/news/research-news/2023/scientists-use-gene-editing-technology-to-produce-first-calf-resistant-to-major-viral-disease/ (accessed on 11 October 2025).
- Gao, Y.; Wu, H.; Wang, Y.; Liu, X.; Chen, L.; Li, Q.; Cui, C.; Liu, X.; Zhang, J.; Zhang, Y. Single Cas9 nickase induced generation of NRAMP1 knockin cattle with reduced off-target effects. Genome Biol. 2017, 18, 13. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Kayode, H.H.; Okesanya, O.J.; Ukoaka, B.M.; Eshun, G.; Mourid, M.R.; Adigun, O.A.; Ogaya, J.B.; Mohamed, Z.O.; Lucero-Prisno III, D.E. CRISPR-Cas systems in the fight against antimicrobial resistance: Current status, potentials, and future directions. Infect. Drug Resist. 2024, 17, 5229–5245. [Google Scholar] [CrossRef]
- Salvesen, H.A.; Grupen, C.G.; McFarlane, G.R. Tackling mosaicism in gene edited livestock. Front. Anim. Sci. 2024, 5, 1368155. [Google Scholar] [CrossRef]
- Fischer, K.; Schnieke, A. How genome editing changed the world of large animal research. Front. Genome Ed. 2023, 5, 1272687. [Google Scholar] [CrossRef]
- Kwon, D.-H.; Gim, G.-M.; Yum, S.-Y.; Jang, G. Current status and future of gene engineering in livestock. BMB Rep. 2024, 57, 50. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.d.J.L.d.; Mpande, C.A.; Helble, M.; Nicholson, M.W.; Cortés, M.d.l.Á.; Ponsa, M.E.P.; Blumenthal, I.R.; Caccavo, F.; Pippo, T.; Rius Sanjuan, J. Investment Opportunities for mRNA Technology in Low-and Middle-Income Countries: Key Findings and Future Perspectives. Vaccines 2025, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhang, J.; Gao, Y.; Yuan, Z.; Zhu, Z.; Wei, Y.; Wu, T.; Han, J.; Zhang, Y. HMEJ-based safe-harbor genome editing enables efficient generation of cattle with increased resistance to tuberculosis. J. Biol. Chem. 2021, 296, 100497. [Google Scholar] [CrossRef]
- Ochwo, S.; Perez, A.M.; Pérez Aguirreburualde, M.S. Beyond accuracy: Leveraging ASSURED criteria for field evaluation of point-of-care tests for food animal diseases. Front. Vet. Sci. 2023, 10, 1239111. [Google Scholar] [CrossRef]
- Velayudhan, B.T.; Naikare, H.K. Point-of-care testing in companion and food animal disease diagnostics. Front. Vet. Sci. 2022, 9, 1056440. [Google Scholar] [CrossRef]
- Coopersmith, M.; Dijkman, R.; Bartlett, M.L.; Currie, R.; Schuurman, S.; Wit, S.d. Development and Laboratory Validation of Rapid, Bird-Side Molecular Diagnostic Assays for Avian Influenza Virus Including Panzootic H5Nx. Microorganisms 2025, 13, 1090. [Google Scholar] [CrossRef]
- Hobbs, E.C.; Colling, A.; Gurung, R.B.; Allen, J. The potential of diagnostic point-of-care tests (POCTs) for infectious and zoonotic animal diseases in developing countries: Technical, regulatory and sociocultural considerations. Transbound. Emerg. Dis. 2021, 68, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Dürr, S.; Bodmer, M. Reducing antimicrobial use by implementing evidence-based, management-related prevention strategies in dairy cows in Switzerland. Front. Vet. Sci. 2021, 7, 611682. [Google Scholar] [CrossRef]
- Bedir, M.; Marouf, S.; Sayed, R.H.; El-Diasty, M.; Hassan, H.M.; Shahien, M.A.; Eid, S.; Aboul-Ella, H.; Eljakee, J. Development and evaluation of a novel lateral flow immunoassay for rapid diagnosis of brucellosis across different animal species. Sci. Rep. 2025, 15, 24149. [Google Scholar] [CrossRef]
- Serafinelli, C.; Fantoni, A.; Alegria, E.C.; Vieira, M. Recent progresses in plasmonic biosensors for point-of-care (POC) devices: A critical review. Chemosensors 2023, 11, 303. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Cvelbar, U.; Abdulhalim, I. A comprehensive review on plasmonic-based biosensors used in viral diagnostics. Commun. Biol. 2021, 4, 70. [Google Scholar] [CrossRef]
- Ferreira, C.; Todorovic, M.; Sugrue, P.; Teixeira, S.; Galvin, P. Emerging sensors and instrumentation systems for bovine health monitoring. Animal 2025, 19, 101527. [Google Scholar] [CrossRef]
- Curti, P.d.F.; Selli, A.; Pinto, D.L.; Merlos-Ruiz, A.; Balieiro, J.C.d.C.; Ventura, R.V. Applications of livestock monitoring devices and machine learning algorithms in animal production and reproduction: An overview. Anim. Reprod. 2023, 20, e20230077. [Google Scholar] [CrossRef]
- Lamanna, M.; Bovo, M.; Cavallini, D. Wearable collar technologies for dairy cows: A systematized review of the current applications and future innovations in precision livestock farming. Animals 2025, 15, 458. [Google Scholar] [CrossRef] [PubMed]
- Rial, C.; Stangaferro, M.; Thomas, M.; Giordano, J. Effect of automated health monitoring based on rumination, activity, and milk yield alerts versus visual observation on herd health monitoring and performance outcomes. J. Dairy Sci. 2024, 107, 11576–11596. [Google Scholar] [CrossRef] [PubMed]
- Reuters. CDC Makes Public Influenza A Wastewater Data to Assist Bird Flu Probe. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/cdc-makes-public-influenza-wastewater-data-assist-bird-flu-probe-2024-05-14/ (accessed on 11 October 2025).
- CDC. National Wastewater Surveillance System (NWSS): Wastewater Monitoring Data. Available online: https://www.cdc.gov/nwss/index.html (accessed on 11 October 2025).
- Song, J.-H.; Son, S.-E.; Kim, H.-W.; Kim, S.-J.; An, S.-H.; Lee, C.-Y.; Kwon, H.-J.; Choi, K.-S. Rapid and specific on-site H5Nx avian influenza diagnosis via RPA and PAM-independent CRISPR-Cas12a assay combined with anti-NP antibody-based viral RNA purification. Front. Vet. Sci. 2025, 12, 1520349. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Zhang, M.; Zhu, M. Rapid detection of FAdV-4 by one-tube RPA-CRISPR/Cas12a assay. Front. Microbiol. 2025, 16, 1541943. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Ma, Y.; Jiang, Y.; Li, Y.; Shi, J.; Deng, G.; Tian, G.; Kong, H.; Wang, X. On-site and visual detection of the H5 subtype avian influenza virus based on RT-RPA and CRISPR/Cas12a. Viruses 2024, 16, 753. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, S.; Xia, N.; Zhang, Y.; Zhang, J.; Liu, A.; Zhang, C.; Chen, N.; Meurens, F.; Zheng, W. Rapid visual detection of african swine fever virus with a CRISPR/Cas12a lateral flow strip based on structural protein gene D117L. Animals 2023, 13, 3712. [Google Scholar] [CrossRef]
- Thompson, J.; Everhart Nunn, S.L.; Sarkar, S.; Clayton, B. Diagnostic screening of bovine mastitis using MALDI-TOF MS direct-spotting of milk and machine learning. Vet. Sci. 2023, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Cuccato, M.; Divari, S.; Sacchi, P.; Girolami, F.; Cannizzo, F.T. MALDI-TOF mass spectrometry profiling of bovine skim milk for subclinical mastitis detection. Front. Vet. Sci. 2022, 9, 1009928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pichpol, D.; Boonyayatra, S. Application of MALDI-TOF MS to Identify and Detect Antimicrobial-Resistant Streptococcus uberis Associated with Bovine Mastitis. Microorganisms 2024, 12, 1332. [Google Scholar] [CrossRef]
- Sarkar, S.; Thompson, J.; Everhart Nunn, S.L.; Clayton, B. 222 High Throughput Screening of Bovine Mastitis from Raw Milk Using Maldi-Tof Ms and Machine Learning. J. Anim. Sci. 2023, 101, 137–138. [Google Scholar] [CrossRef]
- Mitsunaga, T.M.; Nery Garcia, B.L.; Pereira, L.B.R.; Costa, Y.C.B.; da Silva, R.F.; Delbem, A.C.B.; Dos Santos, M.V. Current trends in artificial intelligence and bovine mastitis research: A bibliometric review approach. Animals 2024, 14, 2023. [Google Scholar] [CrossRef]
- Džermeikaitė, K.; Krištolaitytė, J.; Antanaitis, R. Application of Machine Learning Models for the Early Detection of Metritis in Dairy Cows Based on Physiological, Behavioural and Milk Quality Indicators. Animals 2025, 15, 1674. [Google Scholar] [CrossRef] [PubMed]
- Sturm, V.; Efrosinin, D.; Öhlschuster, M.; Gusterer, E.; Drillich, M.; Iwersen, M. Combination of sensor data and health monitoring for early detection of subclinical ketosis in dairy cows. Sensors 2020, 20, 1484. [Google Scholar] [CrossRef]
- Siddique, A.; Panda, S.S.; Khan, S.; Dargan, S.T.; Lewis, S.; Carter, I.; Van Wyk, J.A.; Mahapatra, A.K.; Morgan, E.R.; Terrill, T.H. Innovations in animal health: Artificial intelligence-enhanced hematocrit analysis for rapid anemia detection in small ruminants. Front. Vet. Sci. 2024, 11, 1493403. [Google Scholar] [CrossRef] [PubMed]
- Szalai, S.; Bodnár, Á.; Fébel, H.; Bakony, M.; Jurkovich, V. Rumination Time, Reticulorumen Temperature, and Activity in Relation to Postpartum Health Status in Dairy Cows During Heat Stress. Animals 2025, 15, 1616. [Google Scholar] [CrossRef]
- Vakulya, G.; Hajnal, É.; Udvardy, P.; Simon, G. In-depth development of a versatile rumen bolus sensor for dairy cattle. Sensors 2024, 24, 6976. [Google Scholar] [CrossRef]
- Barto, A.O.; Bailey, D.W.; Trieu, L.L.; Pryor, P.; McCosker, K.D.; Utsumi, S. Monitoring Behavior and Welfare of Cattle in Response to Summer Weather in an Arizona Rangeland Pasture Using a Commercial Rumen Bolus. Animals 2025, 15, 1448. [Google Scholar] [CrossRef]
- Rowe, S.; Zhang, E.; Godden, S.; Vasquez, A.; Nydam, D. Comparison of a machine learning model with a conventional rule-based selective dry cow therapy algorithm for detection of intramammary infections. J. Dairy Sci. 2025, 108, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Rasool, H.M.H.; Chen, Q.; Gong, X.; Zhou, J. CRISPR/Cas system and its application in the diagnosis of animal infectious diseases. FASEB J. 2024, 38, e70252. [Google Scholar] [CrossRef] [PubMed]
- Yasobant, S.; Tadvi, R.; Saxena, D.B. Preparedness for One Health Surveillance System: A qualitative in-depth exploration in Gujarat, India. IJID One Health 2025, 6, 100055. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef]
- Cao, S.; Ma, D.; Xie, J.; Wu, Z.; Yan, H.; Ji, S.; Zhou, M.; Zhu, S. Point-of-care testing diagnosis of African swine fever virus by targeting multiple genes with enzymatic recombinase amplification and CRISPR/Cas12a System. Front. Cell. Infect. Microbiol. 2024, 14, 1474825. [Google Scholar] [CrossRef]
- Kamel, M.; Davidson, J.L.; Schober, J.M.; Fraley, G.S.; Verma, M.S. A paper-based loop-mediated isothermal amplification assay for highly pathogenic avian influenza. Sci. Rep. 2025, 15, 12110. [Google Scholar] [CrossRef]
- Phillips, D.; Conceicao, F.d.; Jong, J.B.d.C.; Rawlin, G.; Mee, P. Stability of Genotube® Swabs for African Swine Fever Virus Detection Using Loop-Mediated Isothermal (LAMP) Laboratory Testing on Samples Stored without Refrigeration. Viruses 2024, 16, 263. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Xie, Z.; Zhao, J.; Hua, J.; Wei, Y.; Li, X.; Li, D.; Luo, S.; Li, M.; Xie, L. Simultaneous differential detection of H5, H7 and H9 subtypes of avian influenza viruses by a triplex fluorescence loop-mediated isothermal amplification assay. Front. Vet. Sci. 2024, 11, 1419312. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef]
- Qin, P.; Park, M.; Alfson, K.J.; Tamhankar, M.; Carrion, R.; Patterson, J.L.; Griffiths, A.; He, Q.; Yildiz, A.; Mathies, R. Rapid and fully microfluidic Ebola virus detection with CRISPR-Cas13a. ACS Sens. 2019, 4, 1048–1054. [Google Scholar] [CrossRef]
- Elbehiry, A.; Aldubaib, M.; Abalkhail, A.; Marzouk, E.; ALbeloushi, A.; Moussa, I.; Ibrahem, M.; Albazie, H.; Alqarni, A.; Anagreyyah, S. How MALDI-TOF mass spectrometry technology contributes to microbial infection control in healthcare settings. Vaccines 2022, 10, 1881. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Hamada, M.; Al-Dubaib, M.; Alyamani, E.; Moussa, I.M.; AlRowaidhan, A.; Hemeg, H.A. Application of MALDI-TOF MS fingerprinting as a quick tool for identification and clustering of foodborne pathogens isolated from food products. New Microbiol. 2017, 40, 269–278. [Google Scholar] [PubMed]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Osman, S.; Edrees, H. Performance of MALDI biotyper compared with Vitek™ 2 compact system for fast identification and discrimination of Staphylococcus species isolated from bovine mastitis. MicrobiologyOpen 2016, 5, 1061–1070. [Google Scholar] [CrossRef]
- Neethirajan, S. Recent advances in wearable sensors for animal health management. Sens. Bio-Sens. Res. 2017, 12, 15–29. [Google Scholar] [CrossRef]
- Yu, Z.; Han, Y.; Cha, L.; Chen, S.; Wang, Z.; Zhang, Y. Design of an intelligent wearable device for real-time cattle health monitoring. Front. Robot. AI 2024, 11, 1441960. [Google Scholar] [CrossRef]
- Grzesiak, W.; Zaborski, D.; Pluciński, M.; Jędrzejczak-Silicka, M.; Pilarczyk, R.; Sablik, P. The Use of Selected Machine Learning Methods in Dairy Cattle Farming: A Review. Animals 2025, 15, 2033. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, S.; Grelet, C.; Leblois, J.; Gengler, N.; Soyeurt, H. Can unsupervised learning methods applied to milk recording big data provide new insights into dairy cow health? J. Dairy Sci. 2022, 105, 6760–6772. [Google Scholar] [CrossRef]
- Akbarein, H.; Taaghi, M.H.; Mohebbi, M.; Soufizadeh, P. Applications and Considerations of Artificial Intelligence in Veterinary Sciences: A Narrative Review. Vet. Med. Sci. 2025, 11, e70315. [Google Scholar] [CrossRef]
- Marzougui, A.; McConnel, C.S.; Adams-Progar, A.; Biggs, T.D.; Ficklin, S.P.; Sankaran, S. Machine learning-derived cumulative health measure for assessing disease impact in dairy cattle. Front. Anim. Sci. 2025, 6, 1532385. [Google Scholar] [CrossRef]
- CDC. About One Health. 2025. Available online: https://www.cdc.gov/one-health/about/index.html (accessed on 11 October 2025).
- Kuhn, C.; Hayibor, K.M.; Acheampong, A.T.; Pires, L.S.A.; Costa-Ribeiro, M.C.V.; Burrone, M.S.; Vásquez-Almazán, C.R.; Radon, K.; Soto, M.T.S.; Salini, A.P.L. How studies on zoonotic risks in wildlife implement the one health approach–A systematic review. One Health 2024, 19, 100929. [Google Scholar] [CrossRef]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, K.J.; Daszak, P. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Offeddu, V.; Cowling, B.J.; Peiris, J.M. Interventions in live poultry markets for the control of avian influenza: A systematic review. One Health 2016, 2, 55–64. [Google Scholar] [CrossRef]
- Zhu, G.; Kang, M.; Wei, X.; Tang, T.; Liu, T.; Xiao, J.; Song, T.; Ma, W. Different intervention strategies toward live poultry markets against avian influenza A (H7N9) virus: Model-based assessment. Environ. Res. 2021, 198, 110465. [Google Scholar] [CrossRef]
- WHO. Emerging Zoonotic Diseases and the One Health Approach: An Overview. Available online: https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/epi_win_digest_4_one-health.pdf?sfvrsn=b9feef83_2 (accessed on 11 October 2025).
- Gass, J.D., Jr. Drivers of Zoonotic Viral Spillover: Understanding Pathways to the Next Pandemic. Zoonotic Dis. 2025, 5, 18. [Google Scholar] [CrossRef]
- Oltean, H.N.; Lipton, B.; Black, A.; Snekvik, K.; Haman, K.; Buswell, M.; Baines, A.E.; Rabinowitz, P.M.; Russell, S.L.; Shadomy, S. Developing a one health data integration framework focused on real-time pathogen surveillance and applied genomic epidemiology. One Health Outlook 2025, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Balta, I.; Lemon, J.; Gadaj, A.; Cretescu, I.; Stef, D.; Pet, I.; Stef, L.; McCleery, D.; Douglas, A.; Corcionivoschi, N. The interplay between antimicrobial resistance, heavy metal pollution, and the role of microplastics. Front. Microbiol. 2025, 16, 1550587. [Google Scholar] [CrossRef]
- Guo, X.; Long, X.; Li, J.; Wu, J.; Zhu, X.; Zhu, Y.; Weng, M.; Zhang, Y. Effects of antibiotics and heavy metals on antibiotic resistance genes and mobile gene elements in agricultural activity. Environ. Sci. Eur. 2025, 37, 87. [Google Scholar] [CrossRef]
- Sharma, P.; Pal, N.; Kumawat, M.; Singh, S.; Das, D.; Tilwari, A.; Prakash, A.; Tiwari, R.R.; Kumar, M. Investigating the antibiotic resistance genes and mobile genetic elements in water systems impacted with anthropogenic pollutants. Environ. Res. 2025, 269, 120814. [Google Scholar] [CrossRef]
- Franklin, A.M.; Weller, D.L.; Durso, L.M.; Bagley, M.; Davis, B.C.; Frye, J.G.; Grim, C.J.; Ibekwe, A.M.; Jahne, M.A.; Keely, S.P. A one health approach for monitoring antimicrobial resistance: Developing a national freshwater pilot effort. Front. Water 2024, 6, 1359109. [Google Scholar] [CrossRef]
- Clarke, L.M.; O’Brien, J.W.; Murray, A.K.; Gaze, W.H.; Thomas, K.V. A review of wastewater-based epidemiology for antimicrobial resistance surveillance. J. Environ. Expo. Assess 2024, 3, 7. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, L.; Li, Y.; Zhang, S.; Xu, X.; Li, Y.; Cao, C.; Xu, J.; Li, S. One Health integrated surveillance: A way forward to accelerate schistosomiasis elimination in China. Sci. One Health 2025, 4, 100114. [Google Scholar] [CrossRef]
- Anderson, M.; Schulze, K.; Cassini, A.; Plachouras, D.; Mossialos, E. A governance framework for development and assessment of national action plans on antimicrobial resistance. Lancet Infect. Dis. 2019, 19, e371–e384. [Google Scholar] [CrossRef]
- WOAH. Towards a Healthier Future for All: Progress in Animal Health to Contain Antimicrobial Resistance. 2024. Available online: https://www.woah.org/app/uploads/2024/10/towards-a-healthier-future-for-all-amr-progress-report-en.pdf (accessed on 11 September 2025).
- Crawley, A.W.; Mercy, K.; Shivji, S.; Lofgren, H.; Trowbridge, D.; Manthey, C.; Tebeje, Y.K.; Clara, A.W.; Landry, K.; Salyer, S.J. An indicator framework for the monitoring and evaluation of event-based surveillance systems. Lancet Glob. Health 2024, 12, e707–e711. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, H.A.; Freeth, F.T.; Bogaardt, C.; Taylor, E.; Reinhardt, J.; Collineau, L.; Prada, J.M.; Hénaux, V. Implementation of One Health surveillance systems: Opportunities and challenges-lessons learned from the OH-EpiCap application. One Health 2024, 18, 100704. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Abramova, A.; Berendonk, T.U.; Coelho, L.P.; Forslund, S.K.; Gschwind, R.; Heikinheimo, A.; Jarquín-Díaz, V.H.; Khan, A.A.; Klümper, U. Towards monitoring of antimicrobial resistance in the environment: For what reasons, how to implement it, and what are the data needs? Environ. Int. 2023, 178, 108089. [Google Scholar] [CrossRef]
- Asaaga, F.A.; Shakeer, I.; Sriram, A.; Chhotaria, K.; Dutta, S.; Narayanaswamy, D.; Amankwaa, G.; Chanda, M.M.; Hoti, S.L.; Young, J.C. Ties that bind: Understanding One Health networks and participation for zoonoses prevention and control in India. One Health Outlook 2024, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- FDA. GFI #187A Heritable Intentional Genomic Alterations in Animals: Risk-Based Approach. 2024. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-187a-heritable-intentional-genomic-alterations-animals-risk-based-approach (accessed on 12 September 2025).
- Copper, S. Following the Framework: Intentional Genomic Alterations in Animals. J. Food L. Pol’y 2022, 18, 117. [Google Scholar] [CrossRef]
- FDA/Federal-Register. Heritable Intentional Genomic Alterations in Animals: The Approval Process; Guidance for Industry; Availability. 2025. Available online: https://www.federalregister.gov/documents/2025/01/07/2024-31532/heritable-intentional-genomic-alterations-in-animals-the-approval-process-guidance-for-industry (accessed on 12 October 2025).
- FDA. Risk Assessment Summary—V-006378 PRLR-SLICK Cattle. 2022. Available online: https://www.fda.gov/media/155706/download (accessed on 11 October 2025).
- Wray-Cahen, D.; Hallerman, E.; Tizard, M. Global regulatory policies for animal biotechnology: Overview, opportunities and challenges. Front. Genome Ed. 2024, 6, 1467080. [Google Scholar] [CrossRef]
- Ledesma, A.V.; Van Eenennaam, A.L. Global status of gene edited animals for agricultural applications. Vet. J. 2024, 305, 106142. [Google Scholar] [CrossRef]
- Gelinsky, E.; Hilbeck, A. European Court of Justice ruling regarding new genetic engineering methods scientifically justified: A commentary on the biased reporting about the recent ruling. Environ. Sci. Eur. 2018, 30, 52. [Google Scholar] [CrossRef]
- Casacuberta, J.; Barro, F.; Braeuning, A.; de Maagd, R.; Epstein, M.M.; Frenzel, T.; Gallois, J.L.; Koning, F.; Messéan, A. New developments in biotechnology applied to animals: An assessment of the adequacy and sufficiency of current EFSA guidance for animal risk assessment. EFSA J. 2025, 23, e9566. [Google Scholar] [PubMed]
- Rothamsted-Research. Precision Breeding Regulations Signed into Law by UK Government. Available online: https://www.rothamsted.ac.uk/news/precision-breeding-regulations-signed-law-uk-government (accessed on 11 October 2025).
- Guardian. Scientists Dismayed as UK Ministers Clear Way for Gene Editing of Crops—But Not Animals. Available online: https://www.theguardian.com/science/2024/nov/02/scientists-dismayed-as-uk-ministers-clear-way-for-gene-editing-of-crops-but-not-animals (accessed on 11 October 2025).
- National Biosafety Authority (NBA). Guidelines for Determining the Regulatory Process of Genome Editing Techniques in Kenya. 2022. Available online: https://healthtechafrica.org/sites/default/files/resources/GENOME-EDITING-GUIDELINES-FINAL-VERSION-25th-Feb-2022-03.pdf (accessed on 11 October 2025).
- Entrican, G.; Bredell, H.; Charlier, J.; Cunningham, A.F.; Jarvis, M.A.; Wood, P.R.; Wren, B.W.; Hope, J.C. Opportunities and challenges for the adoption of novel platform technologies to develop veterinary bacterial vaccines. Vaccine 2025, 54, 127117. [Google Scholar] [CrossRef] [PubMed]
- Koralesky, K.E.; Sirovica, L.V.; Hendricks, J.; Mills, K.E.; von Keyserlingk, M.A.; Weary, D.M. Social acceptance of genetic engineering technology. PLoS ONE 2023, 18, e0290070. [Google Scholar] [CrossRef]
- McFadden, B.R.; Rumble, J.N.; Stofer, K.A.; Folta, K.M. US public opinion about the safety of gene editing in the agriculture and medical fields and the amount of evidence needed to improve opinions. Front. Bioeng. Biotechnol. 2024, 12, 1340398. [Google Scholar] [CrossRef]
- Bearth, A.; Otten, C.D.; Cohen, A.S. Consumers’ perceptions and acceptance of genome editing in agriculture: Insights from the United States of America and Switzerland. Food Res. Int. 2024, 178, 113982. [Google Scholar] [CrossRef]
- Kuo, C.; Koralesky, K.E.; von Keyserlingk, M.A.; Weary, D.M. Gene editing in animals: What does the public want to know and what information do stakeholder organizations provide? Public Underst. Sci. 2024, 33, 725–739. [Google Scholar] [CrossRef]
- Auplish, A.; Tra Vu, T.T.; Pham Duc, P.; Green, A.C.; Tiwari, H.; Housen, T.; Stevenson, M.; Dhand, N. Investigating the workforce capacity and needs for animal disease surveillance and outbreak investigation: A mixed-methods study of veterinary services in Vietnam. Front. Vet. Sci. 2024, 11, 1410606. [Google Scholar] [CrossRef]
- ILRI. ‘Develop, Demonstrate and Deploy’: Building Capacity in DNA Sequencing for Agricultural Biosciences in Africa. 2024. Available online: https://www.ilri.org/news/develop-demonstrate-and-deploy-building-capacity-dna-sequencing-agricultural-biosciences (accessed on 12 September 2025).
- EFSA. New Genomic Techniques. Available online: https://www.efsa.europa.eu/en/topics/new-genomic-techniques (accessed on 11 October 2025).
- WOAH (Formerly OIE). OIE Programme for Veterinary Workforce Development: Strengthening National Veterinary Services for More Effective Animal Health and Veterinary Public Health Systems. 2021. Available online: https://www.woah.org/fileadmin/RC_AFEO_2021/bdp-workforce-development-updated.pdf (accessed on 12 September 2025).
- Hibbard, R.; Mendelson, M.; Page, S.W.; Ferreira, J.P.; Pulcini, C.; Paul, M.C.; Faverjon, C. Antimicrobial stewardship: A definition with a One Health perspective. npj Antimicrob. Resist. 2024, 2, 15. [Google Scholar] [CrossRef]
- Clifford Astbury, C.; Lee, K.M.; Mcleod, R.; Aguiar, R.; Atique, A.; Balolong, M.; Clarke, J.; Demeshko, A.; Labonté, R.; Ruckert, A. Policies to prevent zoonotic spillover: A systematic scoping review of evaluative evidence. Glob. Health 2023, 19, 82. [Google Scholar] [CrossRef]
- Aenishaenslin, C.; Häsler, B.; Ravel, A.; Parmley, E.J.; Mediouni, S.; Bennani, H.; Stärk, K.D.; Buckeridge, D.L. Evaluating the integration of one health in surveillance systems for antimicrobial use and resistance: A conceptual framework. Front. Vet. Sci. 2021, 8, 611931. [Google Scholar] [CrossRef] [PubMed]
- WHX-Insights. mRNA Vaccine Cold Chain Challenges Threaten Widespread Access across African Healthcare Systems. Available online: https://www.worldhealthexpo.com/insights/medical-laboratory/mrna-vaccine-cold-chain-challenges-threaten-widespread-access-across-african-healthcare-systems (accessed on 12 September 2025).
- Pacholec, C.; Flatland, B.; Xie, H.; Zimmerman, K. Harnessing artificial intelligence for enhanced veterinary diagnostics: A look to quality assurance, Part I Model development. Vet. Clin. Pathol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.; Wu, S.; Zaldivar, A.; Barnes, P.; Vasserman, L.; Hutchinson, B.; Spitzer, E.; Raji, I.D.; Gebru, T. Model cards for model reporting. In Proceedings of the Proceedings of the Conference on Fairness, Accountability, and Transparency, Atlanta, GA, USA, 29–31 January 2019; pp. 220–229. [Google Scholar]
- Collins, G.S.; Moons, K.G.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; Van Smeden, M. TRIPOD+ AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 2024, 385, q902. [Google Scholar] [CrossRef]
- Liu, X.; Rivera, S.C.; Moher, D.; Calvert, M.J.; Denniston, A.K.; Ashrafian, H.; Beam, A.L.; Chan, A.-W.; Collins, G.S.; Deeks, A.D.J. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI extension. Lancet Digit. Health 2020, 2, e537–e548. [Google Scholar] [CrossRef]
- van Bavel, B.; Berrang-Ford, L.; Moon, K.; Gudda, F.; Thornton, A.J.; Robinson, R.F.; King, R. Intersections between climate change and antimicrobial resistance: A systematic scoping review. Lancet Planet. Health 2024, 8, e1118–e1128. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhao, J.; Yuan, Y.; Feng, B. Climate change and antimicrobial resistance: A global-scale analysis. BMC Infect. Dis. 2025, 25, 1–15. [Google Scholar] [CrossRef]
- Alhassan, M.Y.; Ahmad, A.A. Antimicrobial resistance in a changing climate: A One Health approach for adaptation and mitigation. Bull. Natl. Res. Cent. 2025, 49, 26. [Google Scholar] [CrossRef]
- Kou, R.; Li, X.; Wang, H.; Wu, Y.; Zou, D.; Hu, J.; Qiu, S.; Yang, L.; Zhang, X. Panel data analysis of the effect of ambient temperature on antimicrobial resistance in China. Sci. Rep. 2025, 15, 18391. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shen, Z.; Zhou, Q.; Ren, J.; Wang, H.; Zhang, D.; Pan, X. Antibiotic resistance genes in the animal manure-amended soil-plant system: Occurrence, transmission, bacterial hosts, and human health risks. Ecotoxicol. Environ. Saf. 2025, 303, 118890. [Google Scholar] [CrossRef]
- Meradji, S.; Basher, N.S.; Sassi, A.; Ibrahim, N.A.; Idres, T.; Touati, A. The Role of Water as a Reservoir for Antibiotic-Resistant Bacteria. Antibiotics 2025, 14, 763. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Drigo, B.; Sarkar, B. Mitigation potential of antibiotic resistance genes in water and soil by clay-based adsorbents. npj Mater. Sustain. 2024, 2, 26. [Google Scholar] [CrossRef]
- Malcom, H.B.; Bowes, D.A. Use of Wastewater to Monitor Antimicrobial Resistance Trends in Communities and Implications for Wastewater-Based Epidemiology: A Review of the Recent Literature. Microorganisms 2025, 13, 2073. [Google Scholar] [CrossRef]
- Punch, R.; Azani, R.; Ellison, C.; Majury, A.; Hynds, P.D.; Payne, S.J.; Brown, R.S. The surveillance of antimicrobial resistance in wastewater from a one health perspective: A global scoping and temporal review (2014–2024). One Health 2025, 21, 101139. [Google Scholar] [CrossRef]
- Craddock, H.A.; Kearney, A.; Fitzpatrick, F.; Finn, C.; Pryce, M.T.; Fitzgerald-Hughes, D. The challenge of reducing antimicrobial resistance (AMR) across the one health landscape: Diverse perspectives on AMR risks and their mitigation in sinks, drains, and wastewater. Sci. Total Environ. 2025, 992, 179935. [Google Scholar] [CrossRef]
- McCorquodale-Bauer, K.; Flores Orozco, D.; Grosshans, R.; Zvomuya, F.; Cicek, N. Reduction of antimicrobial resistance genes in wastewater through phytoremediation. Front. Synth. Biol. 2025, 2, 1513580. [Google Scholar] [CrossRef]
- La Rosa, M.C.; Maugeri, A.; Favara, G.; La Mastra, C.; Magnano San Lio, R.; Barchitta, M.; Agodi, A. The impact of wastewater on antimicrobial resistance: A scoping review of transmission pathways and contributing factors. Antibiotics 2025, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Mediouni, S.; Ndione, C.; Parmley, E.J.; Poder, T.G.; Carabin, H.; Aenishaenslin, C. Systematic review on evaluation tools applicable to One Health surveillance systems: A call for adapted methodology. One Health 2025, 20, 100995. [Google Scholar] [CrossRef]
- WHO. Surveillance and Information Sharing Operational Tool: An Operational Tool of the Tripartite Zoonoses Guide. 2022. Available online: https://iris.who.int/server/api/core/bitstreams/44a70f74-6e54-42d9-899d-d3466c5b5b6e/content (accessed on 11 October 2025).
- Milenkov, M.; Proux, C.; Rasolofoarison, T.L.; Rakotomalala, F.A.; Rasoanandrasana, S.; Rahajamanana, V.L.; Rafalimanana, C.; Ravaoarisaina, Z.; Ramahatafandry, I.T.H.; Westeel, E. Implementation of the WHO Tricycle protocol for surveillance of extended-spectrum β-lactamase producing Escherichia coli in humans, chickens, and the environment in Madagascar: A prospective genomic epidemiology study. Lancet Microbe 2024, 5, 100850. [Google Scholar] [CrossRef] [PubMed]
- Ruppé, E. Lessons from a global antimicrobial resistance surveillance network. Bull. World Health Organ. 2023, 101, 672. [Google Scholar] [CrossRef]
- Kumari, L.S.; Siriwardhana, D.M.; Liyanapathirana, V.; Jinadasa, R.; Wijesinghe, P. Rapid whole genome sequencing for AMR surveillance in low-and middle-income countries: Oxford Nanopore Technology reveals multidrug-resistant Enterobacter cloacae complex from dairy farms in Sri Lanka. BMC Vet. Res. 2025, 21, 351. [Google Scholar] [CrossRef]
- Vallejo-Espín, D.; Galarza-Mayorga, J.; Lalaleo, L.; Calero-Cáceres, W. Beyond clinical genomics: Addressing critical gaps in One Health AMR surveillance. Front. Microbiol. 2025, 16, 1596720. [Google Scholar] [CrossRef]
- Eloe-Fadrosh, E.A.; Mungall, C.J.; Miller, M.A.; Smith, M.; Patil, S.S.; Kelliher, J.M.; Johnson, L.Y.; Rodriguez, F.E.; Chain, P.S.; Hu, B. A practical approach to using the Genomic Standards Consortium MIxS reporting standard for comparative genomics and metagenomics. In Comparative Genomics: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2024; pp. 587–609. [Google Scholar]
- Kim, H.; Park, J.S.; Kim, D.; Kim, H.J.; Shin, J.H.; Kim, Y.A.; Uh, Y.; Kim, S.H.; Shin, J.H.; Jeong, S.H. Standardization of an antimicrobial resistance surveillance network through data management. Front. Cell. Infect. Microbiol. 2024, 14, 1411145. [Google Scholar] [CrossRef]
- Chakrawarti, A.; Eckstrom, K.; Laaguiby, P.; Barlow, J.W. Hybrid Illumina-Nanopore assembly improves identification of multilocus sequence types and antimicrobial resistance genes of Staphylococcus aureus isolated from Vermont dairy farms: Comparison to Illumina-only and R9. 4.1 nanopore-only assemblies. Access Microbiol. 2024, 6, 000766.v3. [Google Scholar] [CrossRef] [PubMed]
- Hallerman, E.; Bredlau, J.; Camargo, L.S.A.; Dagli, M.L.Z.; Karembu, M.; Kovich, D.; Muia, A.N.; Murrone, M.L.; Rocha-Salavarrieta, P.J.; Romero-Aldemita, R. Enabling regulatory policy globally will promote realization of the potential of animal biotechnology. CABI Agric. Biosci. 2024, 5, 25. [Google Scholar] [CrossRef]
- FDA. Intentional Genomic Alterations (IGAs) in Animals: Risk-Reviewed IGAs. 2025. Available online: https://www.fda.gov/animal-veterinary/intentional-genomic-alterations-igas-animals/intentional-genomic-alterations-igas-animals-risk-reviewed-igas (accessed on 11 October 2025).
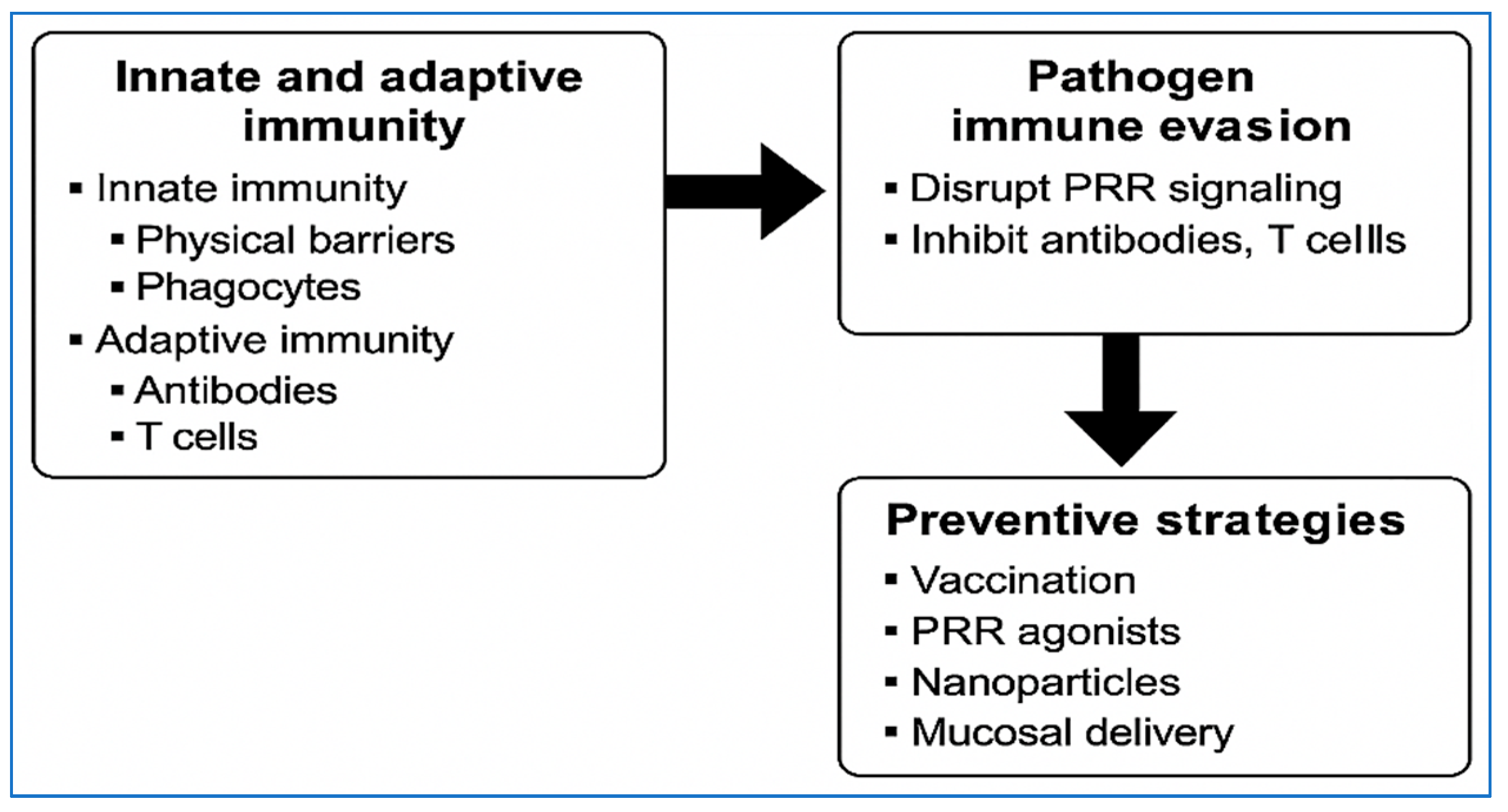
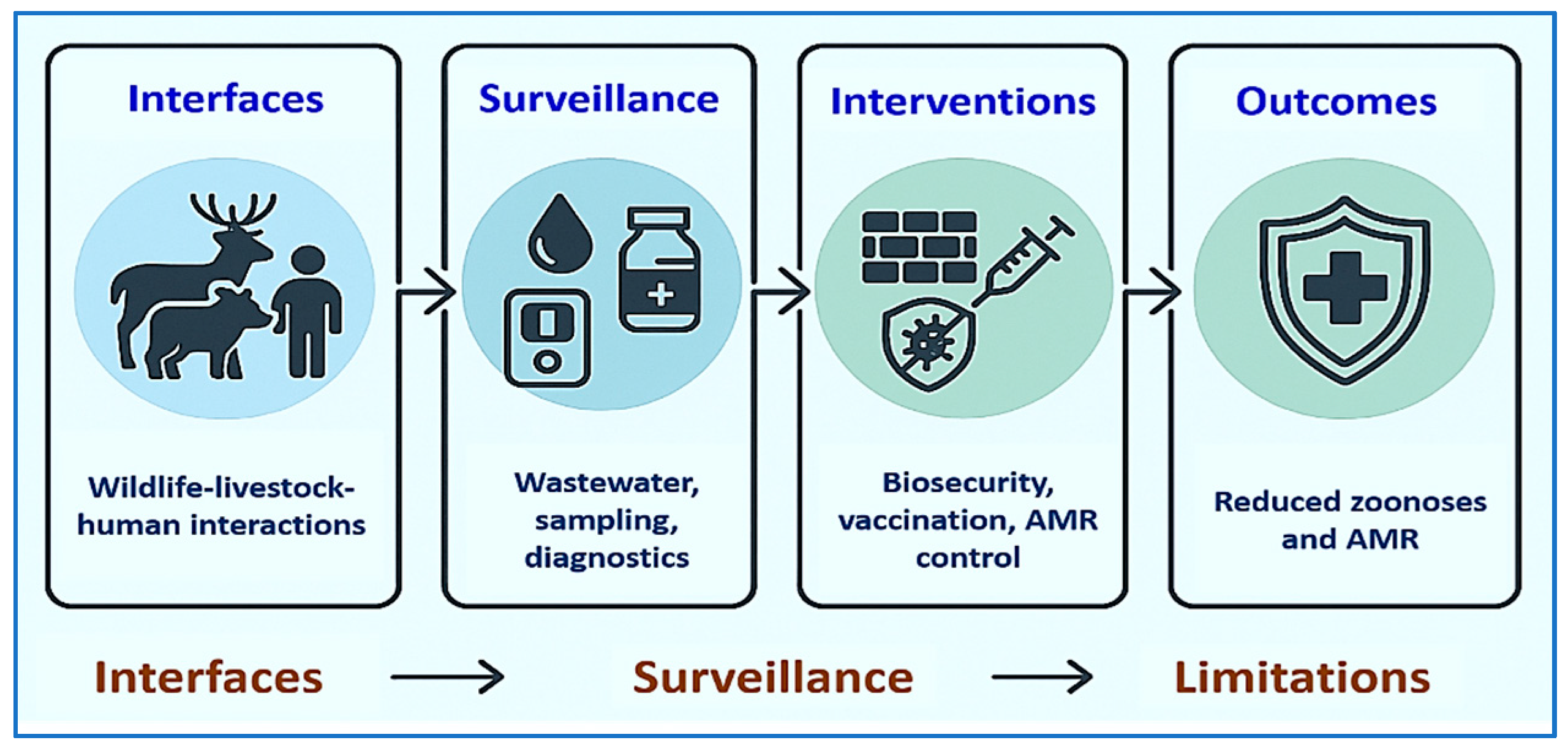
| Domain | Key Feature | Species/Pathogen Context | Preventive Implication | References |
|---|---|---|---|---|
| Innate Immunity | PRR signaling (e.g., TLRs) | Mammals (TLR9), Chickens (TLR21) | Basis for CpG ODN adjuvants; drives trained immunity | [92,93] |
| Trained immunity | Bovine γδ T cells | Epigenetic reprogramming after BCG; potential vaccine enhancement | [44,94] | |
| Adaptive Immunity | Long-lived memory B/T cells | All livestock species | Basis for durable vaccine protection | [41,42] |
| Th1 bias (IFN-γ, TNF-α) | M. bovis | Correlates of protection for intracellular bacteria | [59,95] | |
| Th17 + mucosal T_RM | Enteric & respiratory pathogens | Critical for mucosal vaccines | [48,96] | |
| Species-Specific Features | γδ T cell abundance (15–60%) | Ruminants | Important targets for vaccine design | [43,97] |
| Bursa of Fabricius | Birds (e.g., poultry) | Central to B cell ontogeny | [98] | |
| Human-like immune system | Pigs (~80% overlap) | Translational model for vaccinology | [99,100] | |
| Pathogen Immune Evasion | UPR manipulation, smooth LPS | Brucella spp. | Subverts antigen presentation & autophagy | [51,101] |
| Protein A, MSCRAMMs | S. aureus | Disrupts opsonization & B cell responses | [102,103] | |
| Granuloma complexity | M. bovis | Limits sterilizing immunity | [59,60] | |
| Adjuvants & Platforms | CpG ODNs, MPLA, Poly I:C | Poultry, ruminants, swine | Direct Th bias; cross-serotype protection | [104,105] |
| Nanoparticles (lipid, SAPNs, VLPs) | CSFV, PRV | Improve antigen uptake, stability, dose-sparing | [76,106] | |
| Maternal & Early Life | Colostrum-derived IgG | Neonatal ruminants | Passive transfer; defines vaccination windows | [107,108] |
| Diagnostics | MALDI-TOF MS, AI, biosensors | Herd-level disease control | Rapid pathogen ID & early vaccination planning | [23] |
| Systems Immunology | scRNA-seq, cell atlases | Bovine mastitis, TB | Defines biomarkers, precision vaccines | [90,109] |
| Phage Type | Applications | Immunological Benefits | Limitations | References |
|---|---|---|---|---|
| Natural Phages | Used against Salmonella, E. coli, and S. aureus in poultry, swine, and cattle; mastitis control in dairy systems | Reduce pathogen burden; restore microbiota balance; stimulate innate immune responses (macrophage activation, cytokine release) | Narrow host range; risk of bacterial resistance; limited stability under farm conditions; regulatory hurdles | [220,222,223,224] |
| Engineered Phages | CRISPR-armed phages targeting resistance genes; synthetic phages for broader coverage and lytic activity | High specificity; overcome resistance mechanisms; potential for multivalent action; lower impact on commensals | Complex design and manufacturing; stability and delivery challenges; higher costs; regulatory uncertainty | [211,215,225,226] |
| Target Gene/Pathway | Species | Pathogen/Disease | Editing Approach | Preventive Outcome | Reference |
|---|---|---|---|---|---|
| CD163 (SRCR5 domain removal) | Pig | PRRSV | CRISPR/Cas9-mediated exon deletion | Pigs resistant to PRRSV infection with no detectable viremia post-challenge | [242] |
| ANP32A (point substitution) | Chicken | Avian influenza virus (AIV) | CRISPR/Cas9-mediated point mutation | Near-complete resistance to AIV replication in edited chickens | [233] |
| CD46 | Cattle | BVDV | CRISPR/Cas9 knockout | Reduced susceptibility of edited calves to BVDV infection | [234] |
| NRAMP1 (knock-in) | Cattle | Bovine tuberculosis (M. bovis) | CRISPR/Cas9 nickase + HMEJ strategy | Edited cattle showed enhanced resistance to bTB in macrophage assays | [237,243] |
| Phage-delivered CRISPR antimicrobials | Various livestock-associated bacteria | AMR pathogens | Engineered phages delivering CRISPR/Cas payloads | Targeted removal of antimicrobial resistance genes (ARGs); restored antibiotic sensitivity | [215,238] |
| Platform | Targets/Use | Sample Type | Turnaround Time | Use Cases | Limitations | References |
|---|---|---|---|---|---|---|
| Lateral Flow Immunoassays (LFIA) | Antigens/antibodies (e.g., FMD, Brucella) | Blood, milk, swabs | Minutes | On-farm screening for endemic/zoonotic pathogens | Lower sensitivity than lab-based assays | [276] |
| Isothermal NAAT (LAMP, RPA, ERA + CRISPR) | Pathogen nucleic acids (e.g., ASFV, avian influenza subtypes H5/H7/H9) | Blood, swabs (oropharyngeal, cloacal), serum | ~30–60 min | Field-deployable molecular confirmation; early surveillance; reaction even without perfect lab infrastructure | Risk of contamination (especially when amplification and detection are separated); primer design; sample prep; minimal training required | [277,278,279,280] |
| CRISPR-based assays (e.g., DETECTR, SHERLOCK) | Viral/bacterial DNA or RNA | Swabs, blood | Under 1 h | Highly specific detection of priority pathogens | Still early-stage validation in livestock | [281,282] |
| MALDI-TOF MS | Microbial proteins/antibiotic resistance markers | Cultured isolates, direct clinical samples | Minutes to hours | Rapid pathogen ID and AMR profiling | Database limitations, infrastructure needs | [23,283,284,285] |
| Wearables & Biosensors | Physiological biomarkers (e.g., temp, metabolites) | Animal-attached sensors, saliva, milk | Real-time | Continuous herd health monitoring | Cost, calibration, data integration | [252,286,287] |
| AI & ML Analytics | Big data from diagnostics, sensors, clinical records | Integrated datasets | Real-time to predictive | Outbreak prediction, precision livestock farming | Data standardization, interpretability | [288,289,290,291] |
| Governance Element | Key Metrics/Indicators | Country/Region Example | Reference |
|---|---|---|---|
| Regulatory and Approval Pathways | Number of approvals for gene-edited/vaccine products; time-to-market; clarity of guidance | USA FDA GFI #187A/B; Argentina/Japan policies distinguishing genome editing from GMOs | [312,313,317,318] |
| Economic and Logistical Barriers | Cost per dose; cold chain reliability; vaccine wastage rates; facility capacity | WHO mRNA Technology Transfer Hub (South Africa); livestock vaccination cost studies in LMICs | [242,324,335,336] |
| Ethical, Societal, and Acceptance Issues | Public acceptance rates; trust in regulatory bodies; presence of labeling/traceability policies | UK public dialog on gene editing; US consumer surveys on livestock gene editing | [325,326,327] |
| Workforce and Capacity Gaps | Veterinary staff per 10,000 livestock; training hours per year; retention rates; lab diagnostic capacity | Vietnam field vet training study; ILRI genomics training in Africa; WOAH PVS Pathway | [329,330,332] |
| Policy Integration and One Health Governance | AMU/AMR reduction rates; outbreak frequency; zoonotic spillover tracking; diagnostic lead times; vaccine coverage | Implementation of OH Joint Plan of Action; Canada ISSE framework; national AMR stewardship plans | [30,333,334,335] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzouk, E.; Alajaji, A.I. Preventive Immunology for Livestock and Zoonotic Infectious Diseases in the One Health Era: From Mechanistic Insights to Innovative Interventions. Vet. Sci. 2025, 12, 1014. https://doi.org/10.3390/vetsci12101014
Marzouk E, Alajaji AI. Preventive Immunology for Livestock and Zoonotic Infectious Diseases in the One Health Era: From Mechanistic Insights to Innovative Interventions. Veterinary Sciences. 2025; 12(10):1014. https://doi.org/10.3390/vetsci12101014
Chicago/Turabian StyleMarzouk, Eman, and Ahmed I. Alajaji. 2025. "Preventive Immunology for Livestock and Zoonotic Infectious Diseases in the One Health Era: From Mechanistic Insights to Innovative Interventions" Veterinary Sciences 12, no. 10: 1014. https://doi.org/10.3390/vetsci12101014
APA StyleMarzouk, E., & Alajaji, A. I. (2025). Preventive Immunology for Livestock and Zoonotic Infectious Diseases in the One Health Era: From Mechanistic Insights to Innovative Interventions. Veterinary Sciences, 12(10), 1014. https://doi.org/10.3390/vetsci12101014





